Tooth Decay and Stuff Tooth Decay k Tooth















- Slides: 21

Tooth Decay and Stuff

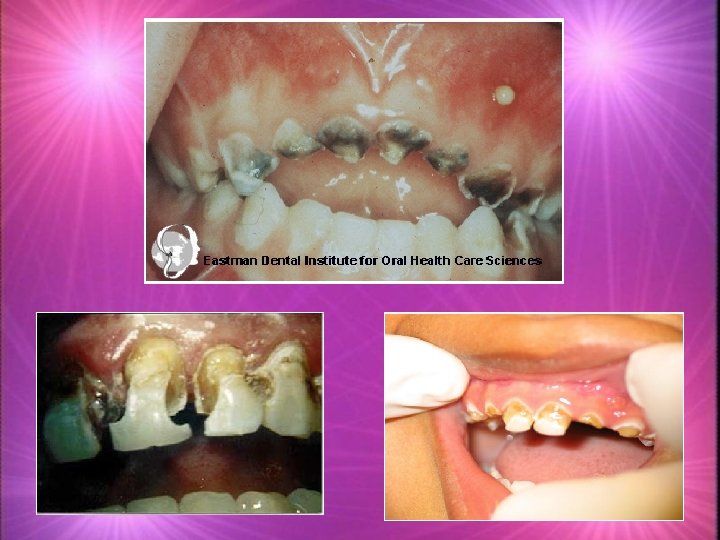
Tooth Decay k. Tooth decay has been present since there have been teeth to decay. k. Tooth decay, is an infectious disease that damages the structures of teeth k. Pain k. Tooth Loss k. Infection k. Death


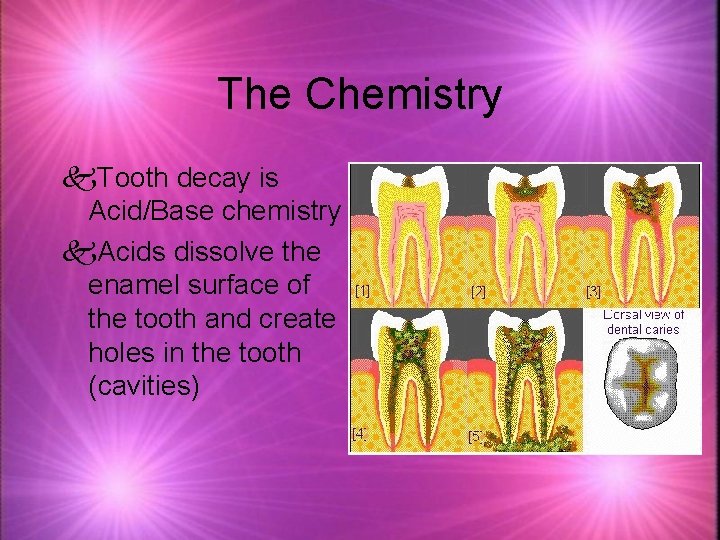
The Chemistry k. Tooth decay is Acid/Base chemistry k. Acids dissolve the enamel surface of the tooth and create holes in the tooth (cavities)

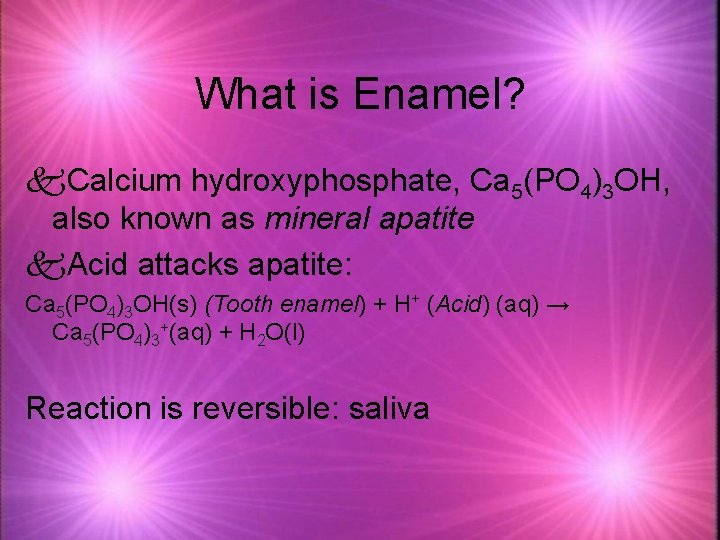
What is Enamel? k. Calcium hydroxyphosphate, Ca 5(PO 4)3 OH, also known as mineral apatite k. Acid attacks apatite: Ca 5(PO 4)3 OH(s) (Tooth enamel) + H+ (Acid) (aq) → Ca 5(PO 4)3+(aq) + H 2 O(l) Reaction is reversible: saliva

Fluoride is your friend k. If fluoride, F-, ions are present in saliva, fluorapatite, Ca 5(PO 4)3 F, also forms. Ca 5(PO 4)3+(aq) + F-(aq) → Ca 5(PO 4)3 F (s) k. Fluorapatite better than apatite itself (fluoride treatments)

Tooth decay, not just candy anymore… k. Not bacterial growth in the mouth, but alterations in internal body chemistry k. But candy doesn’t help!

What is a Dental Filling? Dental material used to restore the function/integrity/morphology of missing tooth structure -Indirect and direct restoration -Numerous types of methods

Amalgams? An amalgam is a mixture of mercury with another metal or alloy. Most metals are soluble in mercury. Amalgams are commonly used in dental fillings. Dental amalgam consists of three solid phases represented by Ag 2 Hg 3, Ag 3 Sn, Sn 8 Hg

Dental Amalgams k Dental amalgam is a stable alloy made by combining elemental mercury, silver, tin, copper and possibly other metallic elements. k ~65% minimum, silver copper ~6% maximum, copper ~2% minimum, zinc ~25% maximum, tin ~45% liquid mercury

Why Dental Amalgams? -Amalgams withstand chewing loads -Useful for restoring molars in the back of the mouth -They are well tolerated by patients, rare occurrences of allergic response.

Why Mercury? k Nearly every metal is soluble in mercury, iron is the only exception k Thus, a malleable paste before solidification can be formed k As silver dissolves, flexible but firm structure is molded k Adheres to tooth enamel k Forms high-strength compound

Primary controversy k. Opponents of dental amalgams with mercury argue the long-term effects of mercury poisoning k. Combination of metals should be safe k. Some action taken before to ban mercury fillings internationally, but FDA, USPHS, CDC-approved k. Not enough research to fully support either side

Controversy

Battery in your mouth? k. Try this at home!: Aluminum foil pressing against amalgam filling will cause pain k. An electrochemical cell is created when aluminum foil contacts a dental amalgam. Aluminum contacts Sn 8 Hg phase i Aluminum becomes the anode and the filling as the cathode. i Saliva acts as an electrolyte. i The contact between the metals acts as a short-circuit

Dental Filling Corrosion k. Dental filling can corrode over time k. Different metals can create an electrical circuit, such as gold and silver.

Electricity in Your mouth k. Silver and Gold have 3 different solid phases (volt potential): k. Ag 2 Hg 3 (0. 85 v) k. Ag 3 Sn (-0. 05 v) k. Sn 8 Hg (-0. 13) k. Experiments you can do at home to create your very own circuit…

Experiments k. No, not that… if you have a filling or braces try chewing on aluminum foil.

The Corrosion k. Sn 8 Hg --> Sn 2+ + 2 ek. O 2(g) + 4 H+(aq) + 4 e- --> 2 H 2 O (l)

Grills Aren’t Dental Fillings!

Sources k http: //www. ada. org/public/topics/fillings. asp#restoring k http: //en. wikipedia. org/wiki/Amalgam k Chang, Raymond. Chemistry; Mc. Graw-Hill: San Francisco, 2002. k http: //en. wikipedia. org/wiki/Dental_fillings#Restoration_classifica tions k http: //en. wikipedia. org/wiki/Dental_amalgam_controversy k http: //www. madsci. org/posts/archives/oct 2000/973002607. Ch. r. html k http: //adr. iadrjournals. org/cgi/reprint/2/1/71. pdf k http: //www. mercurypoisoned. com/dentists_disciplined/Image. De ntist. Newsletter. jpg k http: //lonestartimes. com/images/Weidenhof/grill. jpg k http: //www. seemygrill. com/f/pics/114744405718814 phpr 0 Rvo. L. jpghttp: //farm 1. static. flickr. com/35/72218577_035 bb 4 c 2 b 5. jpg k http: //farm 1. static. flickr. com/35/72218577_035 bb 4 c 2 b 5. jpg