Example of Evaluation Decay of 177 Lu 6


- Slides: 25

Example of Evaluation: Decay of 177 Lu (6. 647 d) Filip G. Kondev kondev@anl. gov 2 nd Workshop for DDEP Evaluators, Bucharest, Romania May 12 -15, 2008

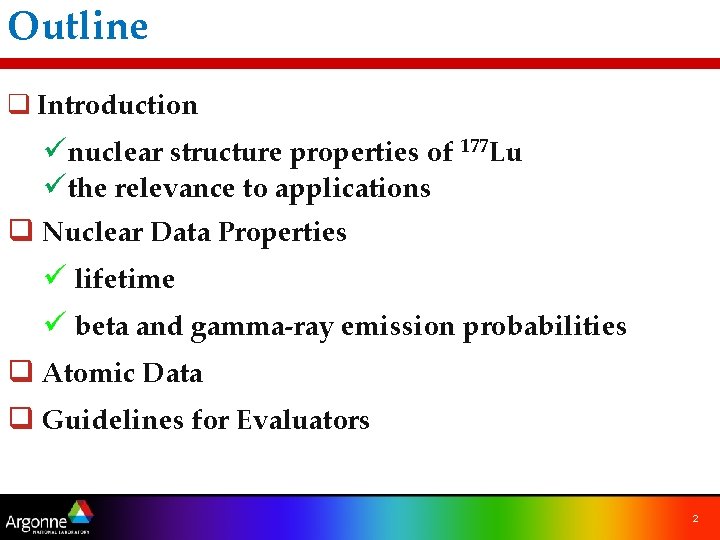
Outline q Introduction ünuclear structure properties of 177 Lu üthe relevance to applications q Nuclear Data Properties ü lifetime ü beta and gamma-ray emission probabilities q Atomic Data q Guidelines for Evaluators 2

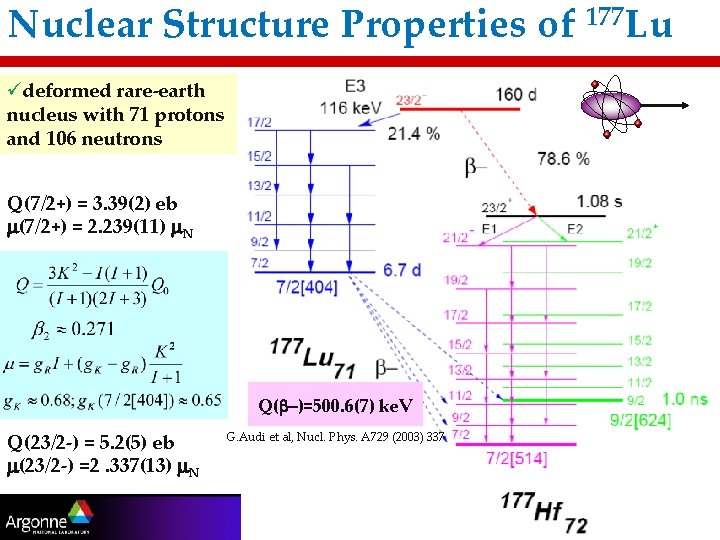
Nuclear Structure Properties of 177 Lu üdeformed rare-earth nucleus with 71 protons and 106 neutrons Q(7/2+) = 3. 39(2) eb m(7/2+) = 2. 239(11) m. N Q(b-)=500. 6(7) ke. V Q(23/2 -) = 5. 2(5) eb m(23/2 -) =2. 337(13) m. N G. Audi et al, Nucl. Phys. A 729 (2003) 337 3

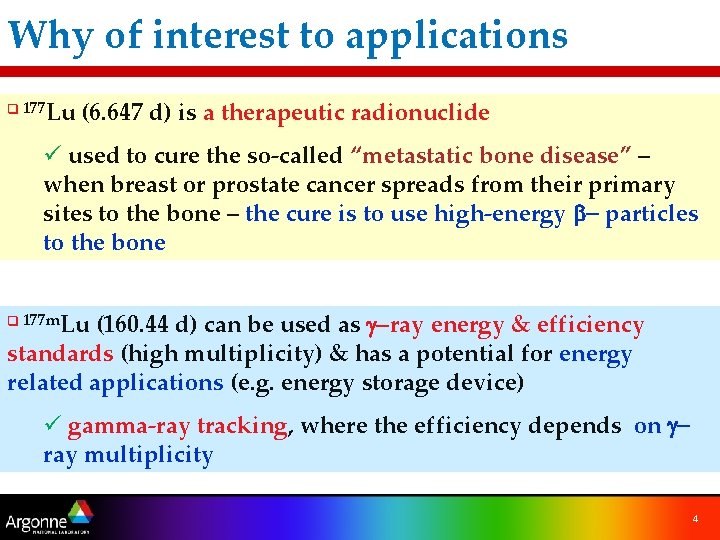
Why of interest to applications Lu (6. 647 d) is a therapeutic radionuclide q 177 ü used to cure the so-called “metastatic bone disease” – when breast or prostate cancer spreads from their primary sites to the bone – the cure is to use high-energy b- particles to the bone Lu (160. 44 d) can be used as g-ray energy & efficiency standards (high multiplicity) & has a potential for energy related applications (e. g. energy storage device) q 177 m ü gamma-ray tracking, where the efficiency depends on gray multiplicity 4

Nuclear Data q Q(b-) ü G. Audi et al, Nucl. Phys. A 729 (2003) 337 ü http: //www. nndc. bnl. gov/qcalc/ q Lifetime ü need to be evaluated q Emission energies & probabilities (b- and g) ü need to know the decay scheme - adopted Ex, Jp, mult (ENSDF) ü a. T – use BRICC (see talk by T. Kibedi) üevaluate Eg, Pg, d, Pbü calculate Eb-, max, log ft 5

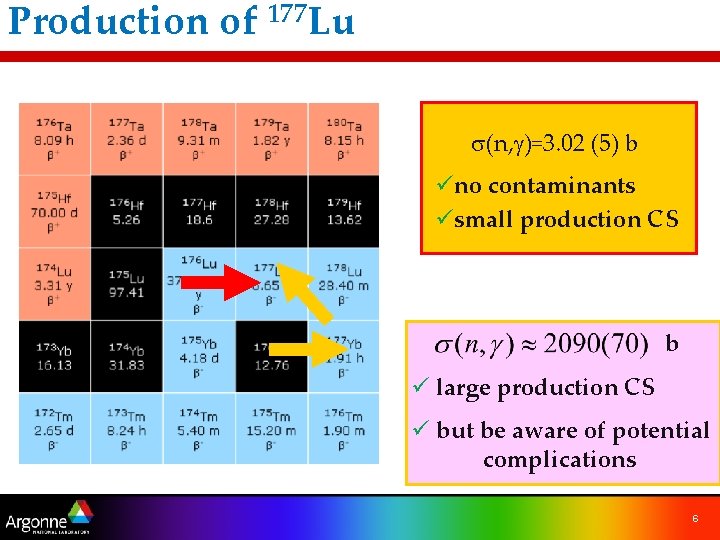
Production of 177 Lu s(n, g)=3. 02 (5) b üno contaminants üsmall production CS b ü large production CS ü but be aware of potential complications 6

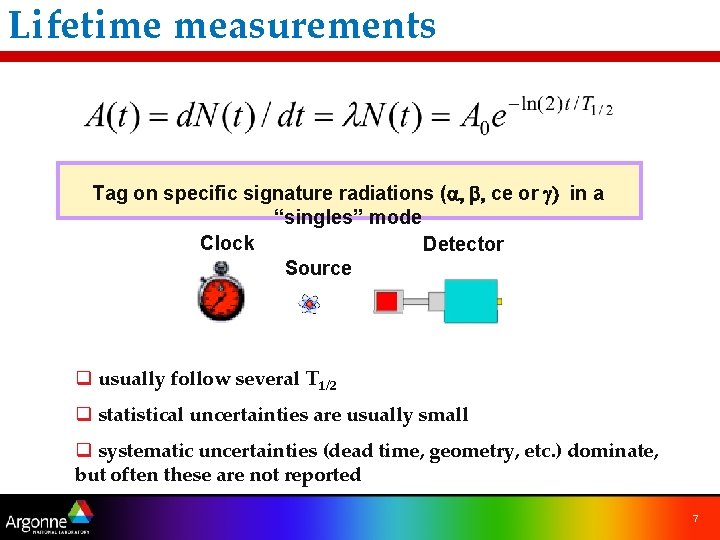
Lifetime measurements Tag on specific signature radiations (a, b, ce or g) in a “singles” mode Clock Detector Source q usually follow several T 1/2 q statistical uncertainties are usually small q systematic uncertainties (dead time, geometry, etc. ) dominate, but often these are not reported 7

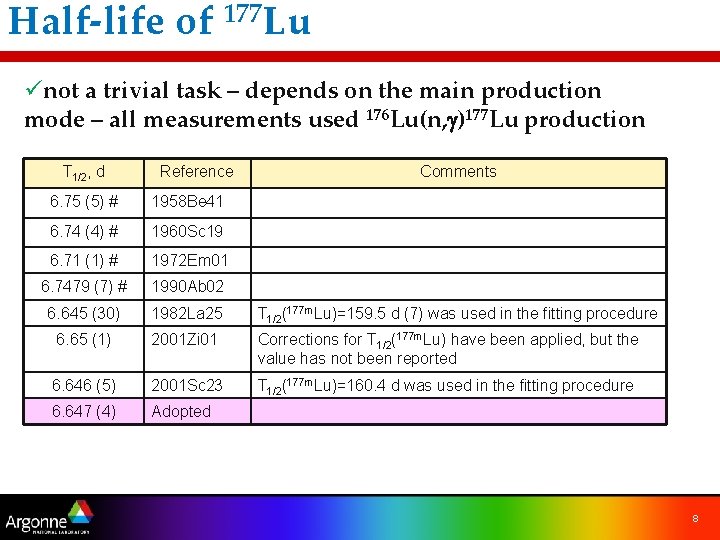
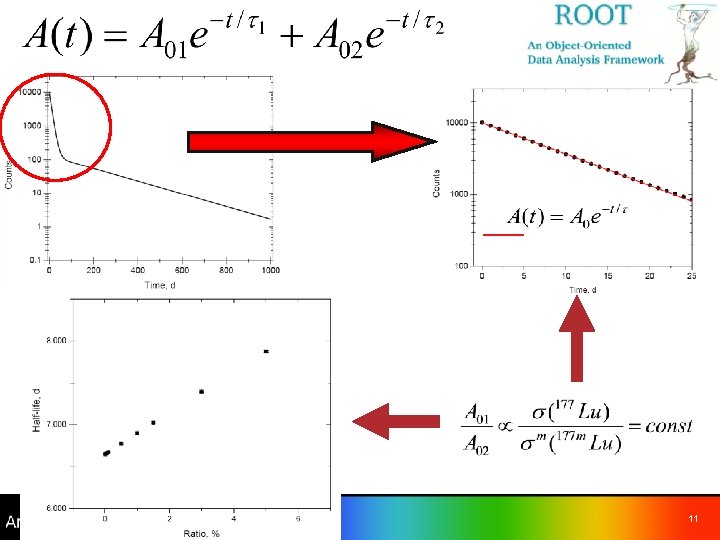
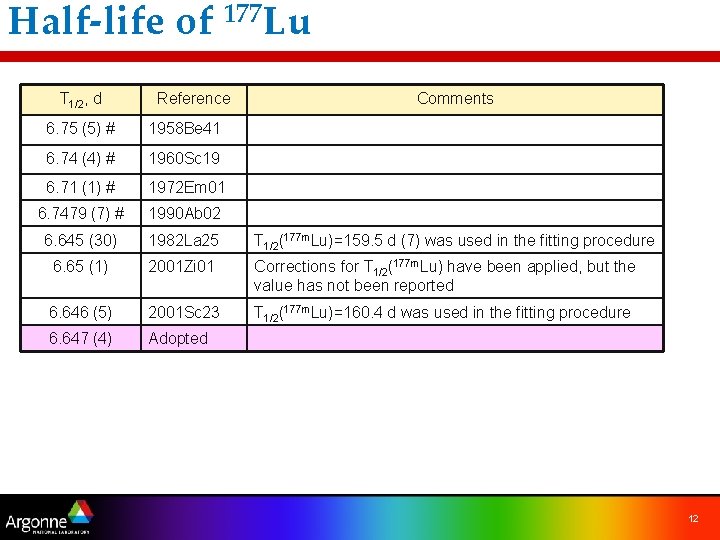
Half-life of 177 Lu ünot a trivial task – depends on the main production mode – all measurements used 176 Lu(n, g)177 Lu production T 1/2, d Reference Comments 6. 75 (5) # 1958 Be 41 6. 74 (4) # 1960 Sc 19 6. 71 (1) # 1972 Em 01 6. 7479 (7) # 1990 Ab 02 6. 645 (30) 1982 La 25 T 1/2(177 m. Lu)=159. 5 d (7) was used in the fitting procedure 6. 65 (1) 2001 Zi 01 Corrections for T 1/2(177 m. Lu) have been applied, but the value has not been reported 6. 646 (5) 2001 Sc 23 T 1/2(177 m. Lu)=160. 4 d was used in the fitting procedure 6. 647 (4) Adopted 8

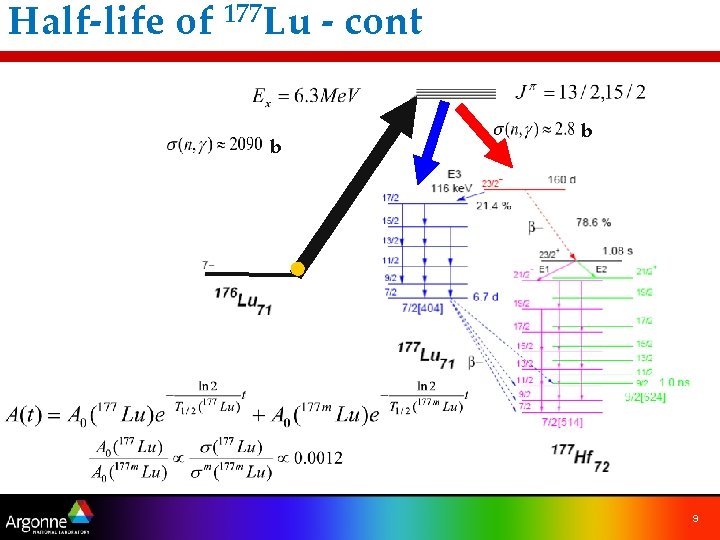
Half-life of 177 Lu - cont b b 9

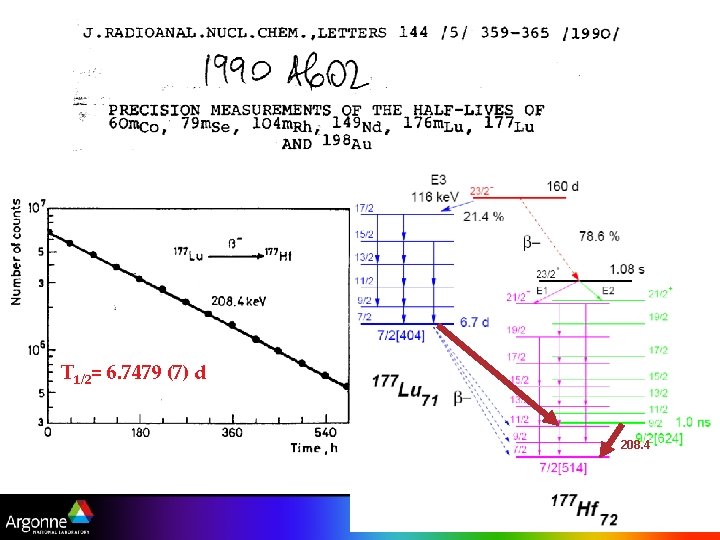
T 1/2= 6. 7479 (7) d 208. 4 10

11

Half-life of 177 Lu T 1/2, d Reference Comments 6. 75 (5) # 1958 Be 41 6. 74 (4) # 1960 Sc 19 6. 71 (1) # 1972 Em 01 6. 7479 (7) # 1990 Ab 02 6. 645 (30) 1982 La 25 T 1/2(177 m. Lu)=159. 5 d (7) was used in the fitting procedure 6. 65 (1) 2001 Zi 01 Corrections for T 1/2(177 m. Lu) have been applied, but the value has not been reported 6. 646 (5) 2001 Sc 23 T 1/2(177 m. Lu)=160. 4 d was used in the fitting procedure 6. 647 (4) Adopted 12

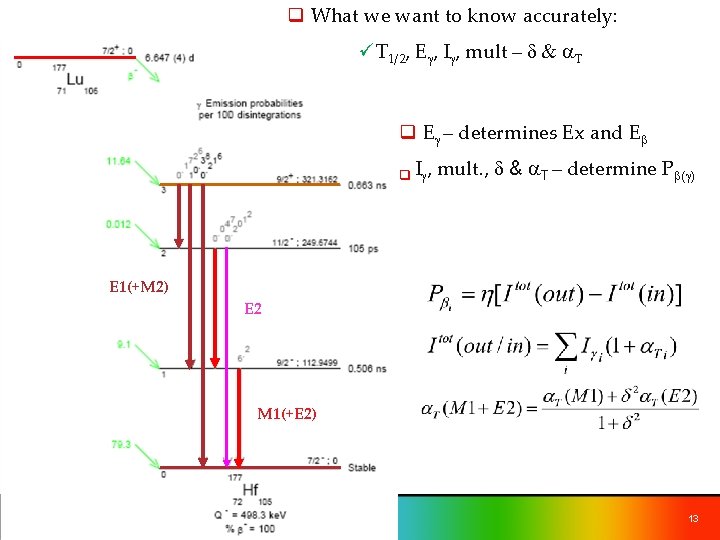
q What we want to know accurately: üT 1/2, Eg, Ig, mult – d & a. T q Eg – determines Ex and Eb q Ig, mult. , d & a. T – determine Pb(g) E 1(+M 2) E 2 M 1(+E 2) 13

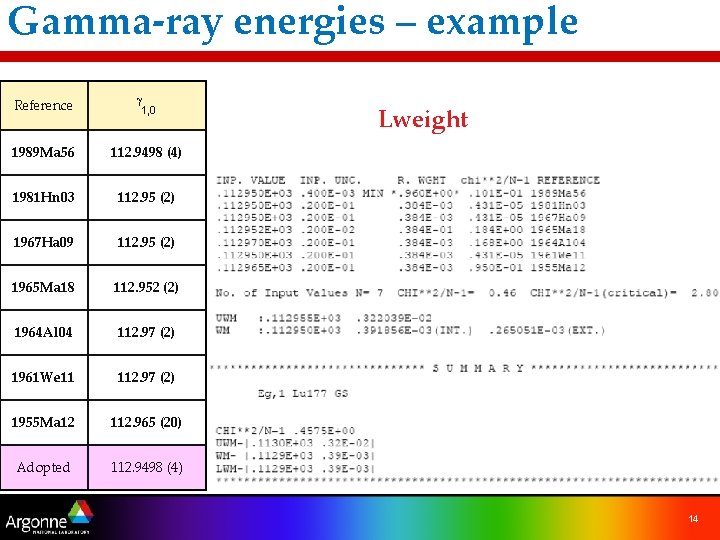
Gamma-ray energies – example Reference g 1, 0 1989 Ma 56 112. 9498 (4) 1981 Hn 03 112. 95 (2) 1967 Ha 09 112. 95 (2) 1965 Ma 18 112. 952 (2) 1964 Al 04 112. 97 (2) 1961 We 11 112. 97 (2) 1955 Ma 12 112. 965 (20) Adopted 112. 9498 (4) Lweight 14

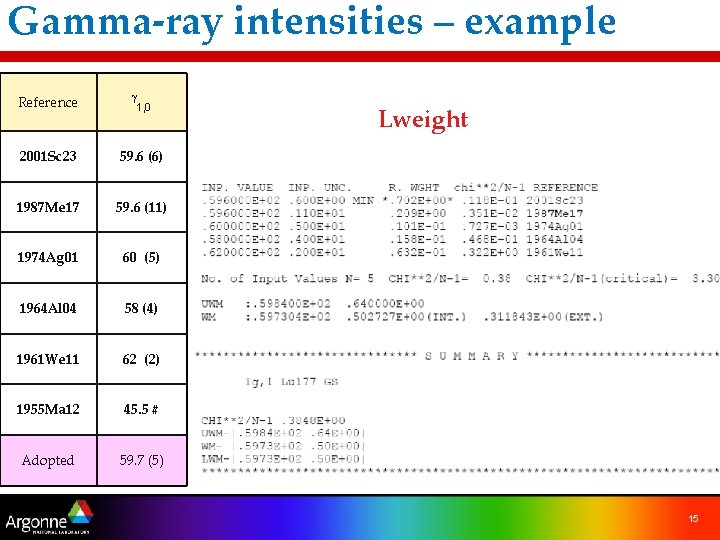
Gamma-ray intensities – example Reference g 1, 0 2001 Sc 23 59. 6 (6) 1987 Me 17 59. 6 (11) 1974 Ag 01 60 (5) 1964 Al 04 58 (4) 1961 We 11 62 (2) 1955 Ma 12 45. 5 # Adopted 59. 7 (5) Lweight 15

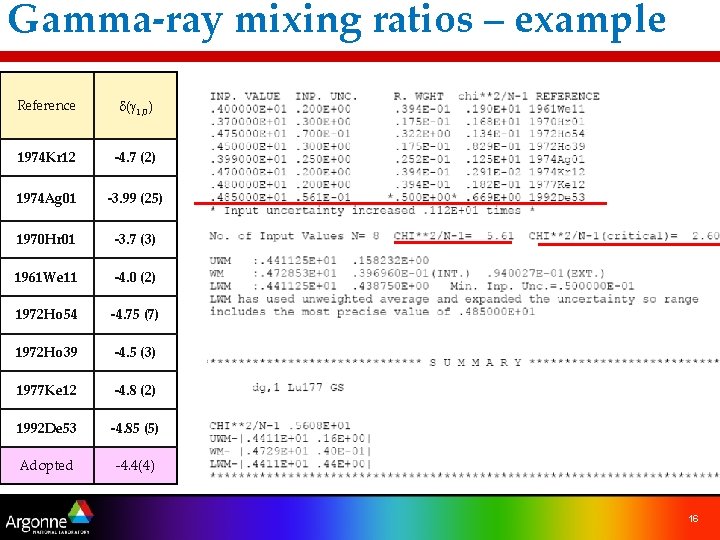
Gamma-ray mixing ratios – example Reference d(g 1, 0) 1974 Kr 12 -4. 7 (2) 1974 Ag 01 -3. 99 (25) 1970 Hr 01 -3. 7 (3) 1961 We 11 -4. 0 (2) 1972 Ho 54 -4. 75 (7) 1972 Ho 39 -4. 5 (3) 1977 Ke 12 -4. 8 (2) 1992 De 53 -4. 85 (5) Adopted -4. 4(4) 16

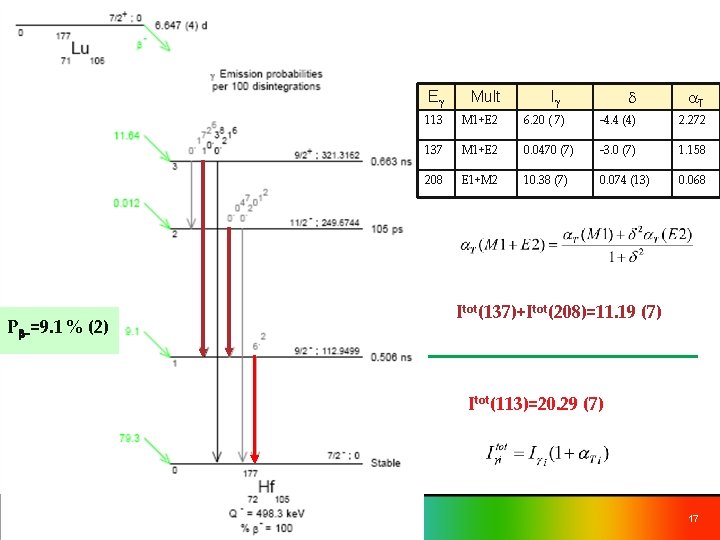
Pb-=9. 1 % (2) d a. T 6. 20 ( 7) -4. 4 (4) 2. 272 M 1+E 2 0. 0470 (7) -3. 0 (7) 1. 158 E 1+M 2 10. 38 (7) 0. 074 (13) 0. 068 Eg Mult 113 M 1+E 2 137 208 Ig Itot(137)+Itot(208)=11. 19 (7) Itot(113)=20. 29 (7) 17

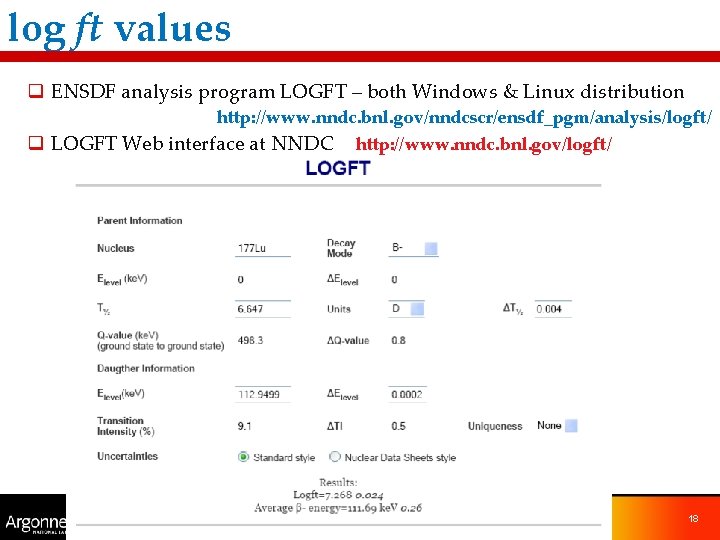
log ft values q ENSDF analysis program LOGFT – both Windows & Linux distribution http: //www. nndc. bnl. gov/nndcscr/ensdf_pgm/analysis/logft/ q LOGFT Web interface at NNDC http: //www. nndc. bnl. gov/logft/ 18

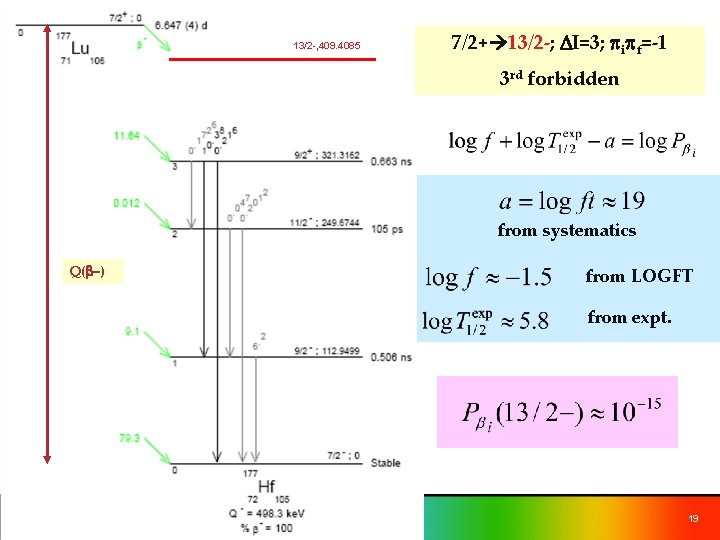
13/2 -, 409. 4085 7/2+ 13/2 -; DI=3; pipf=-1 3 rd forbidden from systematics Q(b-) from LOGFT from expt. 19

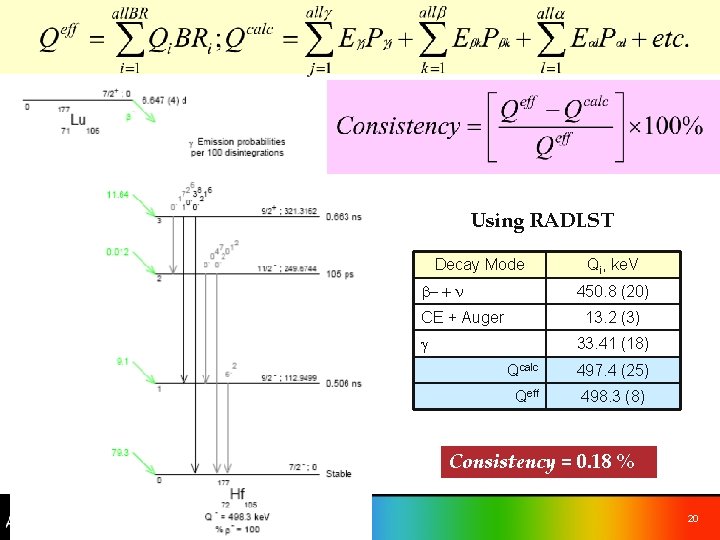
Using RADLST Decay Mode b- + n Qi, ke. V 450. 8 (20) CE + Auger 13. 2 (3) g 33. 41 (18) Qcalc 497. 4 (25) Qeff 498. 3 (8) Consistency = 0. 18 % 20

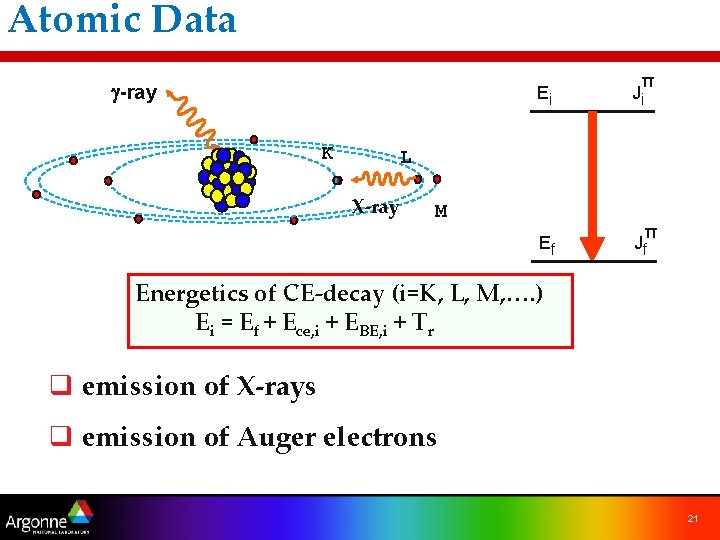
Atomic Data g-ray K Ei π Ji Ef π Jf L X-ray M Energetics of CE-decay (i=K, L, M, …. ) Ei = Ef + Ece, i + EBE, i + Tr q emission of X-rays q emission of Auger electrons 21

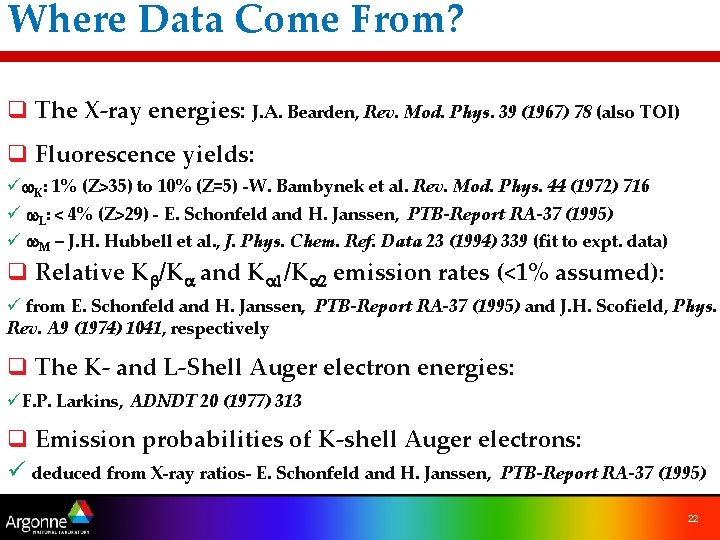
Where Data Come From? q The X-ray energies: J. A. Bearden, Rev. Mod. Phys. 39 (1967) 78 (also TOI) q Fluorescence yields: üw. K: 1% (Z>35) to 10% (Z=5) -W. Bambynek et al. Rev. Mod. Phys. 44 (1972) 716 ü w. L: < 4% (Z>29) - E. Schonfeld and H. Janssen, PTB-Report RA-37 (1995) ü w. M – J. H. Hubbell et al. , J. Phys. Chem. Ref. Data 23 (1994) 339 (fit to expt. data) q Relative Kb/Ka and Ka 1/Ka 2 emission rates (<1% assumed): ü from E. Schonfeld and H. Janssen, PTB-Report RA-37 (1995) and J. H. Scofield, Phys. Rev. A 9 (1974) 1041, respectively q The K- and L-Shell Auger electron energies: üF. P. Larkins, ADNDT 20 (1977) 313 q Emission probabilities of K-shell Auger electrons: ü deduced from X-ray ratios- E. Schonfeld and H. Janssen, PTB-Report RA-37 (1995) 22

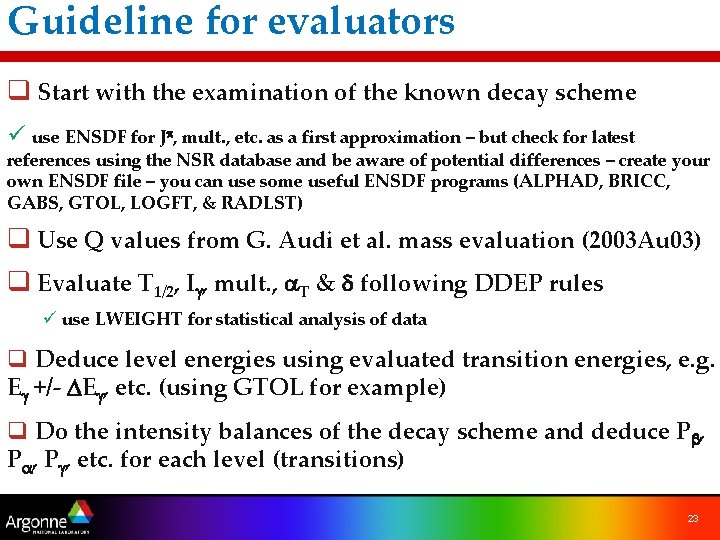
Guideline for evaluators q Start with the examination of the known decay scheme ü use ENSDF for Jp, mult. , etc. as a first approximation – but check for latest references using the NSR database and be aware of potential differences – create your own ENSDF file – you can use some useful ENSDF programs (ALPHAD, BRICC, GABS, GTOL, LOGFT, & RADLST) q Use Q values from G. Audi et al. mass evaluation (2003 Au 03) q Evaluate T 1/2, Ig, mult. , a. T & d following DDEP rules ü use LWEIGHT for statistical analysis of data q Deduce level energies using evaluated transition energies, e. g. Eg +/- DEg, etc. (using GTOL for example) q Do the intensity balances of the decay scheme and deduce Pb, Pa, Pg, etc. for each level (transitions) 23

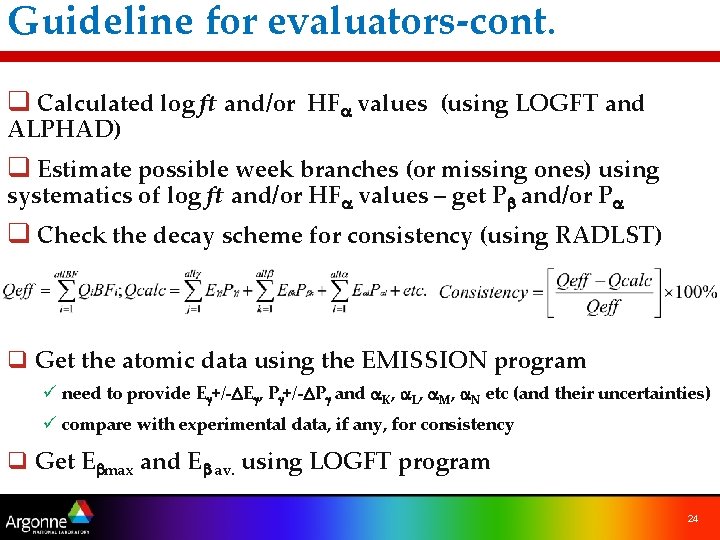
Guideline for evaluators-cont. q Calculated log ft and/or HFa values (using LOGFT and ALPHAD) q Estimate possible week branches (or missing ones) using systematics of log ft and/or HFa values – get Pb and/or Pa q Check the decay scheme for consistency (using RADLST) q Get the atomic data using the EMISSION program ü need to provide Eg+/-DEg, Pg+/-DPg and a. K, a. L, a. M, a. N etc (and their uncertainties) ü compare with experimental data, if any, for consistency q Get Ebmax and Eb av. using LOGFT program 24

Some personal notes … q Be critical to the experimental data you are dealing with! ü as all nuclei are different, so are the experiments q A good evaluation is not just simply averaging numbers! üsometime the most accurate value quoted in the literature is not the best one! q Enjoy what you have been doing! 25
 Lu-177 decay
Lu-177 decay Lu-177 decay
Lu-177 decay Beta plus decay
Beta plus decay Exponential decay factor
Exponential decay factor Stock market vocabulary worksheet
Stock market vocabulary worksheet Lagu pkj 177
Lagu pkj 177 Cs 177
Cs 177 177 rcacs
177 rcacs Krrish bakshi
Krrish bakshi Heights of hot air balloons qualitative or quantitative
Heights of hot air balloons qualitative or quantitative 177 church street
177 church street Multa 177 numeral 5
Multa 177 numeral 5 Commodus achievements
Commodus achievements Frank roorda
Frank roorda Iliad 177
Iliad 177 Psalm 34 zingen
Psalm 34 zingen Cs 177 purdue
Cs 177 purdue Cs 177
Cs 177 Time space convergence example ap human geography
Time space convergence example ap human geography Range definition ap human geography
Range definition ap human geography Exponential decay models
Exponential decay models Decay series example
Decay series example Decay theory example
Decay theory example Truth log example
Truth log example Neuropsychological testing examples
Neuropsychological testing examples Respect is over evaluation of the person
Respect is over evaluation of the person