ERT 4163 CHAPTER 3 FUNDAMENTALS OF MATERIAL AND












- Slides: 72

ERT 416/3 CHAPTER 3: FUNDAMENTALS OF MATERIAL AND ENERGY BALANCES IN BIOPROCESS PLANT MISS. RAHIMAH BINTI OTHMAN (Email: rahimah@unimap. edu. my)

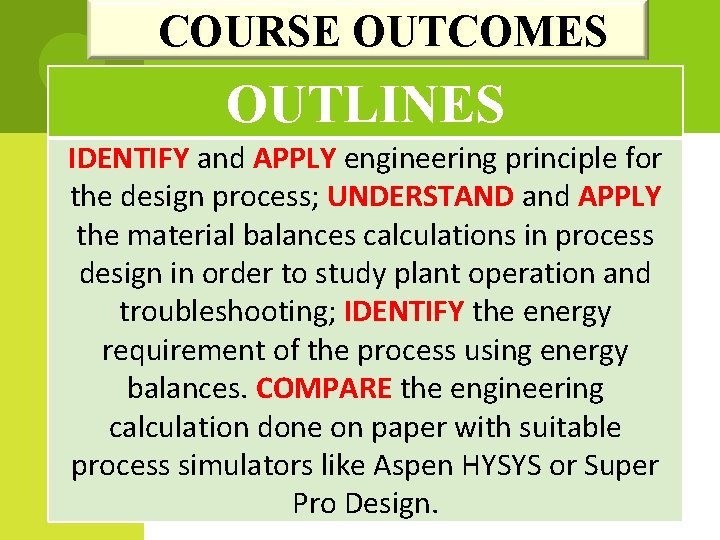
COURSE OUTCOMES OUTLINES IDENTIFY and APPLY engineering principle for the design process; UNDERSTAND and APPLY the material balances calculations in process design in order to study plant operation and troubleshooting; IDENTIFY the energy requirement of the process using energy balances. COMPARE the engineering calculation done on paper with suitable process simulators like Aspen HYSYS or Super Pro Design.

OUTLINES q Engineering principle for the design process. q The material balances calculations in process design- to study plant operation and troubleshooting. q The energy requirement of the process using energy balances. q Comparison between engineering calculation with suitable process simulators like Aspen HYSYS or Super Pro Design.

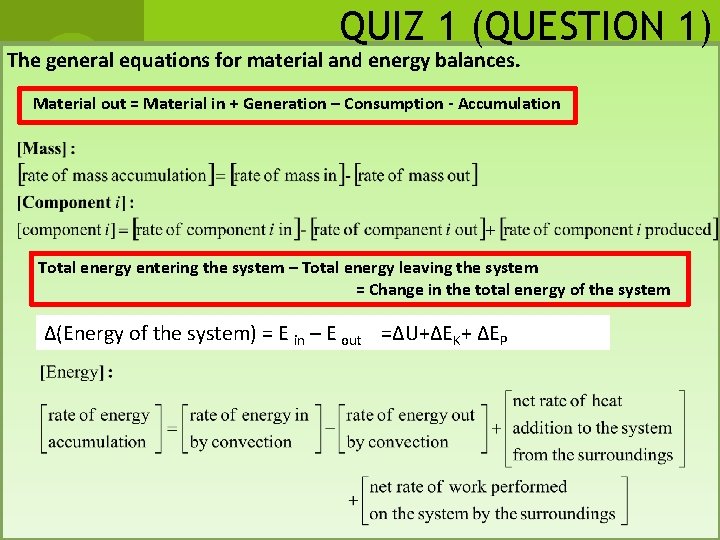
QUIZ 1 (QUESTION 1) The general equations for material and energy balances. Material out = Material in + Generation – Consumption - Accumulation Total energy entering the system – Total energy leaving the system = Change in the total energy of the system ∆(Energy of the system) = E in – E out =∆U+∆EK+ ∆EP

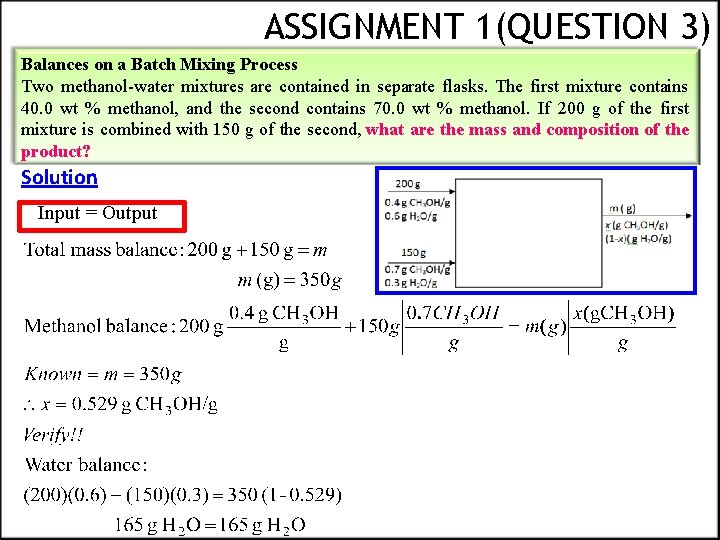
ASSIGNMENT 1(QUESTION 3) Balances on a Batch Mixing Process Two methanol-water mixtures are contained in separate flasks. The first mixture contains 40. 0 wt % methanol, and the second contains 70. 0 wt % methanol. If 200 g of the first mixture is combined with 150 g of the second, what are the mass and composition of the product? Solution Input = Output

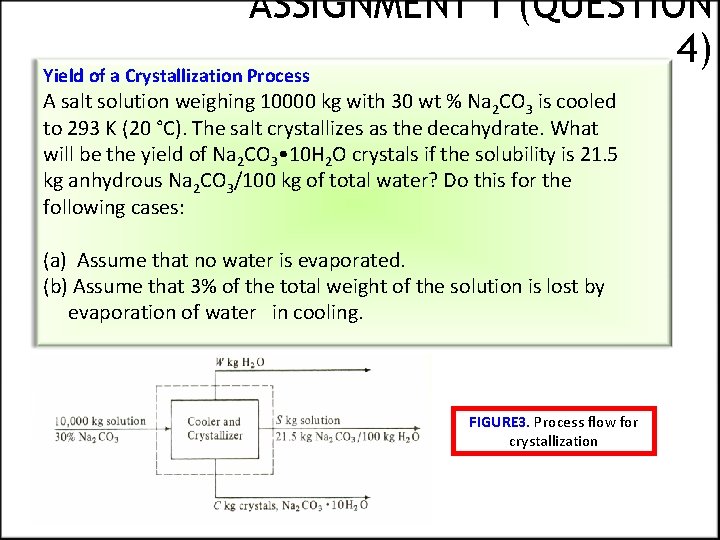
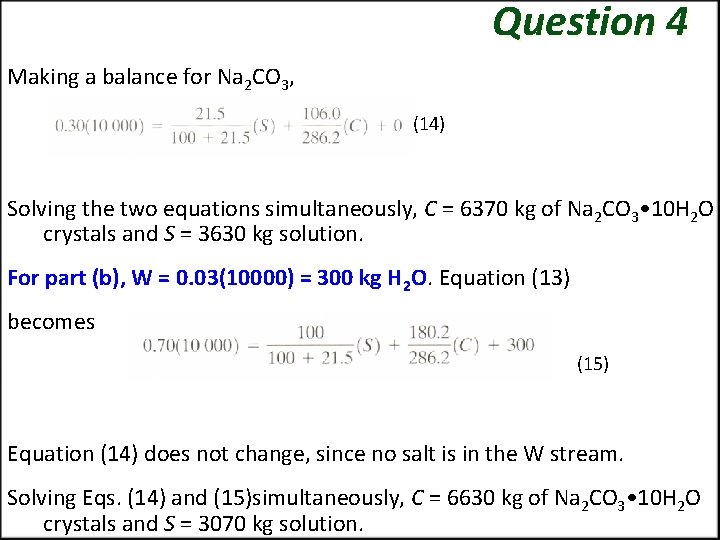
ASSIGNMENT 1 (QUESTION 4) Yield of a Crystallization Process A salt solution weighing 10000 kg with 30 wt % Na 2 CO 3 is cooled to 293 K (20 °C). The salt crystallizes as the decahydrate. What will be the yield of Na 2 CO 3 • 10 H 2 O crystals if the solubility is 21. 5 kg anhydrous Na 2 CO 3/100 kg of total water? Do this for the following cases: (a) Assume that no water is evaporated. (b) Assume that 3% of the total weight of the solution is lost by evaporation of water in cooling. FIGURE 3. Process flow for crystallization

Solution QUESTION 4 The molecular weights are 106. 0 for Na 2 CO 3, 180. 2 for 10 H 20, and 286. 2 for Na 2 CO 3 • 10 H 2 O. The process flow diagram is shown in Fig. 3, with W being kg H 2 O evaporated, S kg solution (mother liquor), and C kg crystals of Na 2 CO 3 • 10 H 2 O. Making a material balance around the dashed line box for water for part (a), where W = 0. (13) where (180. 2)/(286. 2) is wt fraction of water in the crystals.

Question 4 Making a balance for Na 2 CO 3, (14) Solving the two equations simultaneously, C = 6370 kg of Na 2 CO 3 • 10 H 2 O crystals and S = 3630 kg solution. For part (b), W = 0. 03(10000) = 300 kg H 2 O. Equation (13) becomes (15) Equation (14) does not change, since no salt is in the W stream. Solving Eqs. (14) and (15)simultaneously, C = 6630 kg of Na 2 CO 3 • 10 H 2 O crystals and S = 3070 kg solution.

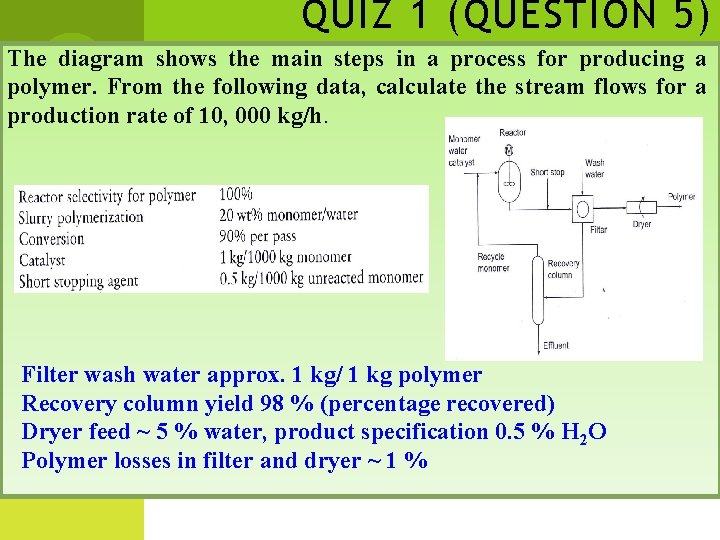
QUIZ 1 (QUESTION 5) The diagram shows the main steps in a process for producing a polymer. From the following data, calculate the stream flows for a production rate of 10, 000 kg/h. Filter wash water approx. 1 kg/ 1 kg polymer Recovery column yield 98 % (percentage recovered) Dryer feed ~ 5 % water, product specification 0. 5 % H 2 O Polymer losses in filter and dryer ~ 1 %

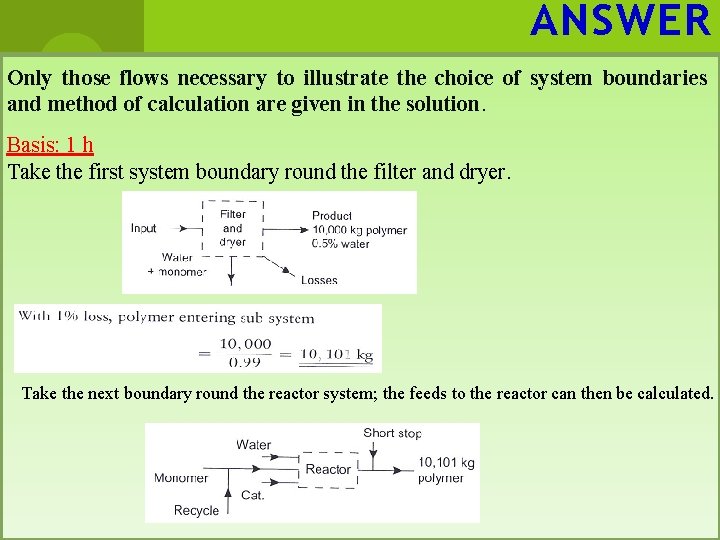
ANSWER Only those flows necessary to illustrate the choice of system boundaries and method of calculation are given in the solution. Basis: 1 h Take the first system boundary round the filter and dryer. Take the next boundary round the reactor system; the feeds to the reactor can then be calculated.

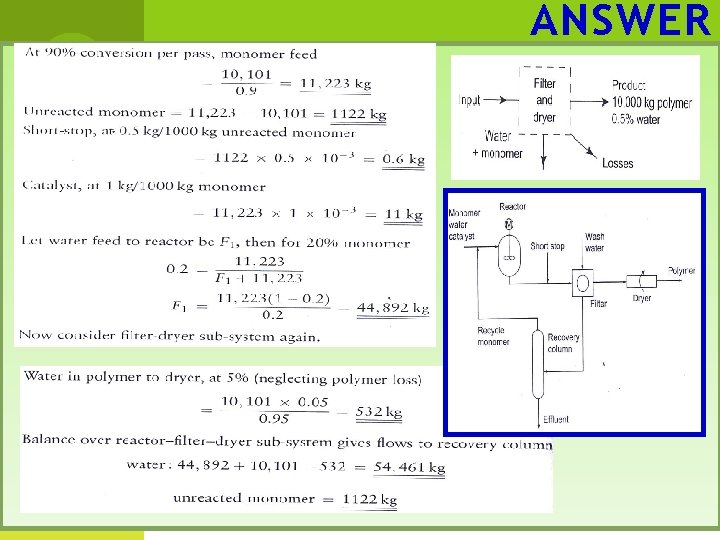
ANSWER

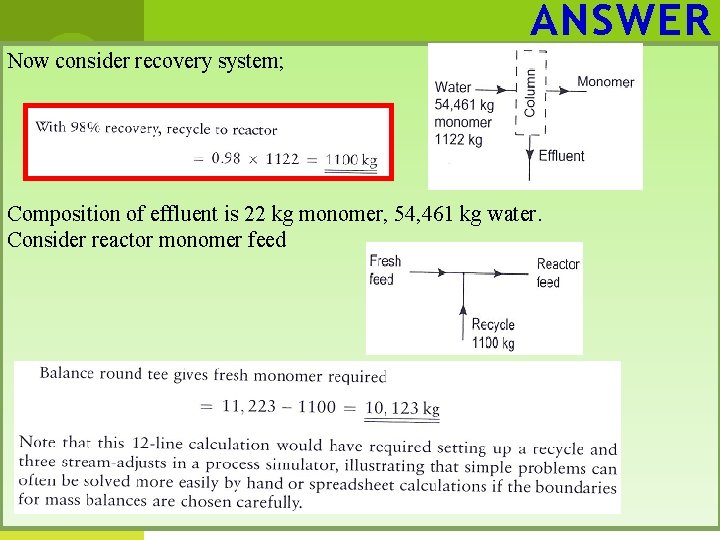
ANSWER Now consider recovery system; Composition of effluent is 22 kg monomer, 54, 461 kg water. Consider reactor monomer feed

OUTLINES q Engineering principle for the design process. q The material balances calculations in process design- to study plant operation and troubleshooting. q The energy requirement of the process using energy balances. q Comparison between engineering calculation with suitable process simulators like Aspen HYSYS or Super Pro Design.

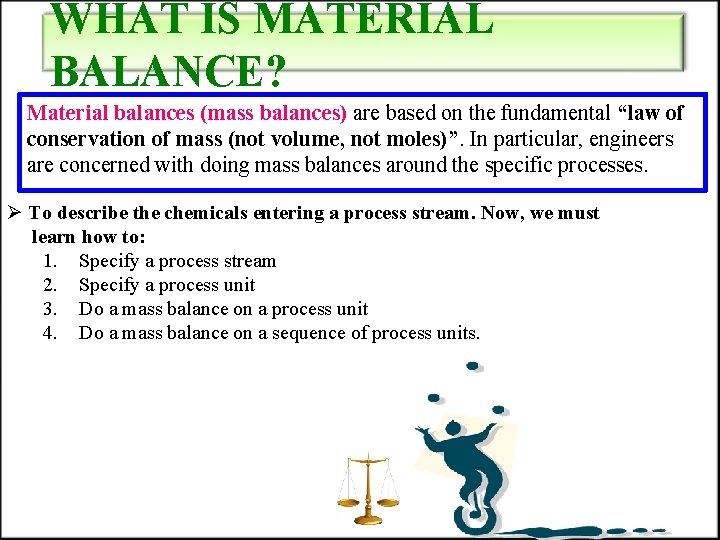
WHAT IS MATERIAL BALANCE? Material balances (mass balances) are based on the fundamental “law of conservation of mass (not volume, not moles)”. In particular, engineers are concerned with doing mass balances around the specific processes. Ø To describe the chemicals entering a process stream. Now, we must learn how to: 1. Specify a process stream 2. Specify a process unit 3. Do a mass balance on a process unit 4. Do a mass balance on a sequence of process units.

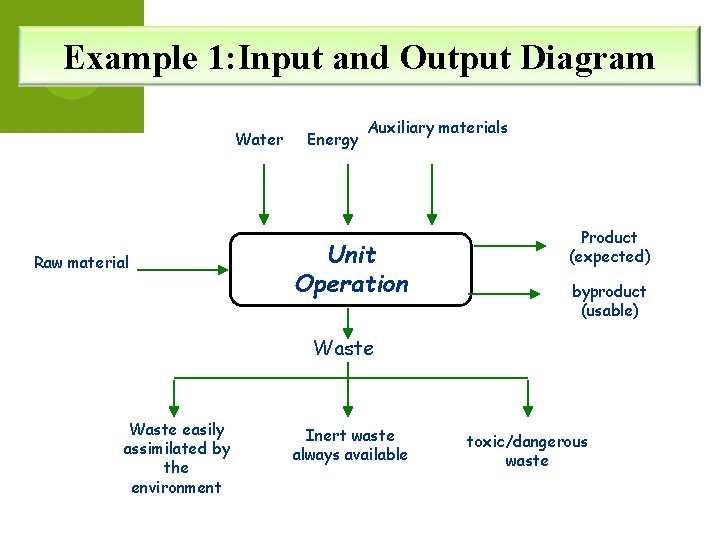
Example 1: Input and Output Diagram Water Raw material Energy Auxiliary materials Unit Operation Product (expected) byproduct (usable) Waste easily assimilated by the environment Inert waste always available toxic/dangerous waste

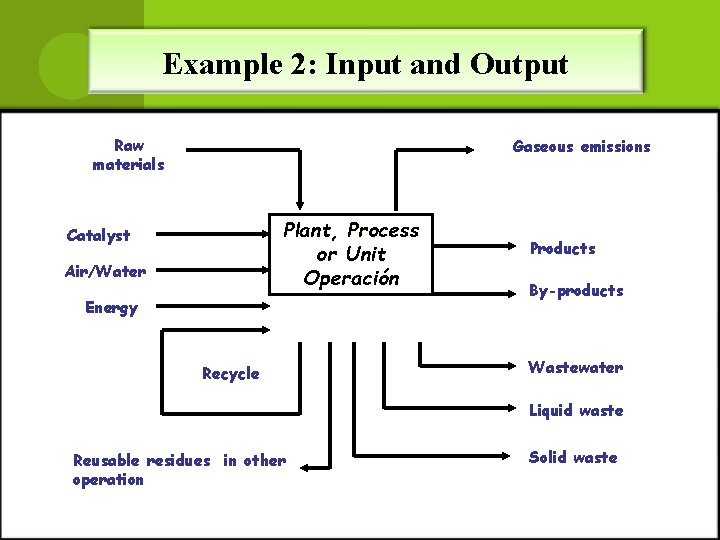
Example 2: Input and Output Raw materials Gaseous emissions Plant, Process or Unit Operación Catalyst Air/Water Energy Recycle Products By-products Wastewater Liquid waste Reusable residues in other operation Solid waste

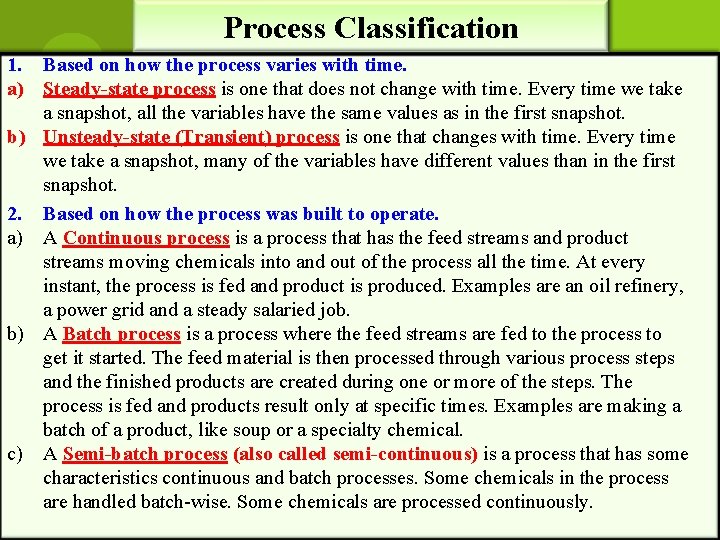
Process Classification 1. Based on how the process varies with time. a) Steady-state process is one that does not change with time. Every time we take a snapshot, all the variables have the same values as in the first snapshot. b) Unsteady-state (Transient) process is one that changes with time. Every time we take a snapshot, many of the variables have different values than in the first snapshot. 2. Based on how the process was built to operate. a) A Continuous process is a process that has the feed streams and product streams moving chemicals into and out of the process all the time. At every instant, the process is fed and product is produced. Examples are an oil refinery, a power grid and a steady salaried job. b) A Batch process is a process where the feed streams are fed to the process to get it started. The feed material is then processed through various process steps and the finished products are created during one or more of the steps. The process is fed and products result only at specific times. Examples are making a batch of a product, like soup or a specialty chemical. c) A Semi-batch process (also called semi-continuous) is a process that has some characteristics continuous and batch processes. Some chemicals in the process are handled batch-wise. Some chemicals are processed continuously.

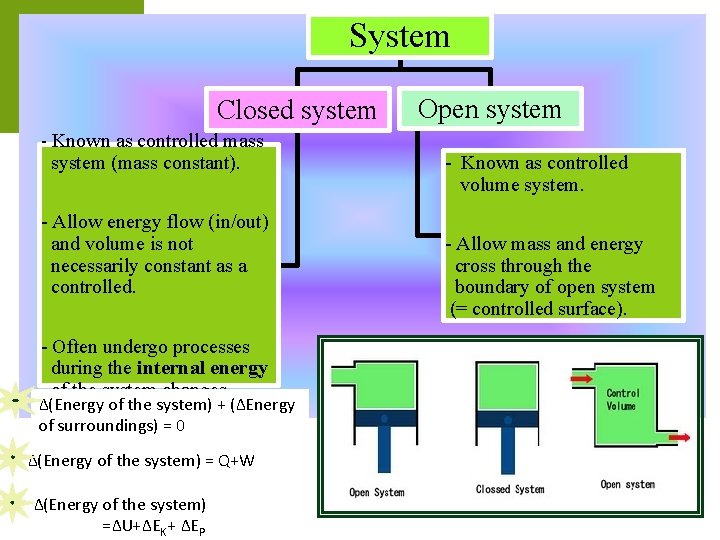
System Closed system - Known as controlled mass system (mass constant). - Allow energy flow (in/out) and volume is not necessarily constant as a controlled. - Often undergo processes during the internal energy of the system changes. ∆(Energy of the system) + (∆Energy of surroundings) = 0 ∆(Energy of the system) = Q+W ∆(Energy of the system) =∆U+∆EK+ ∆EP Open system - Known as controlled volume system. - Allow mass and energy cross through the boundary of open system (= controlled surface).

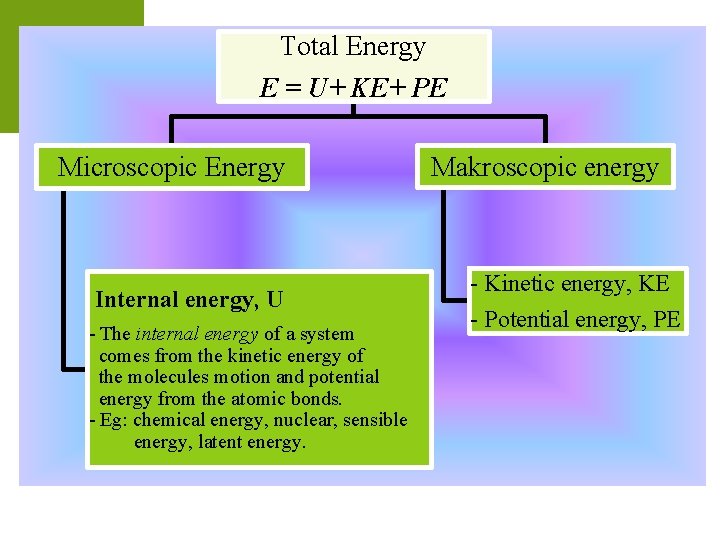
Total Energy E = U+ KE+ PE Microscopic Energy Internal energy, U - The internal energy of a system comes from the kinetic energy of the molecules motion and potential energy from the atomic bonds. - Eg: chemical energy, nuclear, sensible energy, latent energy. Makroscopic energy - Kinetic energy, KE - Potential energy, PE

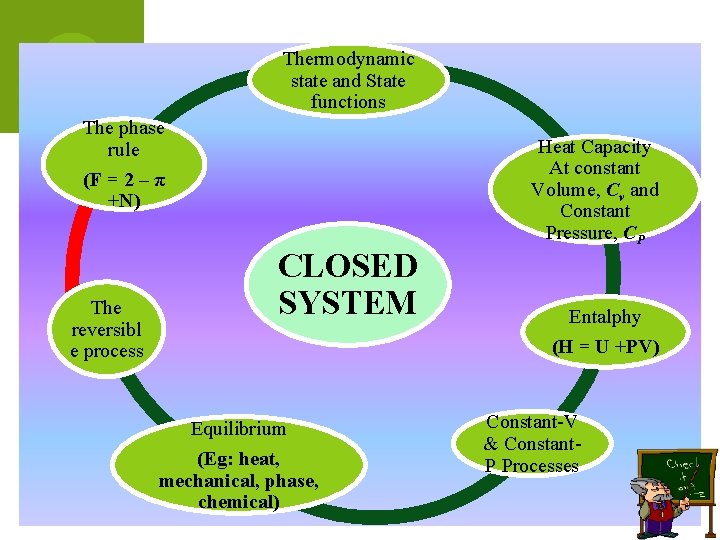
Thermodynamic state and State functions The phase rule Heat Capacity At constant Volume, Cv and Constant Pressure, CP (F = 2 – π +N) The reversibl e process CLOSED SYSTEM Equilibrium (Eg: heat, mechanical, phase, chemical) Entalphy (H = U +PV) Constant-V & Constant. P Processes

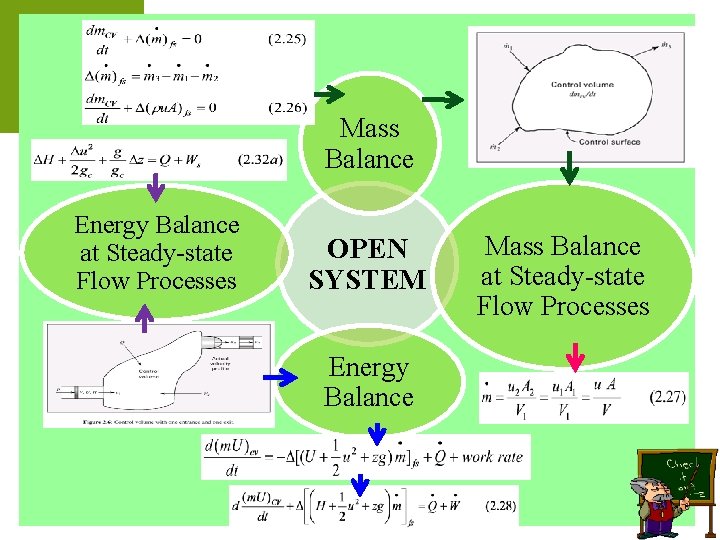
Mass Balance Energy Balance at Steady-state Flow Processes OPEN SYSTEM Energy Balance Mass Balance at Steady-state Flow Processes

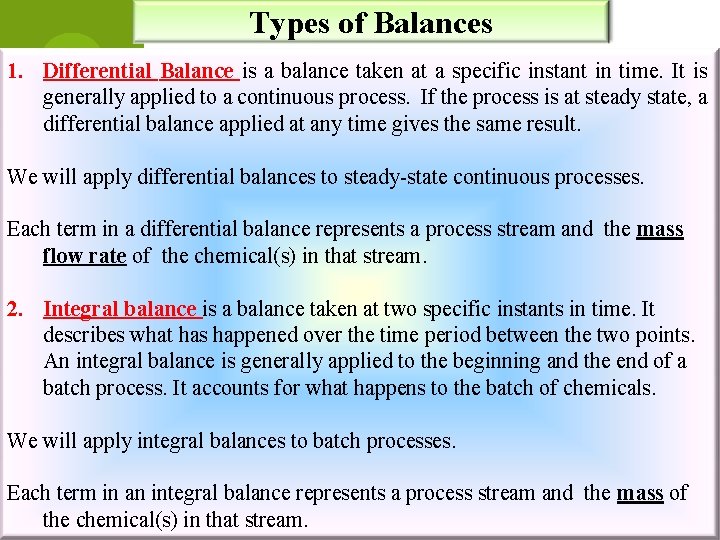
Types of Balances 1. Differential Balance is a balance taken at a specific instant in time. It is generally applied to a continuous process. If the process is at steady state, a differential balance applied at any time gives the same result. We will apply differential balances to steady-state continuous processes. Each term in a differential balance represents a process stream and the mass flow rate of the chemical(s) in that stream. 2. Integral balance is a balance taken at two specific instants in time. It describes what has happened over the time period between the two points. An integral balance is generally applied to the beginning and the end of a batch process. It accounts for what happens to the batch of chemicals. We will apply integral balances to batch processes. Each term in an integral balance represents a process stream and the mass of the chemical(s) in that stream.

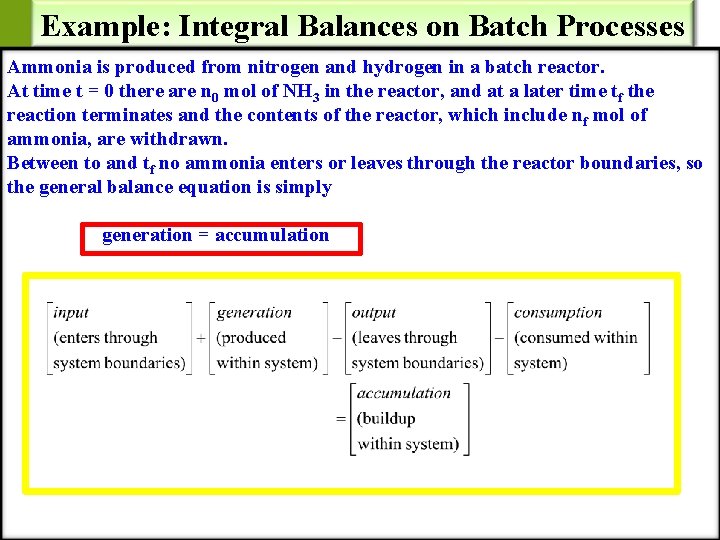
Example: Integral Balances on Batch Processes Ammonia is produced from nitrogen and hydrogen in a batch reactor. At time t = 0 there are n 0 mol of NH 3 in the reactor, and at a later time tf the reaction terminates and the contents of the reactor, which include nf mol of ammonia, are withdrawn. Between to and tf no ammonia enters or leaves through the reactor boundaries, so the general balance equation is simply generation = accumulation

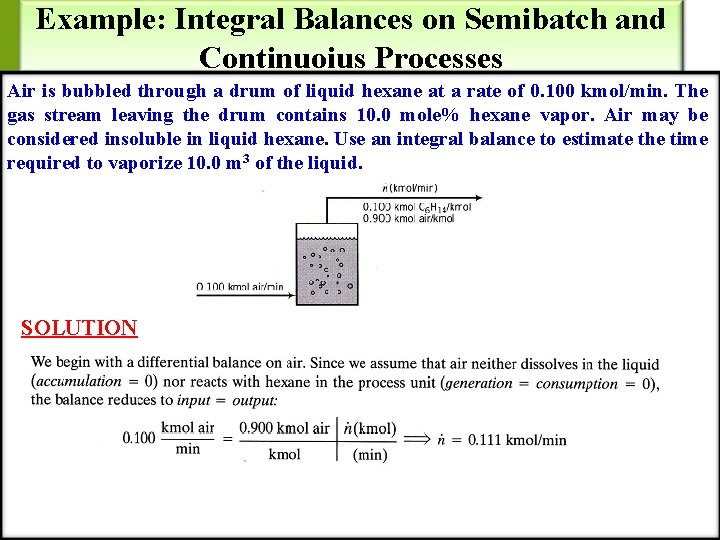
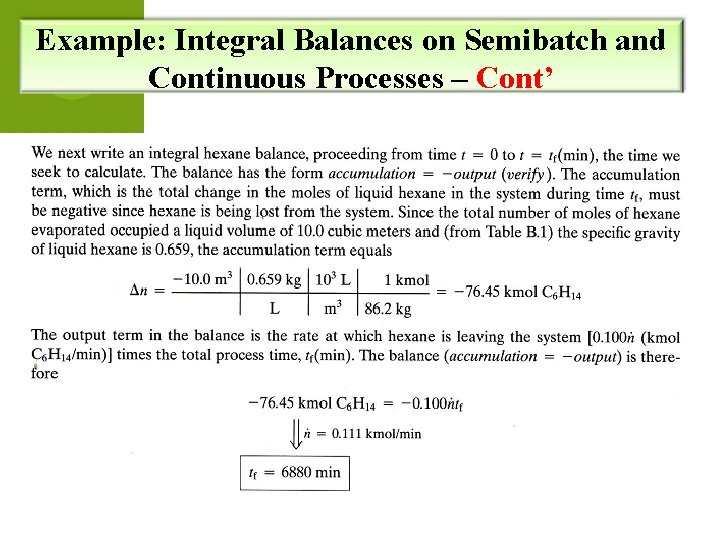
Example: Integral Balances on Semibatch and Continuoius Processes Air is bubbled through a drum of liquid hexane at a rate of 0. 100 kmol/min. The gas stream leaving the drum contains 10. 0 mole% hexane vapor. Air may be considered insoluble in liquid hexane. Use an integral balance to estimate the time required to vaporize 10. 0 m 3 of the liquid. SOLUTION

Example: Integral Balances on Semibatch and Continuous Processes – Cont’

TEST YOURSELF? Example: A process unit involves 3 chemical components. How many mass balances can be written? Solution: We can write 4 balances. We can write a total balance and 3 component balances.

Where am I? Where am I going ? How do I get there? To answer the first question, you need to • Read, study and understand the problem. • Draw a flow sheet for the process. • Label it with all given information, including symbols for the unknowns • Note any special relationships. To accomplish this step, you need to learn • The information needed to specify a stream. • How to use symbols to represent the required stream data. • How to determine the mass of each component in a stream (each mass will be a term in a mass balance)

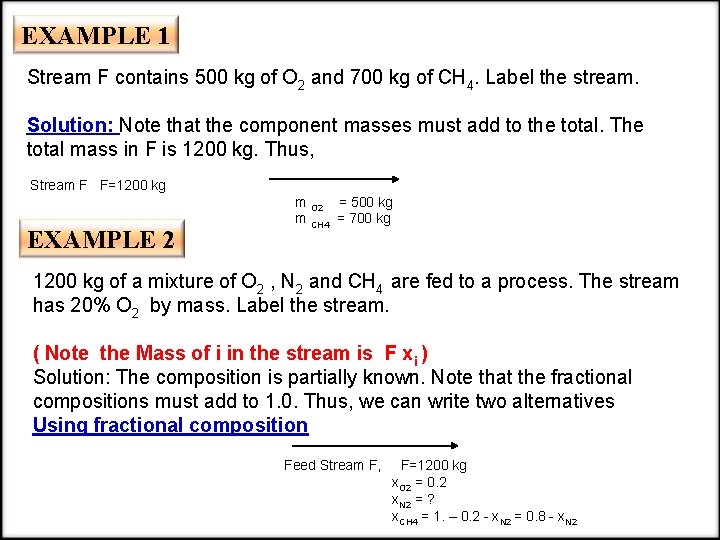
EXAMPLE 1 Stream F contains 500 kg of O 2 and 700 kg of CH 4. Label the stream. Solution: Note that the component masses must add to the total. The total mass in F is 1200 kg. Thus, Stream F F=1200 kg EXAMPLE 2 m O 2 = 500 kg m CH 4 = 700 kg 1200 kg of a mixture of O 2 , N 2 and CH 4 are fed to a process. The stream has 20% O 2 by mass. Label the stream. ( Note the Mass of i in the stream is F xi ) Solution: The composition is partially known. Note that the fractional compositions must add to 1. 0. Thus, we can write two alternatives Using fractional composition Feed Stream F, F=1200 kg x. O 2 = 0. 2 x. N 2 = ? x. CH 4 = 1. – 0. 2 - x. N 2 = 0. 8 - x. N 2

or using component masses Feed Stream F, m. O 2 = 240 kg F=1200 kg m. N 2 = ? m. CH 4 = 1200 - 240 - m. N 2 = 960 - m. N 2 Some Suggestions for Component Labeling • If the stream composition is unknown (or if some of the component masses are known) represent the component masses directly and use a lower case letter for each chemical. Eg. If stream F contains chemicals a, b and c, label the flow rates as; F, a. F, b. F and c. F=F- a. F – Bf • If the stream composition is known from fractional compositions, represent the component masses directly and label as in 2. • If stream composition is partially known with fractional compositions and the total is known, represent the component masses indirectly and use lower case x, y, z for each fractional composition. • Avoid the creation of a product of two unknowns—this will result in a nonlinear equation.

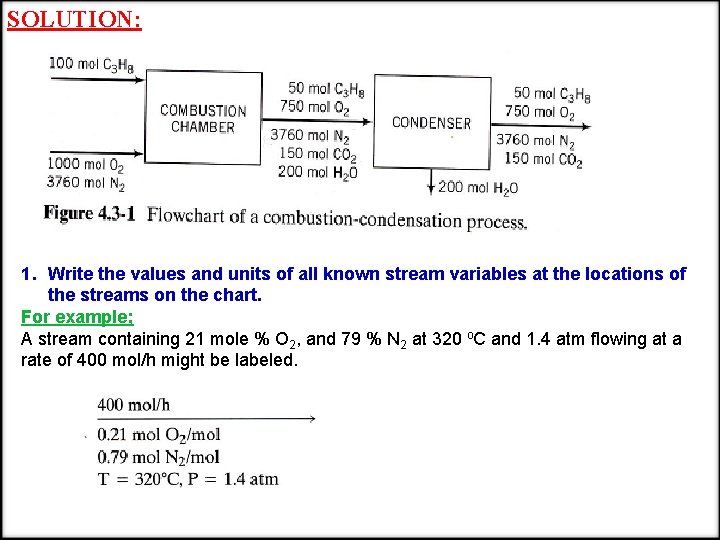
FLOWCHARTS CASE STUDY: The catalytic dehydrogenation of propane is carried out in a continuous packed-bed reactor. One thousand kilograms per hour of pure propane is preheated to a temperature of 670 o. C before it passes into the reactor. The reactor effluent gas, which includes propane, methane, and hydrogen, is cooled from 800 o. C to 110 o. C and fed to an adsorption tower, where the propane and propylene are dissolved in oil. The oil then goes to a stripping tower in which it is heated, releasing the dissolved gases; these gases are recompressed and sent to a distillation column in which the propane and propylene are separated. The propane stream is recycled back to join the feed to the reactor preheater. The product stream from the distillation column contains 98 % propylene, and the recycle stream is 97 % propane. The stripped oil is recycled to the adsorption tower. Draw the flowchart for this system.

SOLUTION: 1. Write the values and units of all known stream variables at the locations of the streams on the chart. For example; A stream containing 21 mole % O 2, and 79 % N 2 at 320 o. C and 1. 4 atm flowing at a rate of 400 mol/h might be labeled.

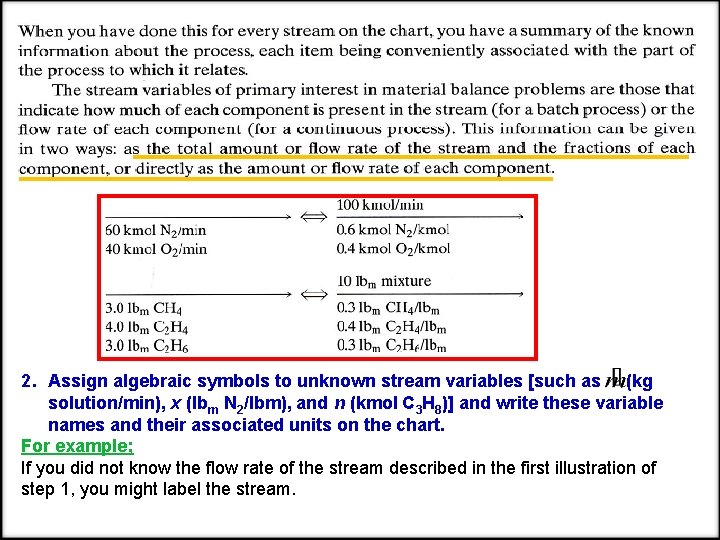
2. Assign algebraic symbols to unknown stream variables [such as (kg solution/min), x (lbm N 2/lbm), and n (kmol C 3 H 8)] and write these variable names and their associated units on the chart. For example; If you did not know the flow rate of the stream described in the first illustration of step 1, you might label the stream.


EXAMPLE

SOLUTION

SOLUTION

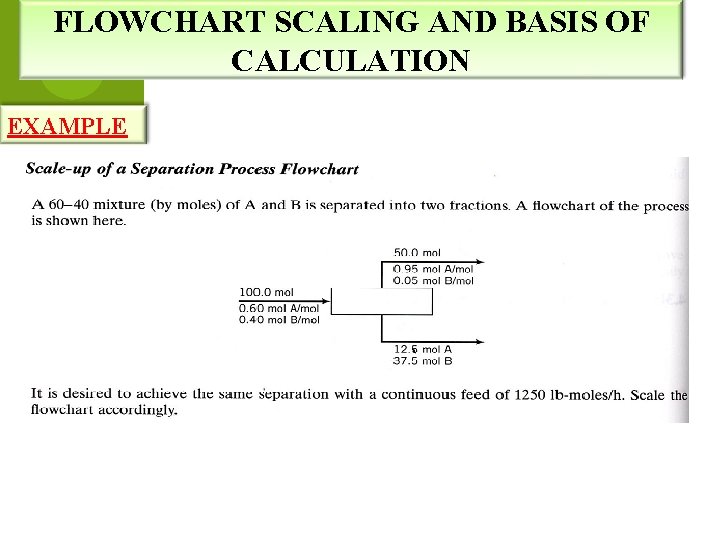
FLOWCHART SCALING AND BASIS OF CALCULATION EXAMPLE

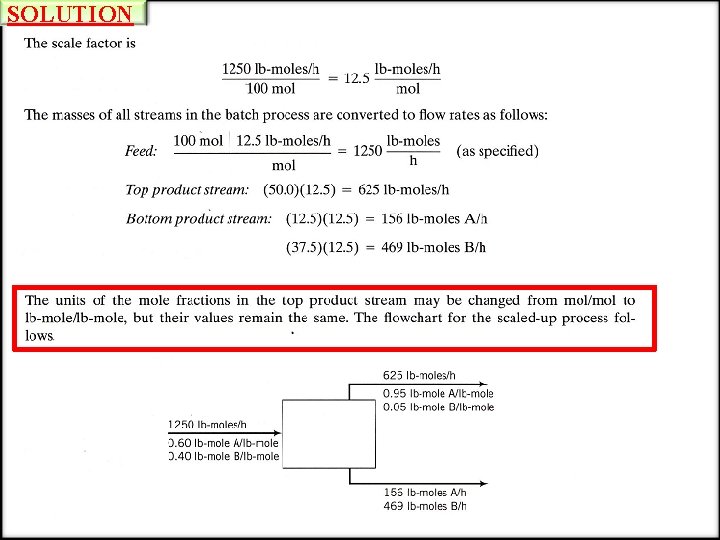
SOLUTION

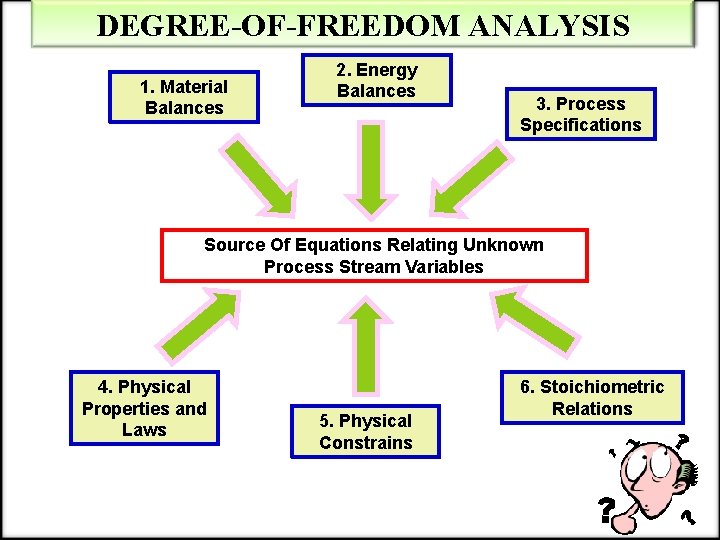
DEGREE-OF-FREEDOM ANALYSIS Equation: ndf (= nunknowns – nindep eqns) Three possibilities: 1. If ndf = 0, there are n independent equations in n unknowns and the problem can in principle be solved. 2. If ndf > 0, there are more unknowns than independent equations relating them, and at least ndf additional variable values must be specified before the remaining variable values can be determined. Either relations have been overlooked or the problem is underspecified and has infinitely many solutions; in either case, plugging into calculations is likely to be a waste of time. 3. If ndf < 0, there are more independent equations than unknowns. Either the flowchart is incompletely labeled or the problem is overspecified with rebundant and possibly inconsistent relations. Again there is little point wasting time trying to solve it until the equations and unknowns are brought into balance.

DEGREE-OF-FREEDOM ANALYSIS 1. Material Balances 2. Energy Balances 3. Process Specifications Source Of Equations Relating Unknown Process Stream Variables 4. Physical Properties and Laws 5. Physical Constrains 6. Stoichiometric Relations

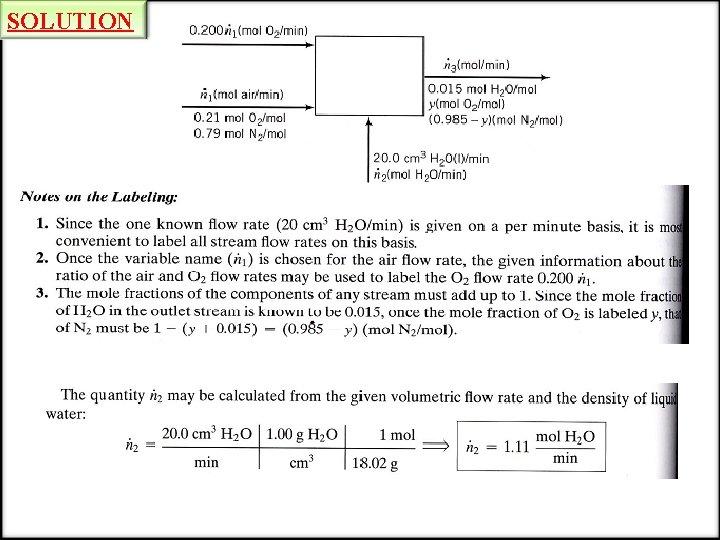
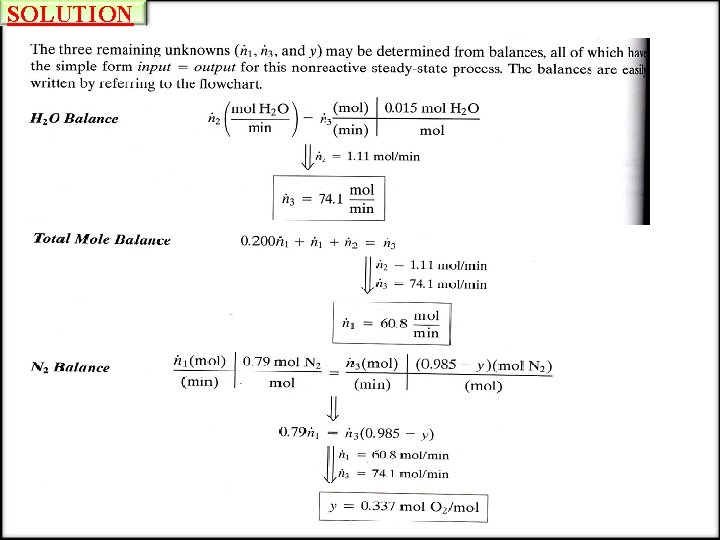
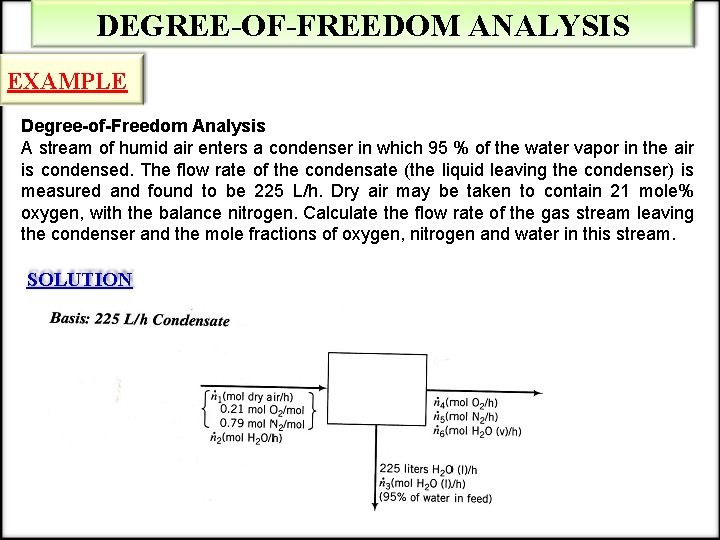
DEGREE-OF-FREEDOM ANALYSIS EXAMPLE Degree-of-Freedom Analysis A stream of humid air enters a condenser in which 95 % of the water vapor in the air is condensed. The flow rate of the condensate (the liquid leaving the condenser) is measured and found to be 225 L/h. Dry air may be taken to contain 21 mole% oxygen, with the balance nitrogen. Calculate the flow rate of the gas stream leaving the condenser and the mole fractions of oxygen, nitrogen and water in this stream. SOLUTION

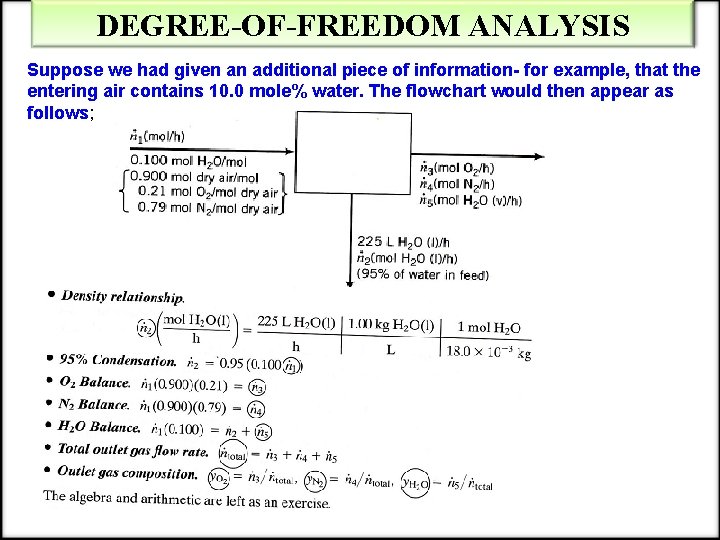
DEGREE-OF-FREEDOM ANALYSIS Suppose we had given an additional piece of information- for example, that the entering air contains 10. 0 mole% water. The flowchart would then appear as follows;

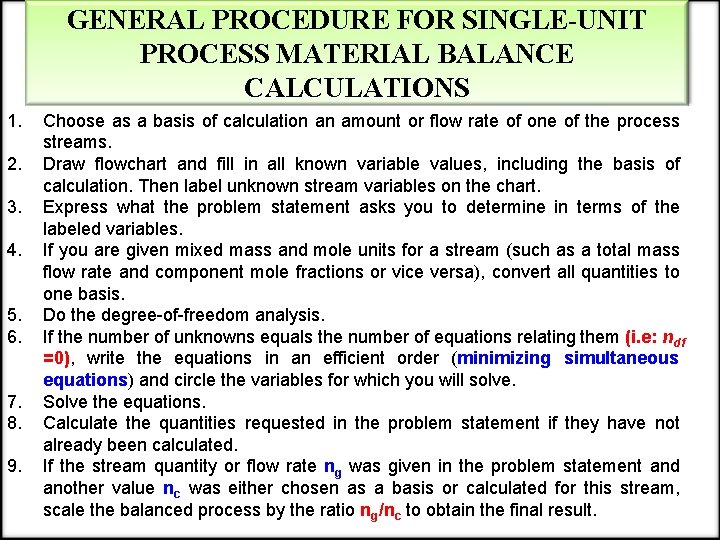
GENERAL PROCEDURE FOR SINGLE-UNIT PROCESS MATERIAL BALANCE CALCULATIONS 1. 2. 3. 4. 5. 6. 7. 8. 9. Choose as a basis of calculation an amount or flow rate of one of the process streams. Draw flowchart and fill in all known variable values, including the basis of calculation. Then label unknown stream variables on the chart. Express what the problem statement asks you to determine in terms of the labeled variables. If you are given mixed mass and mole units for a stream (such as a total mass flow rate and component mole fractions or vice versa), convert all quantities to one basis. Do the degree-of-freedom analysis. If the number of unknowns equals the number of equations relating them (i. e: ndf =0), write the equations in an efficient order (minimizing simultaneous equations) and circle the variables for which you will solve. Solve the equations. Calculate the quantities requested in the problem statement if they have not already been calculated. If the stream quantity or flow rate ng was given in the problem statement and another value nc was either chosen as a basis or calculated for this stream, scale the balanced process by the ratio ng/nc to obtain the final result.

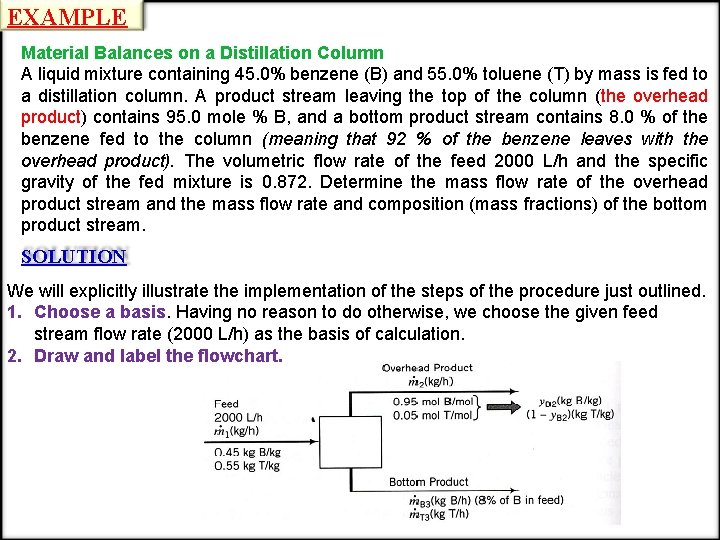
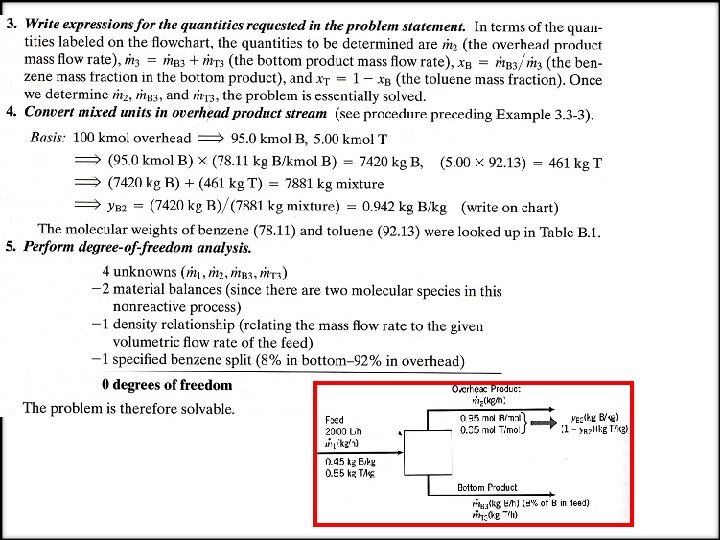
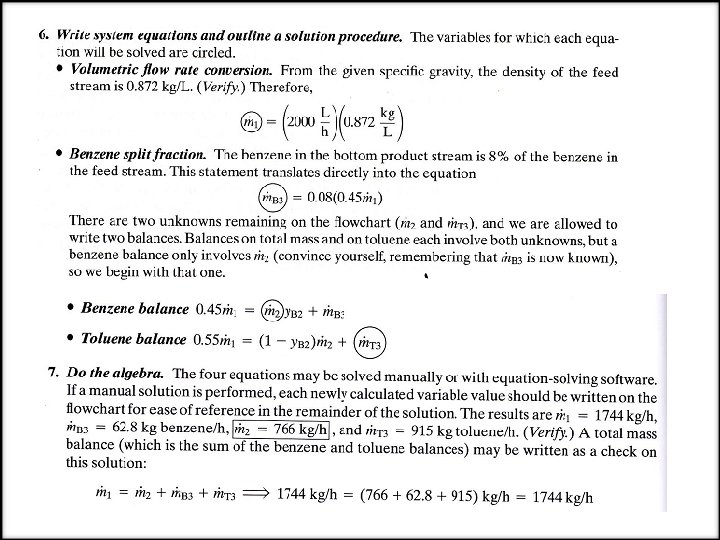
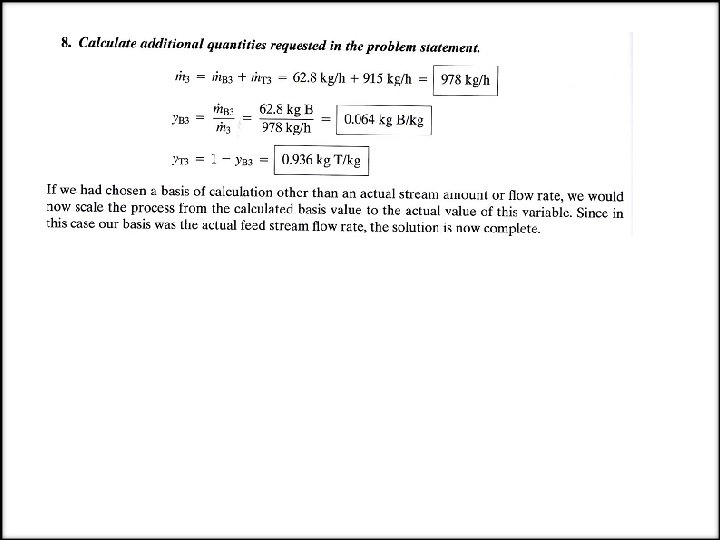
EXAMPLE Material Balances on a Distillation Column A liquid mixture containing 45. 0% benzene (B) and 55. 0% toluene (T) by mass is fed to a distillation column. A product stream leaving the top of the column (the overhead product) contains 95. 0 mole % B, and a bottom product stream contains 8. 0 % of the benzene fed to the column (meaning that 92 % of the benzene leaves with the overhead product). The volumetric flow rate of the feed 2000 L/h and the specific gravity of the fed mixture is 0. 872. Determine the mass flow rate of the overhead product stream and the mass flow rate and composition (mass fractions) of the bottom product stream. SOLUTION We will explicitly illustrate the implementation of the steps of the procedure just outlined. 1. Choose a basis. Having no reason to do otherwise, we choose the given feed stream flow rate (2000 L/h) as the basis of calculation. 2. Draw and label the flowchart.

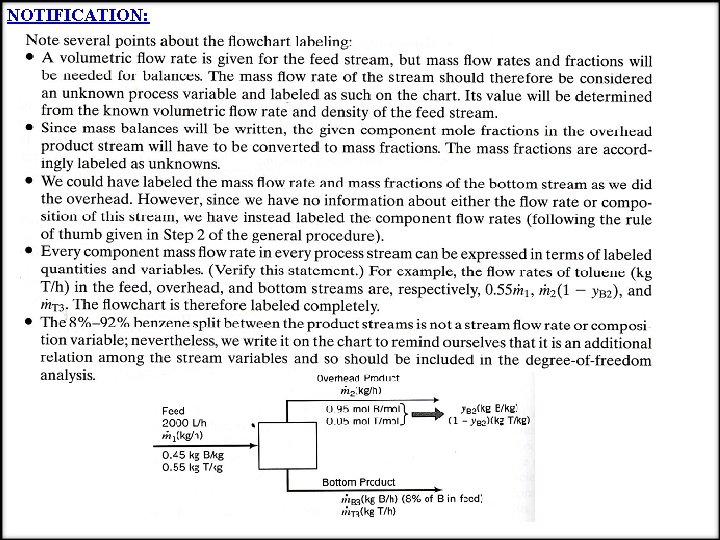
NOTIFICATION:




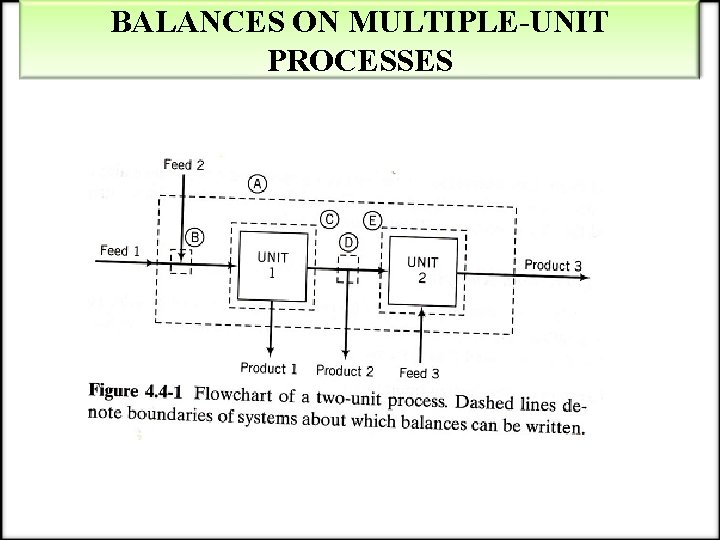
BALANCES ON MULTIPLE-UNIT PROCESSES

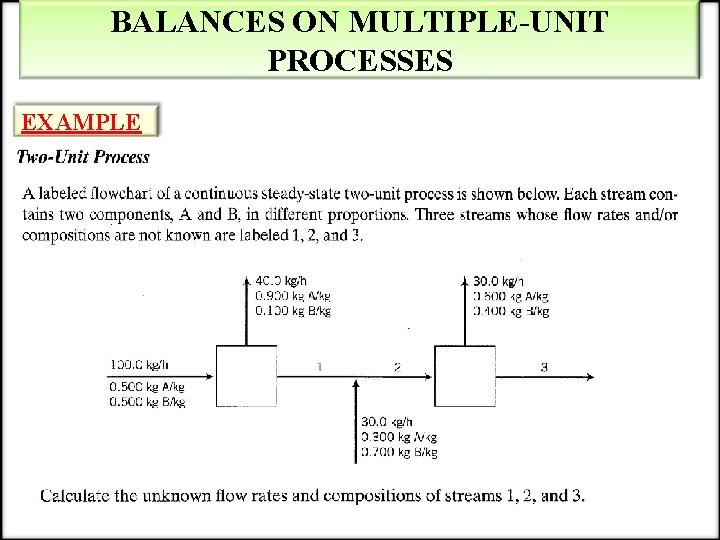
BALANCES ON MULTIPLE-UNIT PROCESSES EXAMPLE

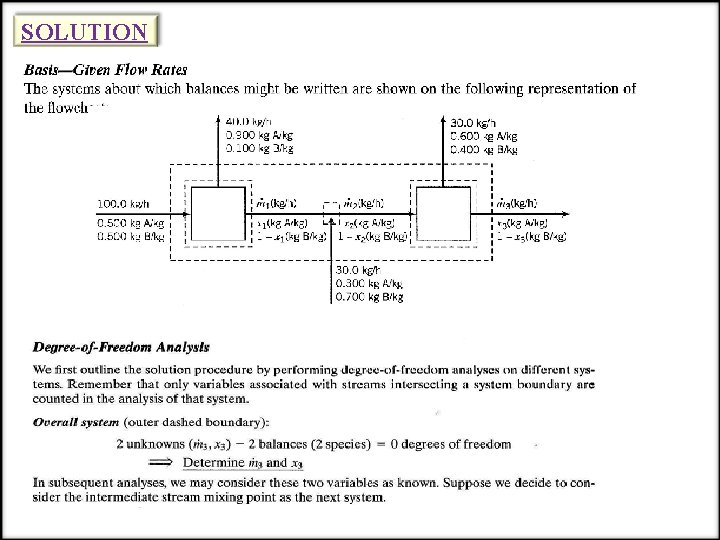
SOLUTION

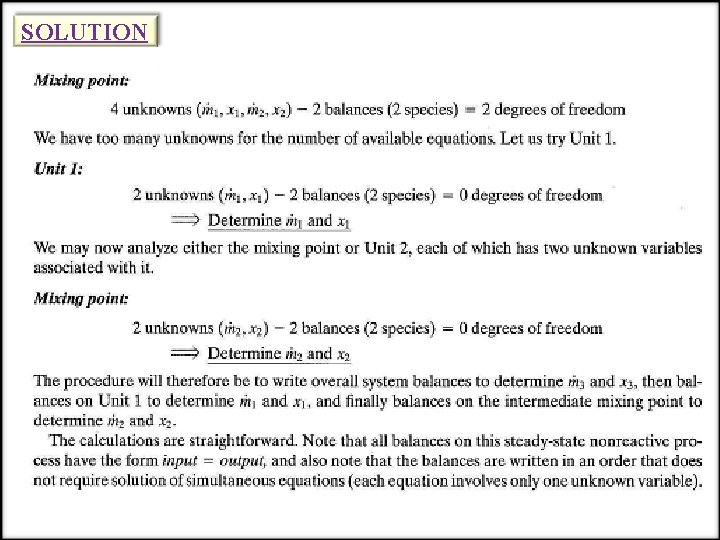
SOLUTION

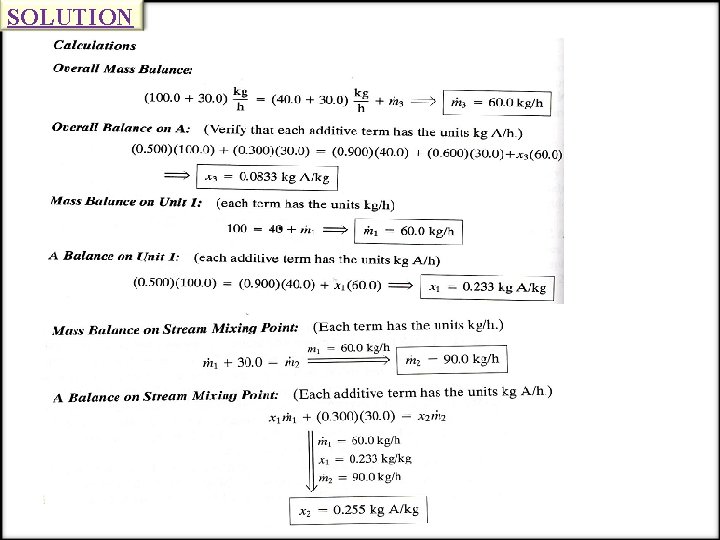
SOLUTION

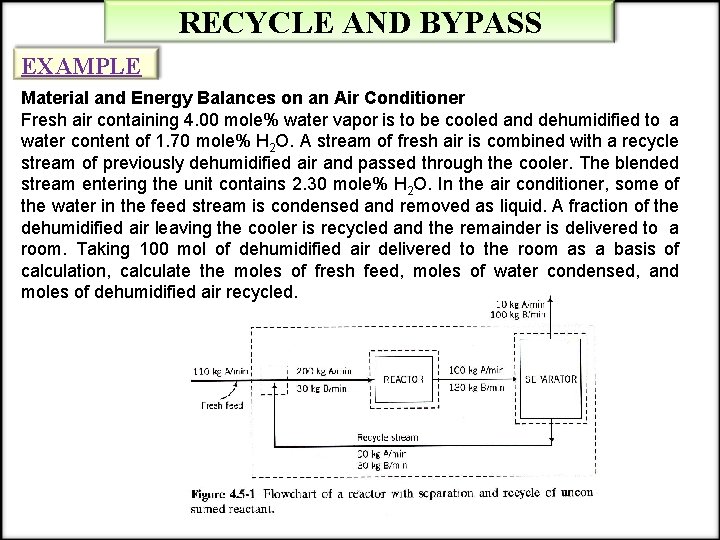
RECYCLE AND BYPASS EXAMPLE Material and Energy Balances on an Air Conditioner Fresh air containing 4. 00 mole% water vapor is to be cooled and dehumidified to a water content of 1. 70 mole% H 2 O. A stream of fresh air is combined with a recycle stream of previously dehumidified air and passed through the cooler. The blended stream entering the unit contains 2. 30 mole% H 2 O. In the air conditioner, some of the water in the feed stream is condensed and removed as liquid. A fraction of the dehumidified air leaving the cooler is recycled and the remainder is delivered to a room. Taking 100 mol of dehumidified air delivered to the room as a basis of calculation, calculate the moles of fresh feed, moles of water condensed, and moles of dehumidified air recycled.

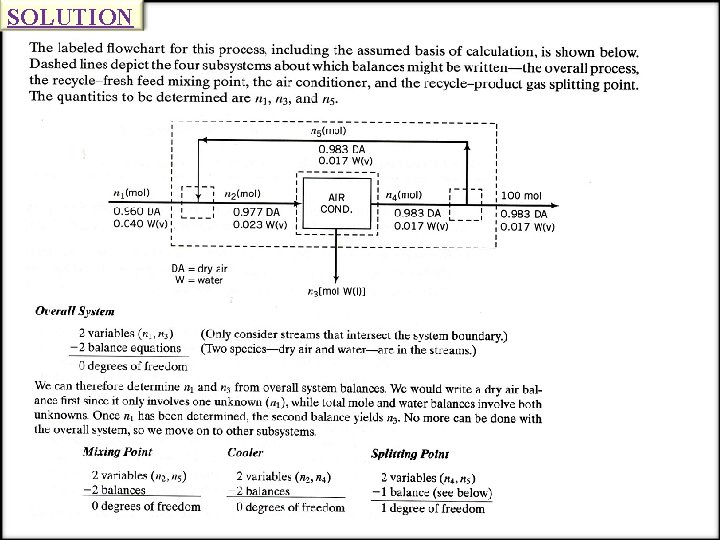
SOLUTION

SOLUTION

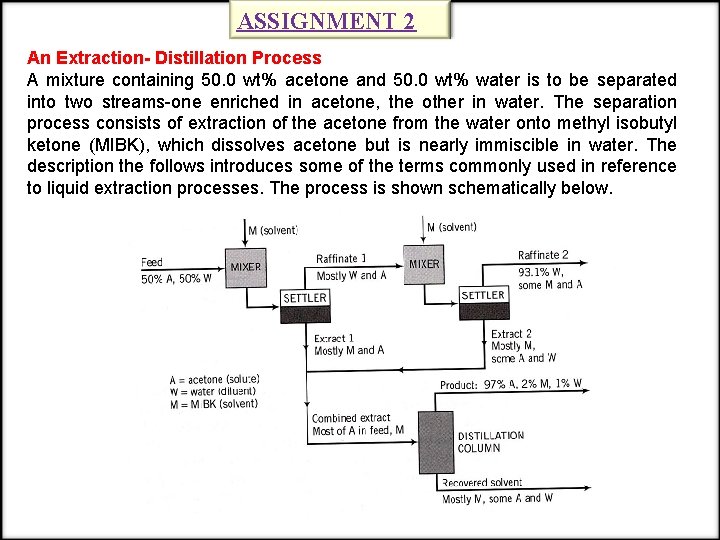
ASSIGNMENT 2 An Extraction- Distillation Process A mixture containing 50. 0 wt% acetone and 50. 0 wt% water is to be separated into two streams-one enriched in acetone, the other in water. The separation process consists of extraction of the acetone from the water onto methyl isobutyl ketone (MIBK), which dissolves acetone but is nearly immiscible in water. The description the follows introduces some of the terms commonly used in reference to liquid extraction processes. The process is shown schematically below.

ASSIGNMENT 2 -CONT’ The acetone (solute)-water(diluent) mixture is first contacted with the MIBK (solvent) in a mixer that provides good contact between the two liquid phases. A portion of the acetone in the feed transfers from the aqueous (water) phase to the organic (MIBK) phase in this step. The mixture passes into a settling tank, where the phases separate and are separately withdrawn. The phase rich in the diluent (water, in the process) is referred to as the raffinate, and the phase rich in the solvent (MIBK) is the extract. The mixer-settler combination is the first stage of this separation process. The raffinate passes to a second extraction stage where it is contacted with a second stream of pure MIBK, leading to the transfer of more acetone. The two phases are allowed to separate in a second settler, and the raffinate from this stage is discarded. The extracts from the two mixer-settler stages are combined and feed to a distillation column. The overhead effluent is rich in acetone and is the process product. The bottom effluent is rich in MIBK and in a real process would be treated further and recycled back to the first extraction stage, but we will not consider recycle in this example.

ASSIGNMENT 2 -CONT’ In a pilot-plant study, for every 100 kg of acetone-water fed to the first extraction stage, 100 kg MIBK is fed to the first stage and 75 kg is fed to the second stage. The extraction from the first stage is found to contain 27. 5 wt% acetone. (All percentages in the remainder of this paragraph are weight percents. ) The secondstage raffinate has a mass of 43. 1 kg and contains 5. 3% acetone, 1. 6 % MIBK, and 93. 1 % water, and the second extract contains 9. 0% acetone, 88. 0 % MIBK, and 3. 0% water. The overhead product from the distillation column contains 2. 0% MIBK, 1. 0% water, and the balance acetone. Taking a basis of calculation of 100 kg acetone-water feed, calculate the masses and compositions (component weight percentages) of the Stage 1 raffinate and extract, the Stage 2 extract, the combined extract, and the distillation overhead and bottoms products.

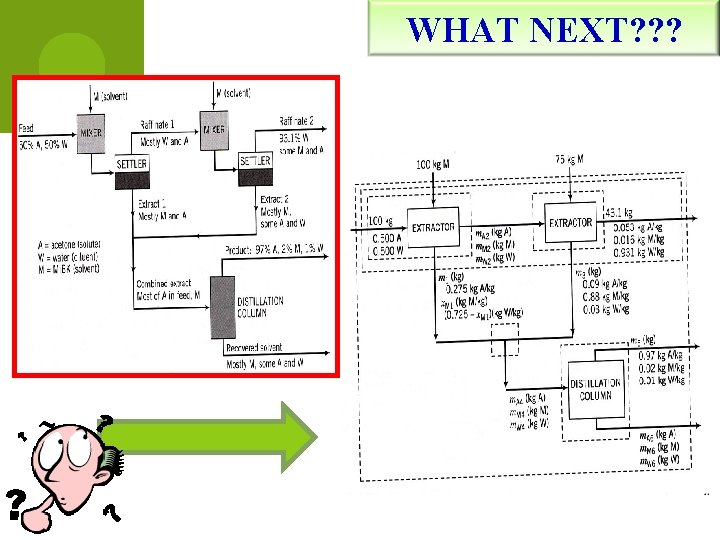
WHAT NEXT? ? ?

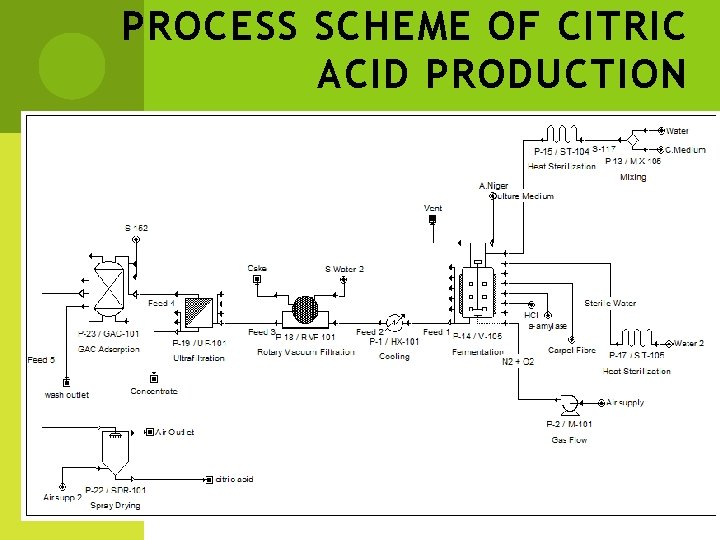
PROCESS SCHEME OF CITRIC ACID PRODUCTION

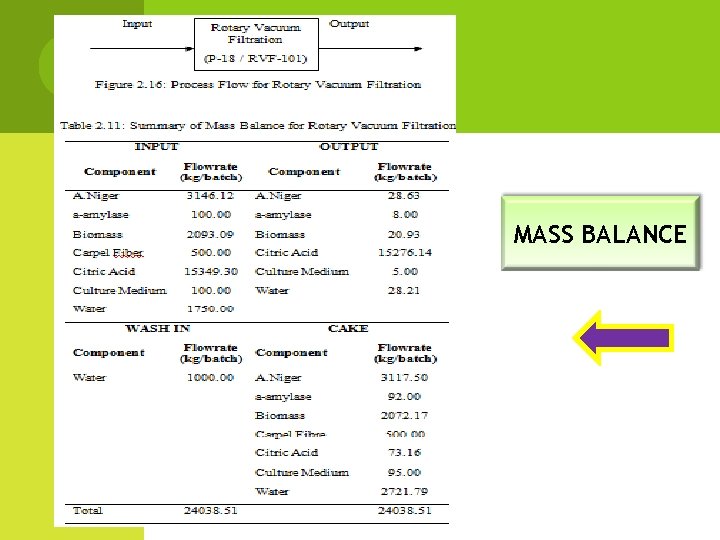
MASS BALANCE

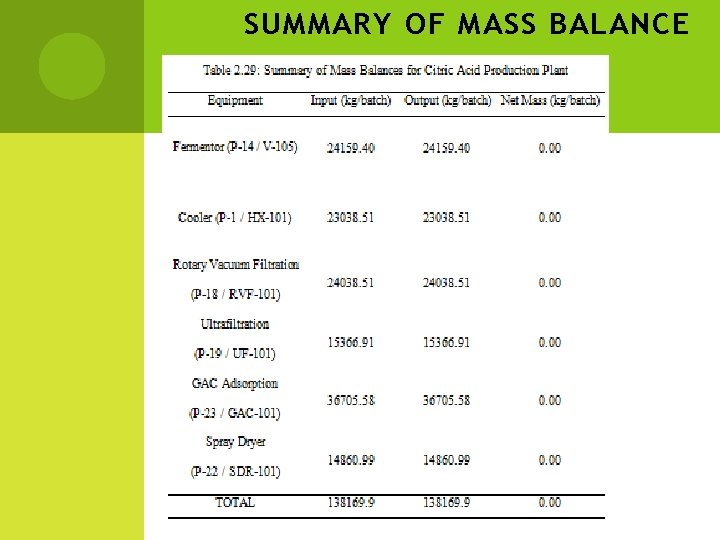
SUMMARY OF MASS BALANCE

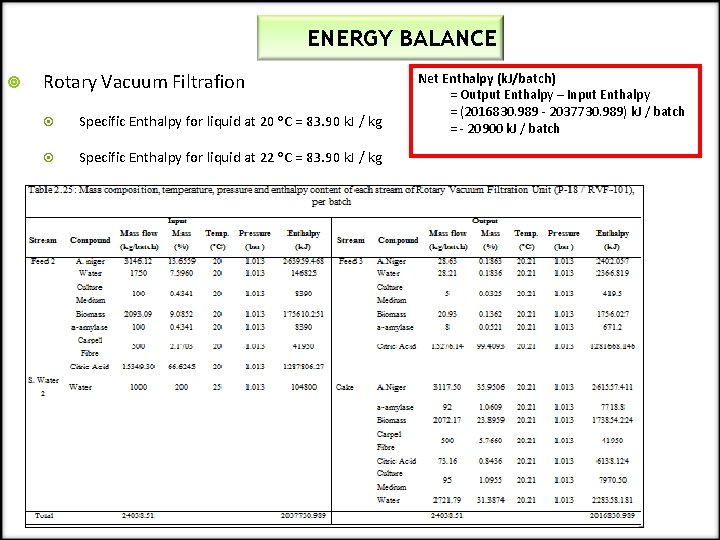
ENERGY BALANCE Rotary Vacuum Filtrafion Specific Enthalpy for liquid at 20 o. C = 83. 90 k. J / kg Specific Enthalpy for liquid at 22 o. C = 83. 90 k. J / kg Net Enthalpy (k. J/batch) = Output Enthalpy – Input Enthalpy = (2016830. 989 - 2037730. 989) k. J / batch = - 20900 k. J / batch

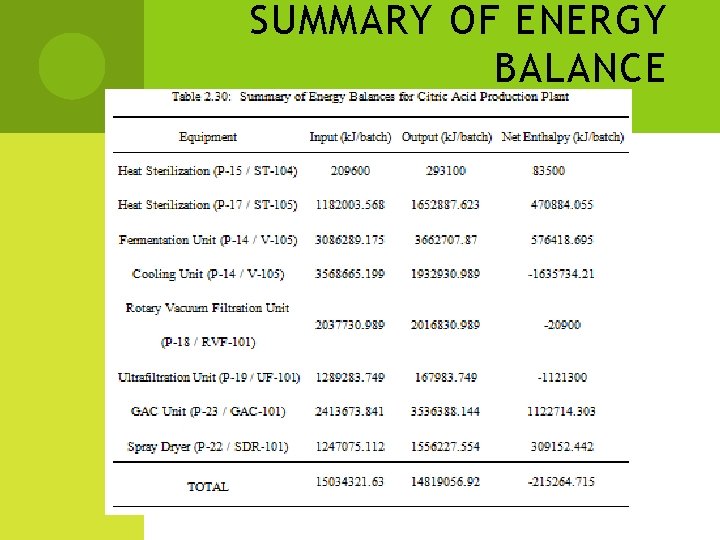
SUMMARY OF ENERGY BALANCE

PART 1 • The importance of the process or product selected from Malaysia Bioprocess Industry point of view. • Information of the chosen process with general data on the plant capacity, total cost in RM (latest currency rate conversion if in $USD, etc. ), location of similar type of units and capacity in Malaysia or outside Malaysia. • Write down at least 3 different alternatives of the process scheme for the production of the desired bio-product. • Choice of the best process supported by data, safety and environmental issues and economical indicators, market analysis, flexibility and controllability. • Process chemistry, reactions, kinetics, thermodynamics data and other physical and chemical properties data (MSDS Sheet of all the raw materials, products and by -products in the process). • Choose the capacity of the plant based on the literature background and provide the justification. Give the accurate reference and complete description of the process. References must be quoted in the standard format. • Selection of the plan site location. • Conceptual design process. • Analysis for the most economical load of the plant. • The general profitability analysis of the plant. • Environmental effects and risks of the plant.

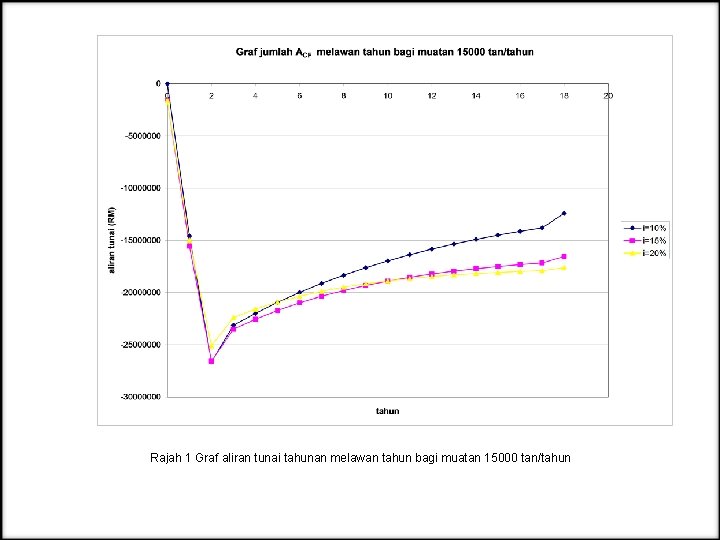
Rajah 1 Graf aliran tunai tahunan melawan tahun bagi muatan 15000 tan/tahun

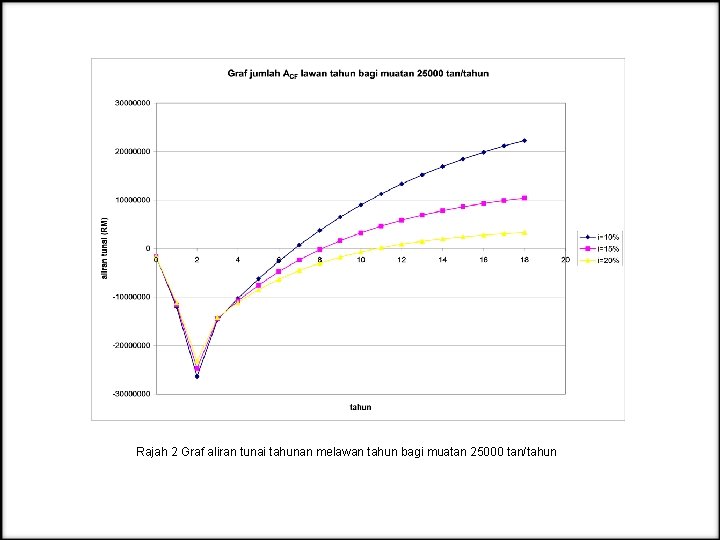
Rajah 2 Graf aliran tunai tahunan melawan tahun bagi muatan 25000 tan/tahun

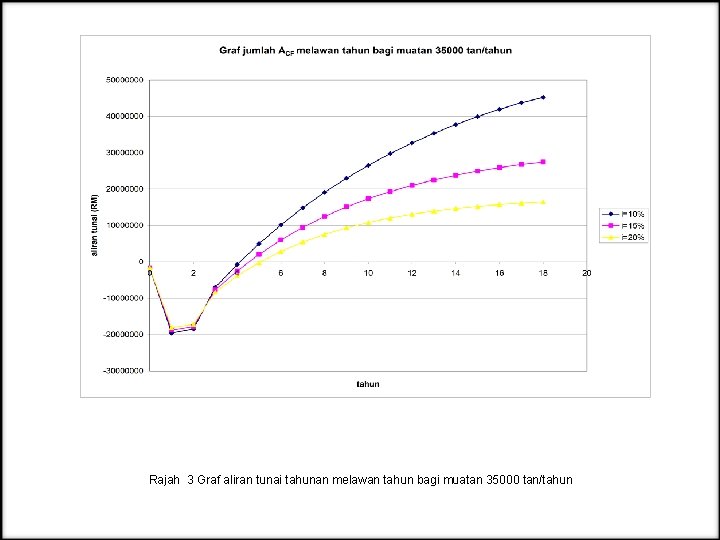
Rajah 3 Graf aliran tunai tahunan melawan tahun bagi muatan 35000 tan/tahun

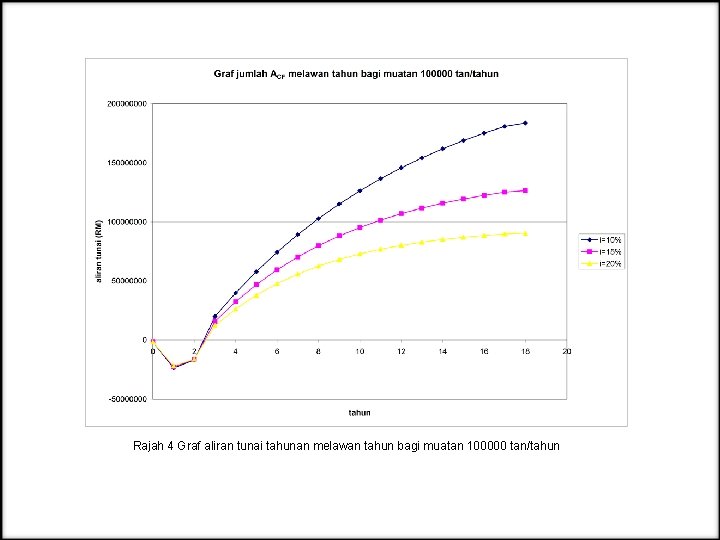
Rajah 4 Graf aliran tunai tahunan melawan tahun bagi muatan 100000 tan/tahun

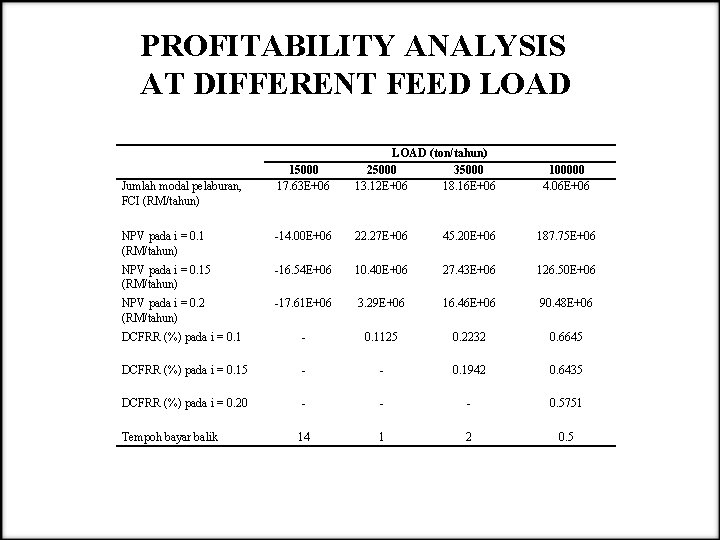
PROFITABILITY ANALYSIS AT DIFFERENT FEED LOAD 15000 17. 63 E+06 LOAD (ton/tahun) 25000 35000 13. 12 E+06 18. 16 E+06 NPV pada i = 0. 1 (RM/tahun) -14. 00 E+06 22. 27 E+06 45. 20 E+06 187. 75 E+06 NPV pada i = 0. 15 (RM/tahun) -16. 54 E+06 10. 40 E+06 27. 43 E+06 126. 50 E+06 NPV pada i = 0. 2 (RM/tahun) -17. 61 E+06 3. 29 E+06 16. 46 E+06 90. 48 E+06 DCFRR (%) pada i = 0. 1 - 0. 1125 0. 2232 0. 6645 DCFRR (%) pada i = 0. 15 - - 0. 1942 0. 6435 DCFRR (%) pada i = 0. 20 - - - 0. 5751 14 1 2 0. 5 Jumlah modal pelaburan, FCI (RM/tahun) Tempoh bayar balik 100000 4. 06 E+06

THANK YOU Prepared by, MISS RAHIMAH OTHMAN
 Pt = p.ert merupakan rumus dari pertumbuhan penduduk
Pt = p.ert merupakan rumus dari pertumbuhan penduduk Transfer function of pid controller is
Transfer function of pid controller is Ert tool
Ert tool Ert diagram
Ert diagram Ert erp definition
Ert erp definition Ert erp definition
Ert erp definition Ert 1 programa
Ert 1 programa Ert products malaysia
Ert products malaysia Intermediate beam design
Intermediate beam design Maximum material condition and least material condition
Maximum material condition and least material condition Pop culture examples
Pop culture examples What is real culture
What is real culture Example of material culture
Example of material culture Examples of household materials useful and harmful
Examples of household materials useful and harmful Variance analysis meaning
Variance analysis meaning Tire wheel and wheel bearing fundamentals
Tire wheel and wheel bearing fundamentals Chapter 1 health and wellness fundamentals
Chapter 1 health and wellness fundamentals Forensic science fundamentals and investigations chapter 6
Forensic science fundamentals and investigations chapter 6 Understanding health and wellness
Understanding health and wellness Fundamentals of nursing chapter 1
Fundamentals of nursing chapter 1 Fundamentals of electric circuits chapter 4 solutions
Fundamentals of electric circuits chapter 4 solutions Fundamentals of corporate finance chapter 6 solutions
Fundamentals of corporate finance chapter 6 solutions Digital fundamentals chapter 4
Digital fundamentals chapter 4 Chapter 24 magnetism magnetic fundamentals answers
Chapter 24 magnetism magnetic fundamentals answers 9
9 Fundamentals of electric circuits chapter 7 solutions
Fundamentals of electric circuits chapter 7 solutions Fundamentals of building construction chapter summaries
Fundamentals of building construction chapter summaries Computer fundamentals chapter 1
Computer fundamentals chapter 1 Fundamentals of corporate finance, chapter 1
Fundamentals of corporate finance, chapter 1 Fundamentals of thermal-fluidsciences chapter 1 problem 13p
Fundamentals of thermal-fluidsciences chapter 1 problem 13p Introduction to information systems 6th edition
Introduction to information systems 6th edition Fundamentals of information systems chapter 1
Fundamentals of information systems chapter 1 Fundamentals of nursing chapter 17 vital signs
Fundamentals of nursing chapter 17 vital signs Driven meaning
Driven meaning Chapter 2 lab java fundamentals
Chapter 2 lab java fundamentals Fundamentals of nursing chapter 15 critical thinking
Fundamentals of nursing chapter 15 critical thinking Examples of the respiratory system
Examples of the respiratory system Chapter 39 electrical fundamentals
Chapter 39 electrical fundamentals Fundamentals of thermal-fluidsciences chapter 2 problem 11p
Fundamentals of thermal-fluidsciences chapter 2 problem 11p Fundamentals of thermal-fluidsciences chapter 2 problem 64p
Fundamentals of thermal-fluidsciences chapter 2 problem 64p Chapter 15 critical thinking in nursing practice
Chapter 15 critical thinking in nursing practice Fundamentals of thermal-fluidsciences chapter 1 problem 19p
Fundamentals of thermal-fluidsciences chapter 1 problem 19p Fundamentals of thermal-fluidsciences chapter 1 problem 16p
Fundamentals of thermal-fluidsciences chapter 1 problem 16p Fundamentals of nursing chapter 16
Fundamentals of nursing chapter 16 Grashof number
Grashof number Fundamentals of corporate finance, chapter 1
Fundamentals of corporate finance, chapter 1 Fundamentals of thermal-fluidsciences chapter 2 problem 30p
Fundamentals of thermal-fluidsciences chapter 2 problem 30p Fundamentals of thermal-fluidsciences chapter 2 problem 28p
Fundamentals of thermal-fluidsciences chapter 2 problem 28p Chapter 23 computer system fundamentals
Chapter 23 computer system fundamentals Heat transfer
Heat transfer Mta security fundamentals practice exam
Mta security fundamentals practice exam Logic and computer design fundamentals
Logic and computer design fundamentals Logic and computer design fundamentals
Logic and computer design fundamentals Logic and computer design fundamentals
Logic and computer design fundamentals Logic and computer design fundamentals
Logic and computer design fundamentals Activity and exercise fundamentals of nursing
Activity and exercise fundamentals of nursing Chapter 74 ase questions
Chapter 74 ase questions Fundamentals of data and signals
Fundamentals of data and signals Customer service and sales fundamentals
Customer service and sales fundamentals Which neuron is rare
Which neuron is rare Fluid mechanics fundamentals and applications
Fluid mechanics fundamentals and applications Pearson physics
Pearson physics Productivity output input
Productivity output input Process instrumentation ppt
Process instrumentation ppt Fundamentals of planning and developing tourism
Fundamentals of planning and developing tourism Fundamentals of data and signals
Fundamentals of data and signals Introduction to futures and options
Introduction to futures and options Applications of plasmonics
Applications of plasmonics Fundamentals of food and beverage service operation
Fundamentals of food and beverage service operation Evolution and fundamentals of business
Evolution and fundamentals of business Pain management goals for nurses
Pain management goals for nurses Critical radius of insulation for cylinder
Critical radius of insulation for cylinder Nervous
Nervous