ENTC 4390 MEDICAL IMAGING STRUCTURE OF MATTER l





















- Slides: 40

ENTC 4390 MEDICAL IMAGING STRUCTURE OF MATTER

l l Since the late 1920 s it has been understood that electrons in an atom do not behave exactly like tiny moons orbiting a planet-like nucleus. Their behavior Is described more accurately if, instead of defining them as point particles in orbits with specific velocities and positions, they are defined as entities whose behavior is described by wave functions.

l While a wave function itself is not directly observable, calculations may be performed with this function to predict the location of the electron. • In contrast to the calculations of classical mechanics in which properties such as force, mass, acceleration, and so on, are entered into equations to yield a definite answer for a quantity such as position in space, quantum mechanical calculations yield probabilities.

l At a particular location in space, for example, the square of the amplitude of a particle’s wave function yields the probability that the particle will appear at that location. • • However, it is important to emphasize that the probability of finding the electron at other locations, even in the middle of the nucleus, is not zero. This particular result explains a certain form of radioactive decay in which a nucleus captures an electron. • This event is not explainable by classical mechanics, but can be explained with quantum mechanics.

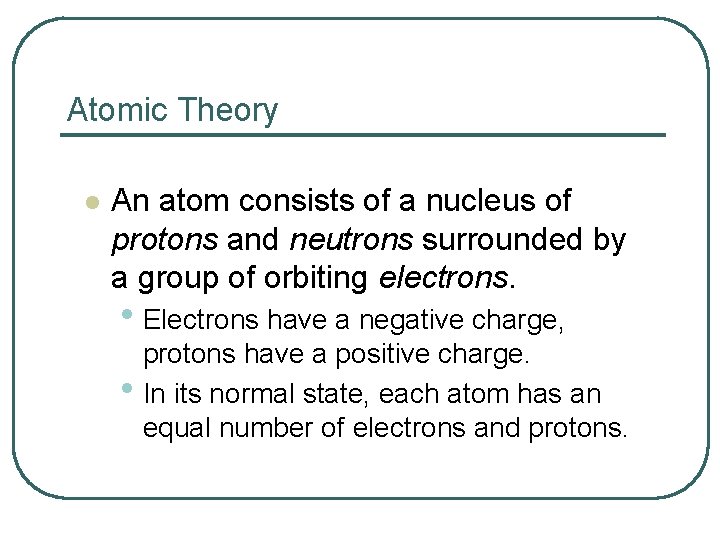
Atomic Theory l An atom consists of a nucleus of protons and neutrons surrounded by a group of orbiting electrons. • Electrons have a negative charge, protons have a positive charge. • In its normal state, each atom has an equal number of electrons and protons.


Atomic Theory l l l Electrons orbit the nucleus in discrete orbits called shells. These shells are designated by letters K, L, M, N, etc. Only certain numbers of electrons can exist within any given shell.

Atomic Theory l l The outermost shell of an atom is called the valence shell. The electrons in this shell are called valence electrons. No element can have more than eight valence electrons. The number of valence electrons affects its electrical properties.

l The binding energy of an electron (Eb) is defined as the energy required to completely separate the electron from the atom. • • When energy is measured in the macroscopic world of everyday experience, units such as joules and kilowatt -hours are used. In the microscopic world, the electron-volt is a more convenient unit of energy • One electron volt is the kinetic energy imparted to an electron accelerated across a potential difference (i. e. , voltage) of 1 Volt.

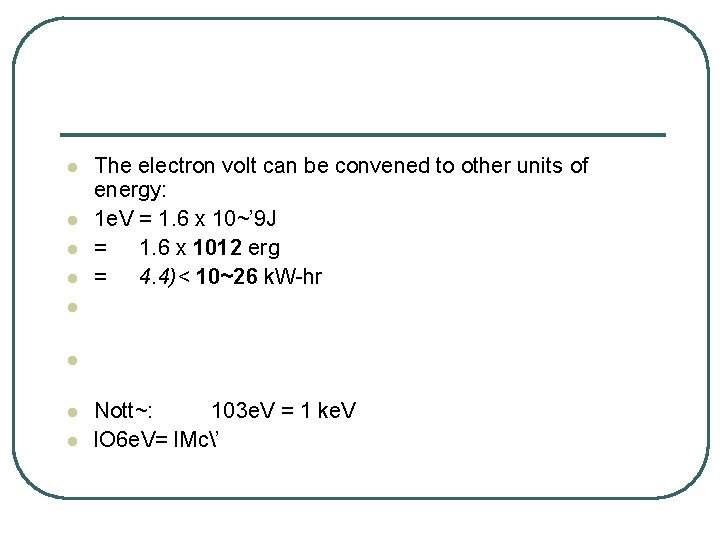
l l The electron volt can be convened to other units of energy: 1 e. V = 1. 6 x 10~’ 9 J = 1. 6 x 1012 erg = 4. 4)< 10~26 k. W-hr l l Nott~: 103 e. V = 1 ke. V l. O 6 e. V= l. Mc’

l l The electron volt describes potential as well as kintnic energy. The binding energy of an electron in an atom is a form o. F potential energy

l l An electron in an inner shell of an atom is attracted to the nucleus by a force greater than that exerted by the nucleus on an electron farther away. An electron may be moved from one shell to another shell that is farther from the nucleus only if energy is supplied by an external source. • Binding energy is negative (i. e. , written with a minus sign) because it represents an amount of energy that must be supplied to remove an electron from an atom. The energy that must be imparted to an atom to move an electron from an inner shell to an outer shell is equal to the arithmetic difference in binding energy between the two shells.

l For example, the binding energy is 13. 5 e. V for an electron in the K shell of hydrogen and is 3. 4 e. V for an electron in the L shell. • The energy required to move an electron from the K to the L shell in hydrogen is ( 3. 4 e. V) ( 13. 5 e. V) = 10. 1 e. V

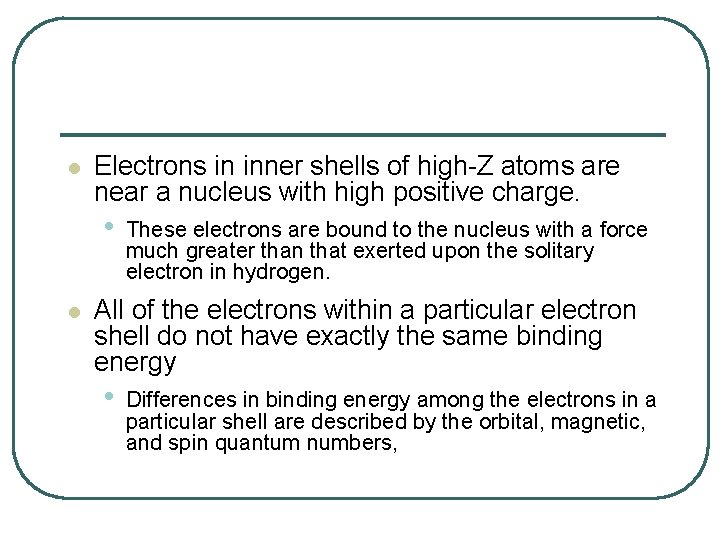
l Electrons in inner shells of high-Z atoms are near a nucleus with high positive charge. • l These electrons are bound to the nucleus with a force much greater than that exerted upon the solitary electron in hydrogen. All of the electrons within a particular electron shell do not have exactly the same binding energy • Differences in binding energy among the electrons in a particular shell are described by the orbital, magnetic, and spin quantum numbers,

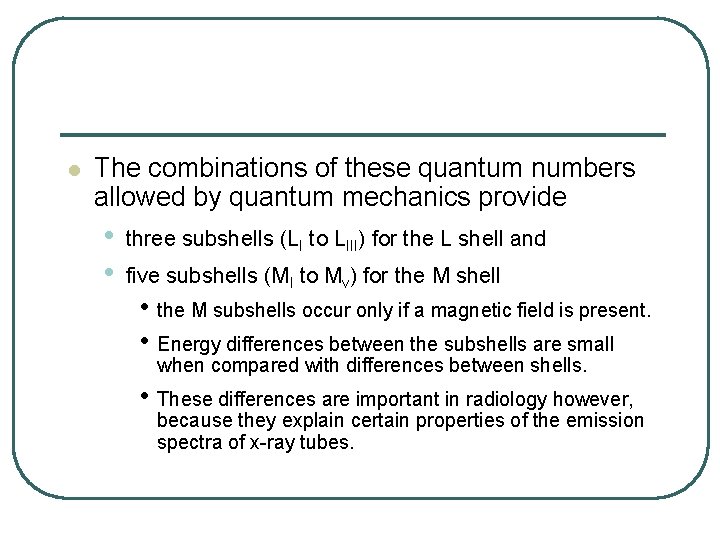
l The combinations of these quantum numbers allowed by quantum mechanics provide • • three subshells (LI to LIII) for the L shell and five subshells (MI to Mv) for the M shell • the M subshells occur only if a magnetic field is present. • Energy differences between the subshells are small when compared with differences between shells. • These differences are important in radiology however, because they explain certain properties of the emission spectra of x-ray tubes.

l l Various processes can cause an electron to be ejected from an electron shell. When an electron is removed from a shell, a vacancy or “hole” is left in the shell • • (i. e. , a quantum “address” is left vacant. An electron may move from one shell to another to fill the vacancy • This movement, termed an electron transition, involves a

Conductors l Materials that have large numbers of free electrons are called conductors. • Metals are generally good conductors because they have few loosely bound valence electrons. • Silver, gold, copper, and aluminum are excellent conductors.

Insulators l Materials that do not conduct because their valence shells are full or almost full are called insulators. • Glass, porcelain, plastic, and rubber are good insulators. • If high enough voltage is applied, an insulator will break down and conduct.

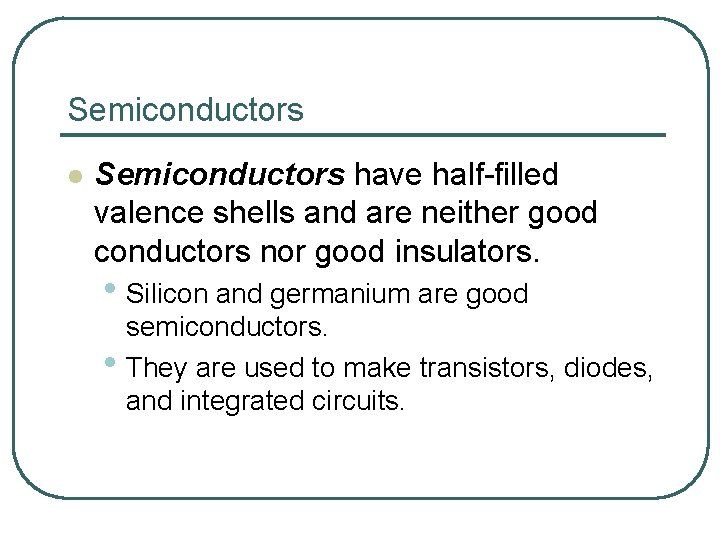
Semiconductors l Semiconductors have half-filled valence shells and are neither good conductors nor good insulators. • Silicon and germanium are good semiconductors. • They are used to make transistors, diodes, and integrated circuits.

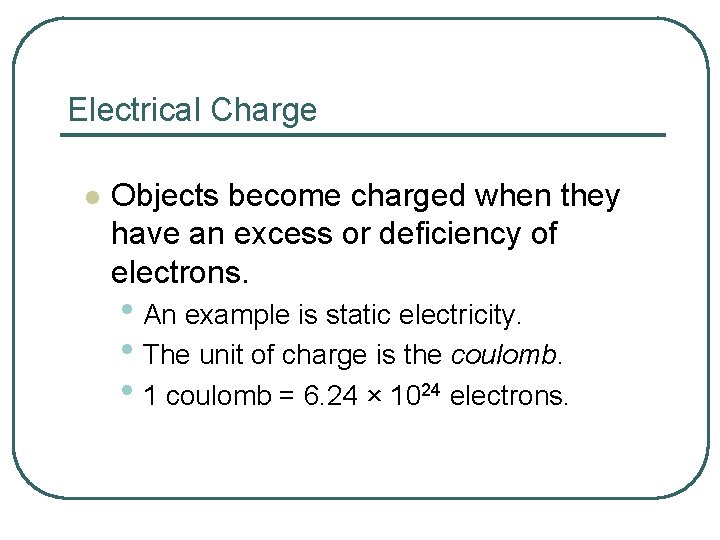
Electrical Charge l Objects become charged when they have an excess or deficiency of electrons. • An example is static electricity. • The unit of charge is the coulomb. • 1 coulomb = 6. 24 × 1024 electrons.

ENTC 4390 THE NUCLEUS

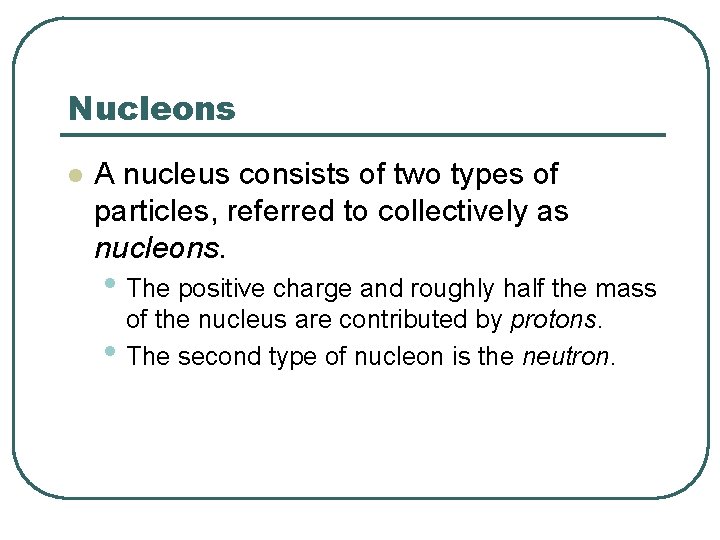
Nucleons l A nucleus consists of two types of particles, referred to collectively as nucleons. • The positive charge and roughly half the mass • of the nucleus are contributed by protons. The second type of nucleon is the neutron.

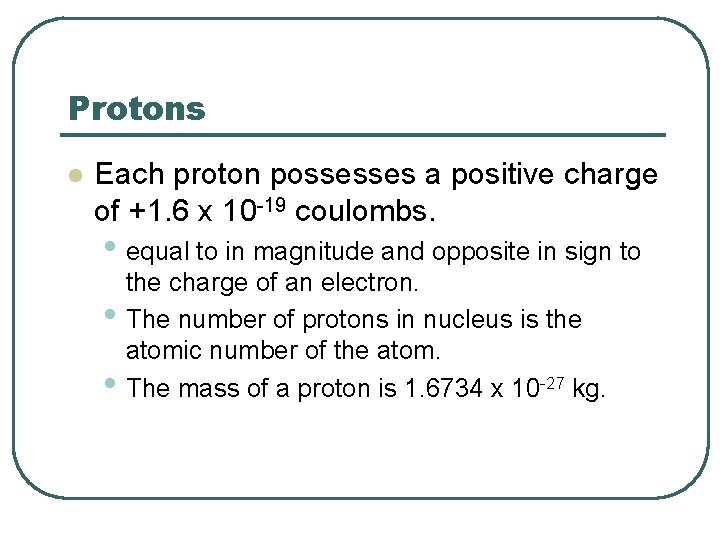
Protons l Each proton possesses a positive charge of +1. 6 x 10 -19 coulombs. • equal to in magnitude and opposite in sign to • • the charge of an electron. The number of protons in nucleus is the atomic number of the atom. The mass of a proton is 1. 6734 x 10 -27 kg.

Neutrons l Neutrons are uncharged particles with a mass of 1. 6747 x 10 -27 kg. • Outside the nucleus, neutrons are unstable • and divide into protons, electrons, and antineurtrinos. The number of neutrons in in a nucleus is the neutron number N for the nucleus.

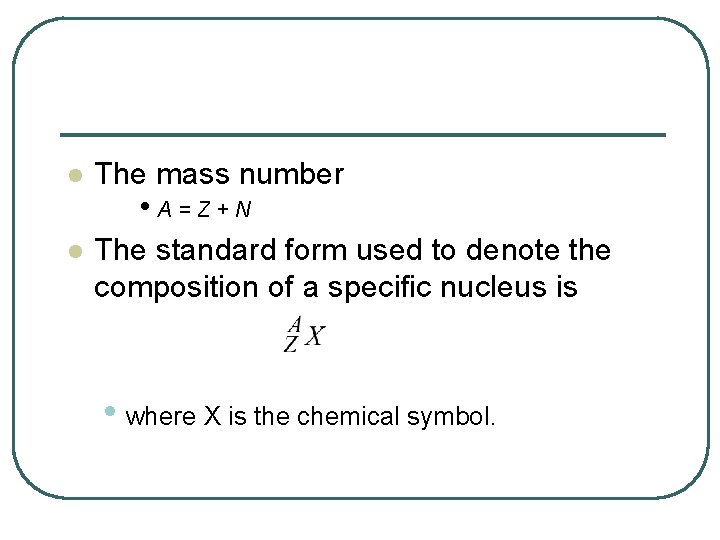
l The mass number l The standard form used to denote the composition of a specific nucleus is • A=Z+N • where X is the chemical symbol.

Isotopes l Isotopes are atoms that possess the same number of protons but a varying number of neutrons. • Isotopes of hydrogen are • 1 H—protium • 2 H—deuterium • 3 H—tritium

ENTC 4390 NUCLEAR FISSION & FUSION

Nuclear Power l l Nuclear power may be produced in two ways Nuclear fission involves the splitting of an atom into two fragments, particles, and the release of energy Nuclear fusion involves the combination of two nuclei into a single, more massive nuclei, plus energy Stars are powered by nuclear fusion

Nuclear Fusion l l Nuclear Fusion has been used since the early 1950’s in Hydrogen bombs These are the most powerful type of nuclear weapon We have not yet devised a method of utilizing the power of nuclear fusion in the laboratory, nor in any commercial reactor Therefore, we will not further consider fusion in this course

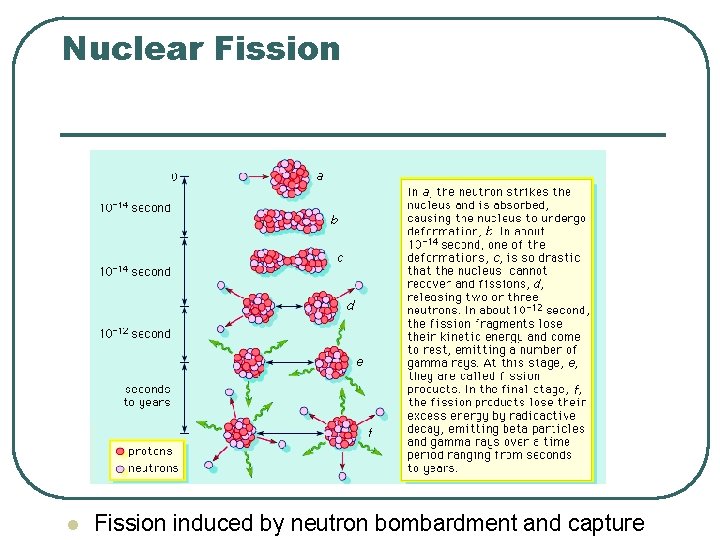
Nuclear Fission l Fission induced by neutron bombardment and capture

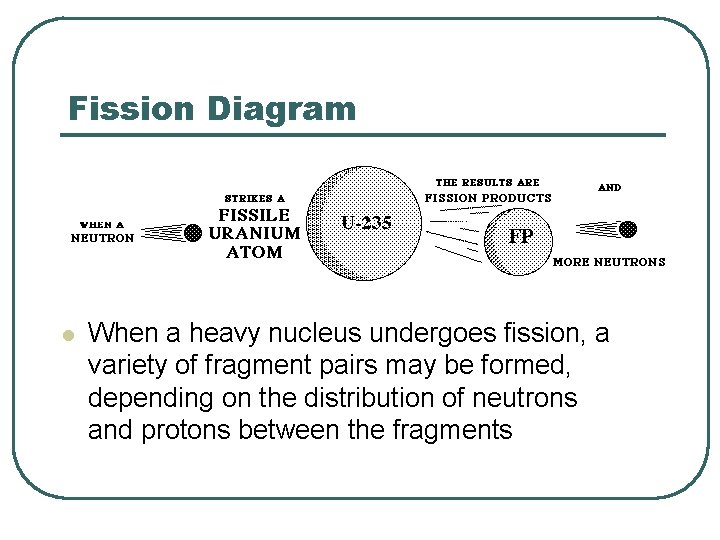
Fission Diagram l When a heavy nucleus undergoes fission, a variety of fragment pairs may be formed, depending on the distribution of neutrons and protons between the fragments

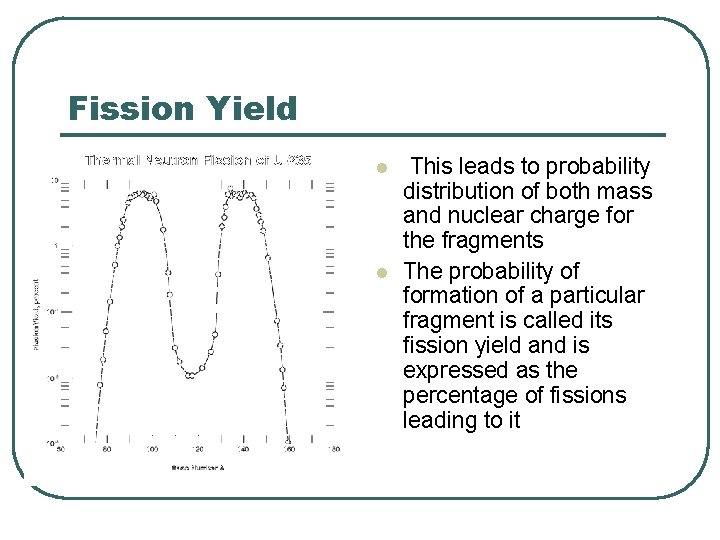
Fission Yield l l This leads to probability distribution of both mass and nuclear charge for the fragments The probability of formation of a particular fragment is called its fission yield and is expressed as the percentage of fissions leading to it

Fission Products l A fission product is any of the lighter atomic nuclei formed by splitting heavier nuclei (nuclear fission), including both the primary nuclei directly produced (fission fragments) and the nuclei subsequently generated by their radioactive decay

Fission Fragment Decay l l Fission fragments are highly unstable because of their abnormally large number of neutrons compared with protons Consequently, they undergo successive radioactive decays by emitting neutrons, by converting neutrons into protons, antineutrinos, and ejected electrons (beta decay), and by radiating energy (gamma decay)

Fission of l l 235 U One of the many known fission reactions of uranium-235 induced by absorbing a neutron results, for example, in two extremely unstable fission fragments, a barium and a krypton nucleus These fragments almost instantaneously release three neutrons between themselves, becoming barium-144 and krypton-89

Barium Decay l By repeated beta decay, the barium-144 in turn is converted step by step to other fission products • Lanthanum-144 • Cerium-144 • Praseodymium-144 • Eventually relatively stable neodymium-144

Krypton l krypton-89 is similarly transformed by repeated beta decay to: • Rubidium-89 • Strontium-89 • To stable yttrium-89

Fission Product Identification l l Fission products are identified by their chemical properties and by their radioactive properties, such as their halflives and the kinds of particles they emit The multiple decays mean fission products are highly radioactive and therefore quite dangerous

Why are Fission Products Radioactive? l l l To maintain stability, the neutron-toproton (n/p) ratio in nuclei must increase with increasing proton number The ratio remains at unity up to the element calcium, with 20 protons It then gradually increases until it reaches a value of about 1. 5 for the heaviest elements

 Frc control system
Frc control system Entc
Entc Ohiohealth berger hospital mammography circleville
Ohiohealth berger hospital mammography circleville Spie
Spie Radiology medical terms
Radiology medical terms Fourier
Fourier Medical imaging software
Medical imaging software Conclusion of marginalization
Conclusion of marginalization Body quadrants and organs
Body quadrants and organs Section 1 composition of matter
Section 1 composition of matter Grey matter of nervous system
Grey matter of nervous system Composition of matter section 1
Composition of matter section 1 Chapter 2 section 1 classifying matter answers
Chapter 2 section 1 classifying matter answers Brain falx
Brain falx Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Gray matter and white matter
Gray matter and white matter What does grey matter do
What does grey matter do Flow energy review
Flow energy review Ptal california medical board
Ptal california medical board Gbmc medical records
Gbmc medical records Difference between medical report and medical certificate
Difference between medical report and medical certificate Torrance memorial outpatient lab
Torrance memorial outpatient lab Cartersville medical center medical records
Cartersville medical center medical records Ishellextinit
Ishellextinit Gabriela barreto lemos
Gabriela barreto lemos Affordable hyperspectral imaging
Affordable hyperspectral imaging Live cell imaging applications
Live cell imaging applications Curved mirrors
Curved mirrors Focused imaging learning
Focused imaging learning Direct imaging subsystem
Direct imaging subsystem Hardware independent imaging
Hardware independent imaging Digital images definition
Digital images definition Digital imaging artist
Digital imaging artist Support implementation
Support implementation Chapter 41 intraoral imaging
Chapter 41 intraoral imaging Is2000 the advanced imaging solution
Is2000 the advanced imaging solution Imaging near redwood city
Imaging near redwood city Picture perfect imaging
Picture perfect imaging Nuclear imaging techniques
Nuclear imaging techniques Coulomb explosion imaging
Coulomb explosion imaging Iac princeton
Iac princeton