Energy Reaction Kinetics NGSS PS 1 A PS













![General form for a rate law expression Rate = k m n [A] [B] General form for a rate law expression Rate = k m n [A] [B]](https://slidetodoc.com/presentation_image/3878418d4f4f86b76a35faaafc922e3a/image-35.jpg)

![Experiment number [A] (M) [B] (M) Initial rate (M/s) 1 0. 100 4. 0 Experiment number [A] (M) [B] (M) Initial rate (M/s) 1 0. 100 4. 0](https://slidetodoc.com/presentation_image/3878418d4f4f86b76a35faaafc922e3a/image-37.jpg)
![Solution part A Looking at experiment 1 and 2 keeping [A] constant, doubling [B]; Solution part A Looking at experiment 1 and 2 keeping [A] constant, doubling [B];](https://slidetodoc.com/presentation_image/3878418d4f4f86b76a35faaafc922e3a/image-38.jpg)
![Solution part B k = rate [A]2 = 4. 0 x 10 -5 M/s Solution part B k = rate [A]2 = 4. 0 x 10 -5 M/s](https://slidetodoc.com/presentation_image/3878418d4f4f86b76a35faaafc922e3a/image-39.jpg)
![Solution part C Rate = k[A]2 (4. 0 x 10 -3 M-1 s-1)(0. 050 Solution part C Rate = k[A]2 (4. 0 x 10 -3 M-1 s-1)(0. 050](https://slidetodoc.com/presentation_image/3878418d4f4f86b76a35faaafc922e3a/image-40.jpg)


- Slides: 42

Energy Reaction Kinetics NGSS: PS 1. A , PS 1. B, PS 3. A, PS 3. B, PS 3. D Chapter 17


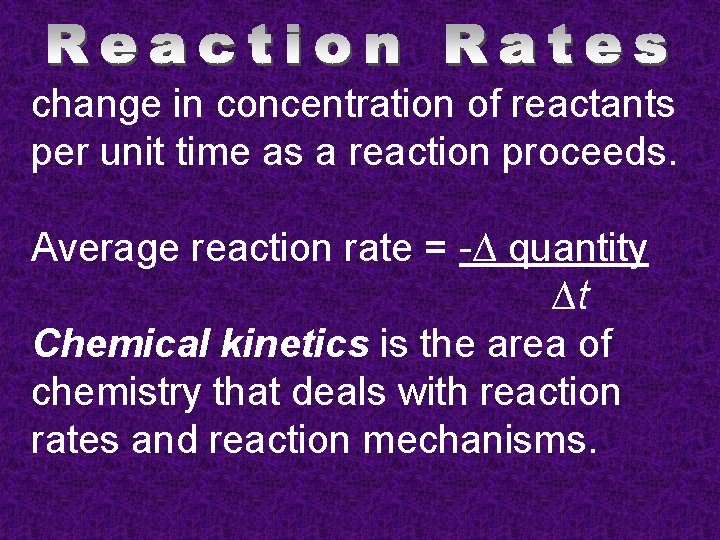
change in concentration of reactants per unit time as a reaction proceeds. Average reaction rate = -∆ quantity ∆t Chemical kinetics is the area of chemistry that deals with reaction rates and reaction mechanisms.

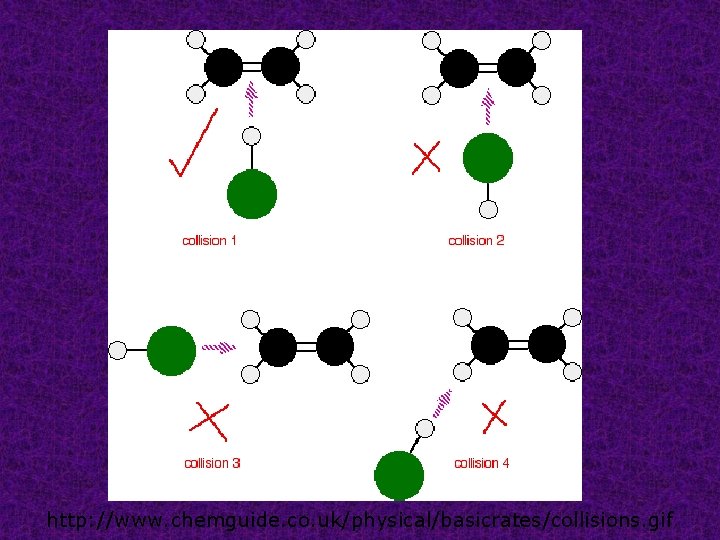
Collision theory states that in order for reactions to occur between substances their particles must collide with sufficient energy and with a favorable orientation.


http: //www. chemguide. co. uk/physical/basicrates/collisions. gif

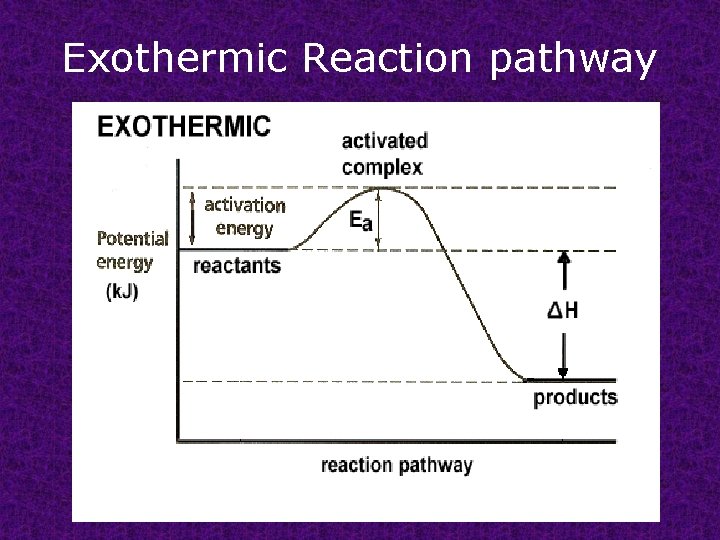
Exothermic Reaction pathway

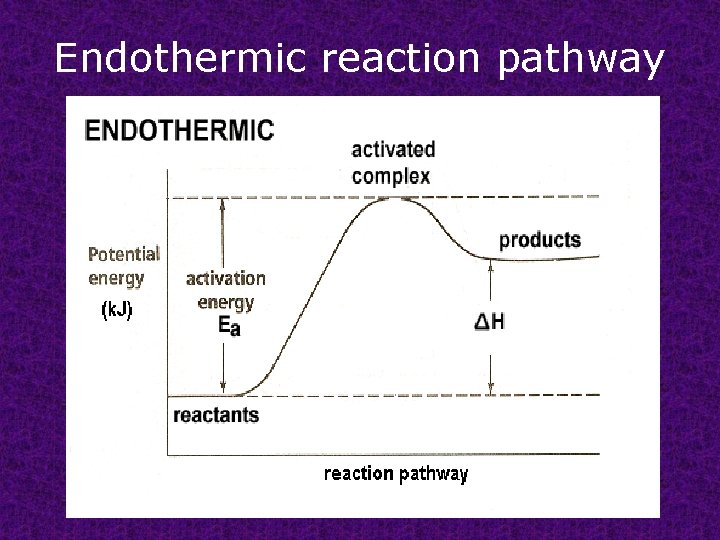
Endothermic reaction pathway

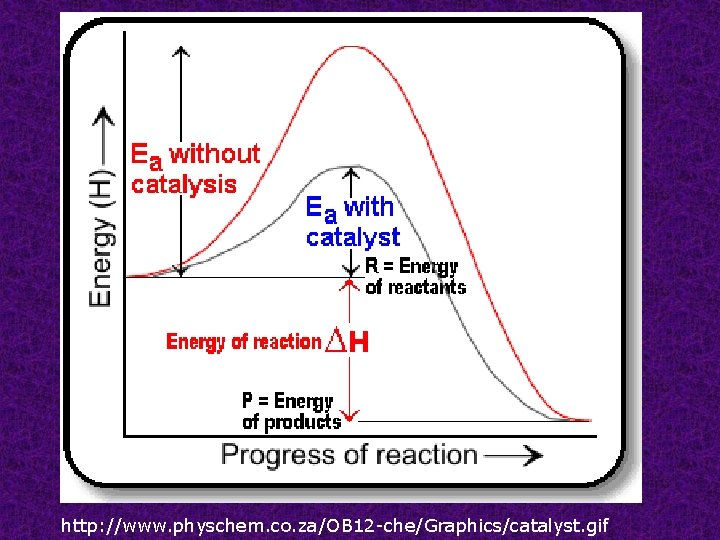
Activation energy : minimum energy required to transform the reactants into an activated complex.

Activated complex transitional structure that results from an effective collision and that persists while old bonds are breaking and new bonds are forming.

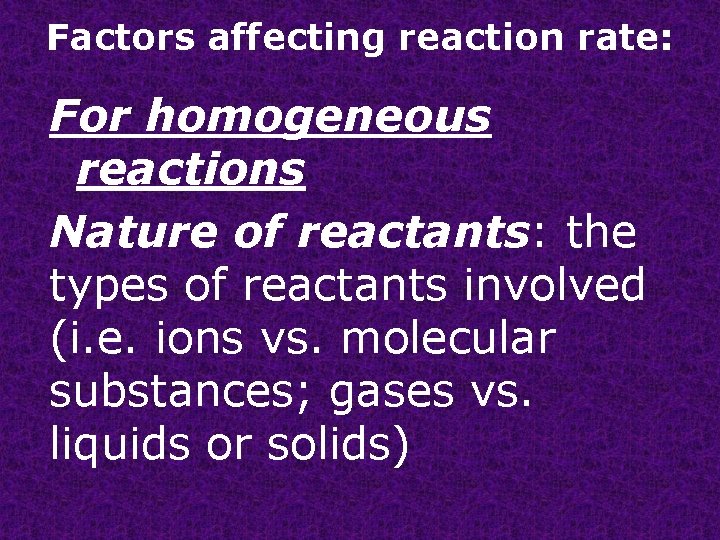
Factors affecting reaction rate: For homogeneous reactions Nature of reactants: the types of reactants involved (i. e. ions vs. molecular substances; gases vs. liquids or solids)

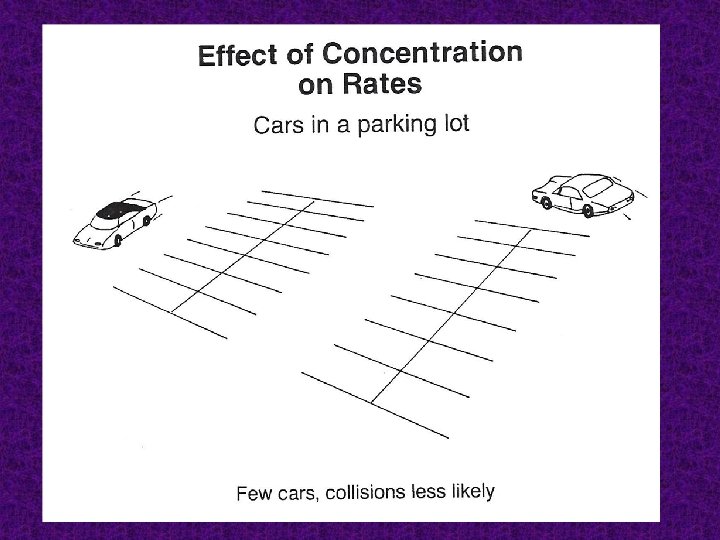
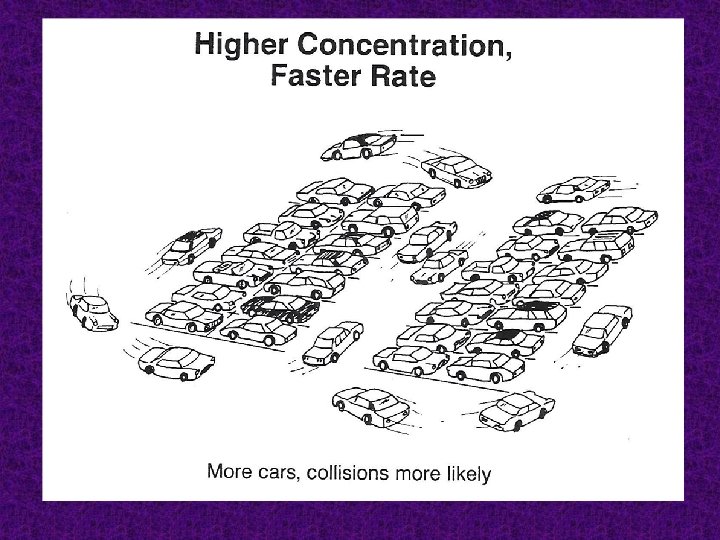
Concentration: the higher the concentration of one or more of the reactants results the faster the reaction rate.



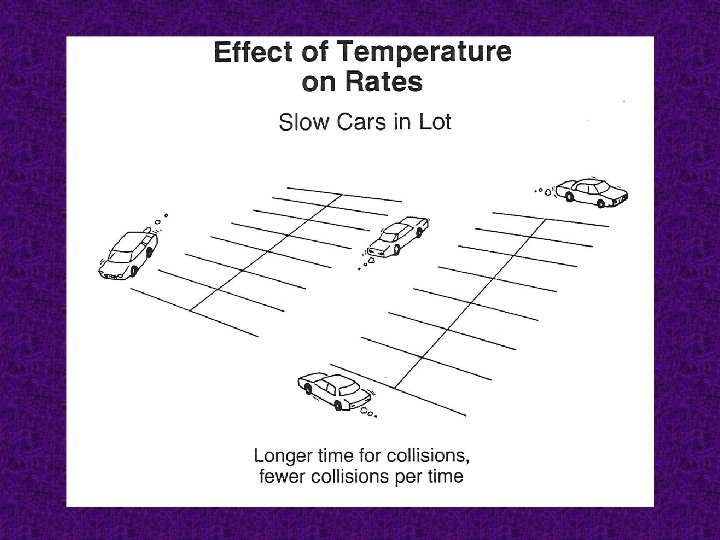
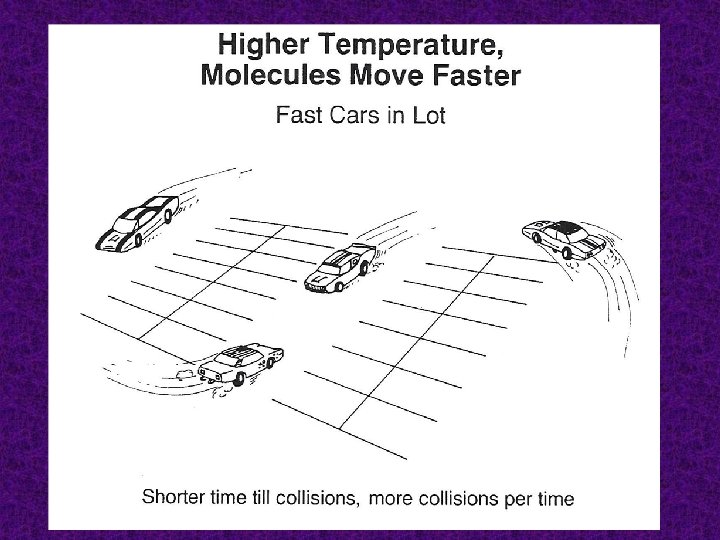
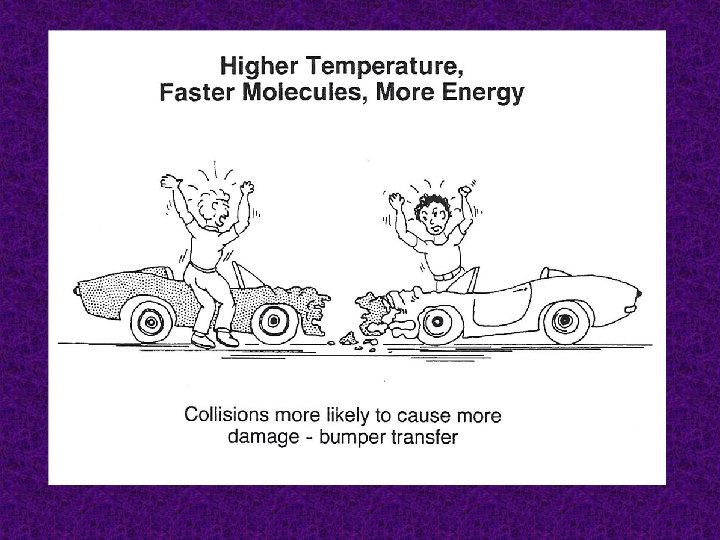
Temperature An increase in temperature increases the number of particles that have enough energy to form the activated complex. In general, for every 10 K (10˚C) rise in temperature, there is a doubling of the rate.




Catalysts substances that change the rate of the reaction without being consumed. Homogeneous catalysts are of the same phase as the reactants while heterogeneous catalysts are of a different phase as the reactants.

http: //www. physchem. co. za/OB 12 -che/Graphics/catalyst. gif

Enzyme animation: http: //www. youtube. com/wat ch? v=CZD 5 xs. OKres (2: 01)

For heterogeneous reactions: surface area is also important. (i. e. wood chips vs. a log) (Solid wood with gaseous oxygen)

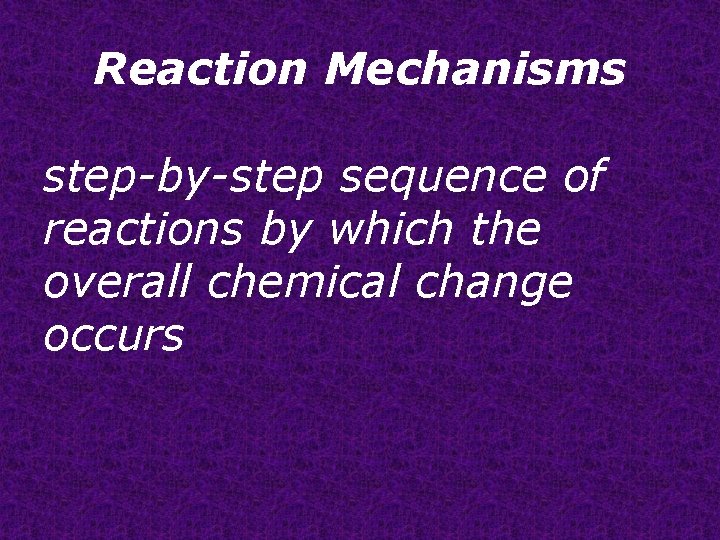
Reaction Mechanisms step-by-step sequence of reactions by which the overall chemical change occurs

Rate-determining step. The slowest step in multiple step chemical reaction. This is determined experimentally or (for our purposes) you will be given this information.

Intermediates Species that appear in some steps but not in the net ionic equation.

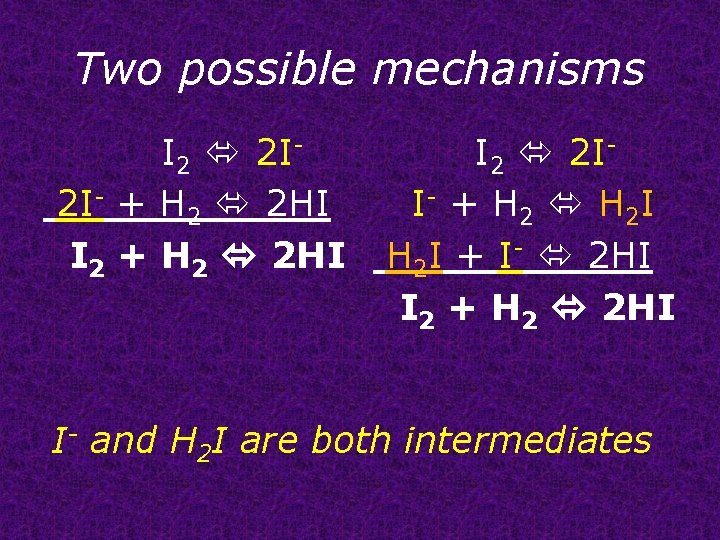
Two possible mechanisms I 2 2 I- I 2 2 I 2 I- + H 2 2 HI I- + H 2 I I 2 + H 2 2 HI H 2 I + I- 2 HI I 2 + H 2 2 HI I- and H 2 I are both intermediates

Reaction Rate Laws A rate law is an equation that relates reaction rate and concentrations of reactants. It is specific for reactions at specific temperatures.

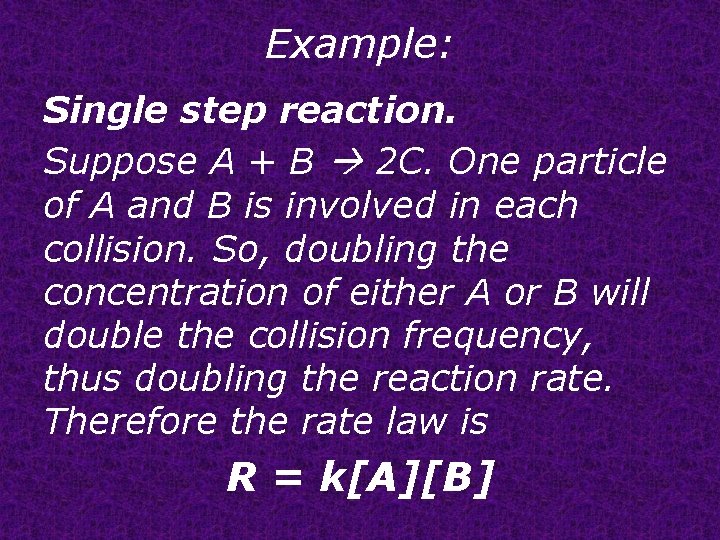
Example: Single step reaction. Suppose A + B 2 C. One particle of A and B is involved in each collision. So, doubling the concentration of either A or B will double the collision frequency, thus doubling the reaction rate. Therefore the rate law is R = k[A][B]

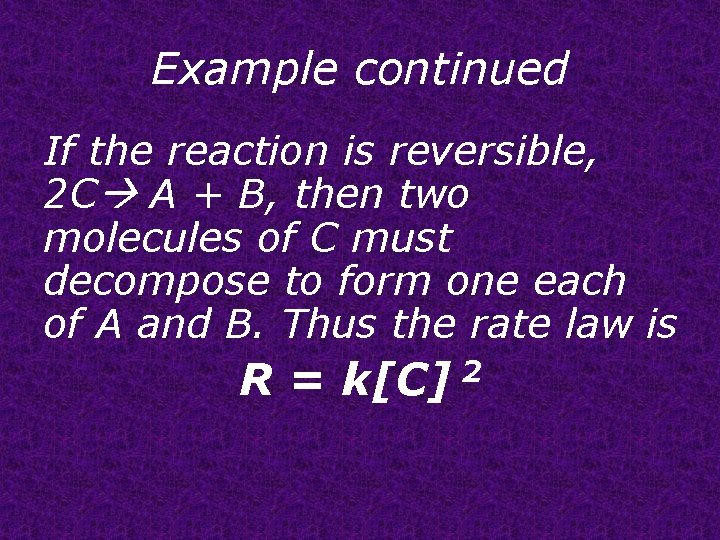
Example continued If the reaction is reversible, 2 C A + B, then two molecules of C must decompose to form one each of A and B. Thus the rate law is R = k[C] 2

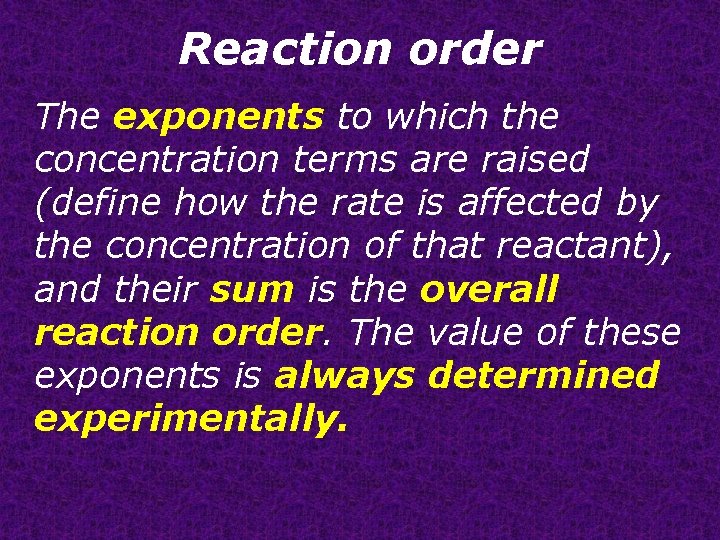
Reaction order The exponents to which the concentration terms are raised (define how the rate is affected by the concentration of that reactant), and their sum is the overall reaction order. The value of these exponents is always determined experimentally.

zero-order changing the concentration of one reactant has no effect on the rate, then it is zero-order for that reactant

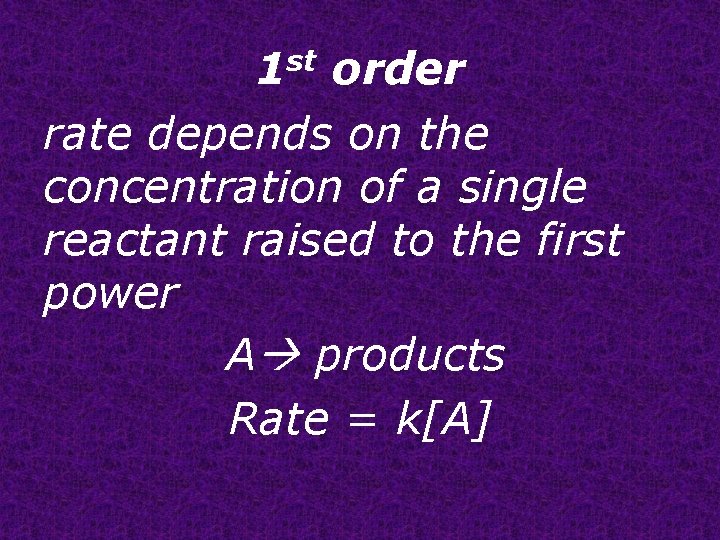
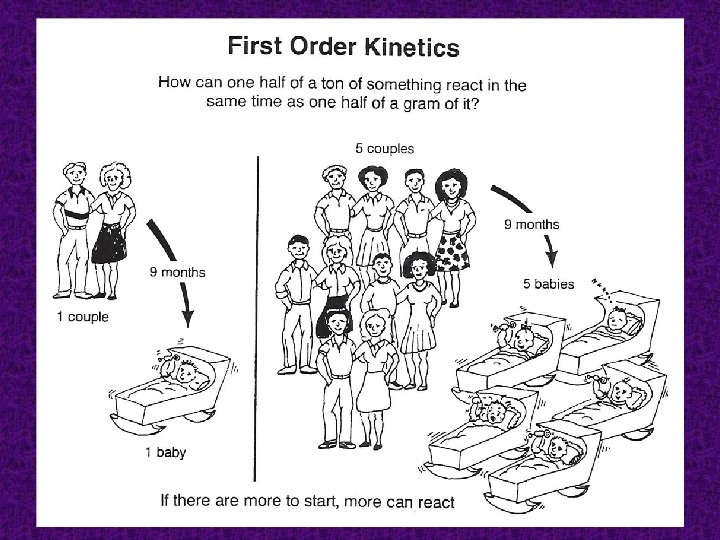
1 st order rate depends on the concentration of a single reactant raised to the first power A products Rate = k[A]


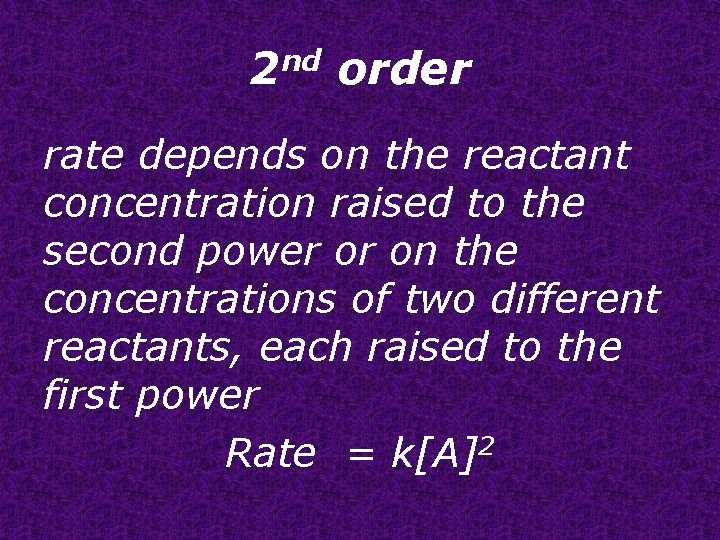
2 nd order rate depends on the reactant concentration raised to the second power or on the concentrations of two different reactants, each raised to the first power Rate = k[A]2
![General form for a rate law expression Rate k m n A B General form for a rate law expression Rate = k m n [A] [B]](https://slidetodoc.com/presentation_image/3878418d4f4f86b76a35faaafc922e3a/image-35.jpg)
General form for a rate law expression Rate = k m n [A] [B] Determine values for m and n

Example problem The initial rate of a reaction A + B C was measured for several different starting concentrations of A and B, with the results given here:
![Experiment number A M B M Initial rate Ms 1 0 100 4 0 Experiment number [A] (M) [B] (M) Initial rate (M/s) 1 0. 100 4. 0](https://slidetodoc.com/presentation_image/3878418d4f4f86b76a35faaafc922e3a/image-37.jpg)
Experiment number [A] (M) [B] (M) Initial rate (M/s) 1 0. 100 4. 0 x 10 -5 2 0. 100 0. 200 4. 0 x 10 -5 3 0. 200 0. 100 16. 0 x 10 -5 Using these data, determine (a) the rate law for the reaction; (b) the magnitude of the rate constant; (c) the rate of the reaction when [A] = 0. 050 M and [B]= 0. 100 M.
![Solution part A Looking at experiment 1 and 2 keeping A constant doubling B Solution part A Looking at experiment 1 and 2 keeping [A] constant, doubling [B];](https://slidetodoc.com/presentation_image/3878418d4f4f86b76a35faaafc922e3a/image-38.jpg)
Solution part A Looking at experiment 1 and 2 keeping [A] constant, doubling [B]; no effect on rate. Order of B is 0 (n=0). Looking at experiments 1 and 3 keeping [B] constant, doubling [A]; rate increased by a factor of 4, i. e. [A]2 (m=2). rate = k[A]2
![Solution part B k rate A2 4 0 x 10 5 Ms Solution part B k = rate [A]2 = 4. 0 x 10 -5 M/s](https://slidetodoc.com/presentation_image/3878418d4f4f86b76a35faaafc922e3a/image-39.jpg)
Solution part B k = rate [A]2 = 4. 0 x 10 -5 M/s (0. 100 M)2 = 4. 0 x 10 -3 M-1 s-1
![Solution part C Rate kA2 4 0 x 10 3 M1 s10 050 Solution part C Rate = k[A]2 (4. 0 x 10 -3 M-1 s-1)(0. 050](https://slidetodoc.com/presentation_image/3878418d4f4f86b76a35faaafc922e3a/image-40.jpg)
Solution part C Rate = k[A]2 (4. 0 x 10 -3 M-1 s-1)(0. 050 M)2 = 1. 0 x 10 -5 M/s

Isaacs TEACH https: //www. youtube. com/watch? v=m. BTSw. Jn. Z 6 Sk - collision theory 5: 12 https: //www. youtube. com/watch? v=ac. ZOZU 9 Xwm. M – potential energy diagrams 6: 54 https: //www. youtube. com/watch? v=1 j. F 6 y. Oz. Zbws – rate of reaction and rate laws 11: 14 (continue watching through the accelerated class section!) https: //www. youtube. com/watch? v=S 1 s. Wv. COTUl 8 – factors that affect reaction rate 10: 38 (continue watching through the accelerated class section!) https: //www. youtube. com/watch? v=e. EFJpm. Fk-9 A – reaction mechanisms (and rate limiting step) 4: 31 Crash Course Chemistry: https: //www. youtube. com/watch? v=7 q. OFt. L 3 VEBc Kinetics: Chemistry's Demolition Derby - 9: 57 This also introduces/reviews some equilibrium concepts!

Bozeman Science https: //www. youtube. com/watch? v=WDXz. VI 8 Smf. E The Rate Law 8: 43 (Don’t worry about finding slope of curves – beyond what you need to know) – watch this one before the on the Rate Constant https: //www. youtube. com/watch? v=6 m. Aq. X 31 RRJU The rate of Reactions 6: 24 (you do NOT need to know anything about Beers Law!) https: //www. youtube. com/watch? v=q. D 7 PDOhqbp. M Enthalpy of Reaction 8: 02 Review of Chapter 16 material (calorimetry, Hess’s law) https: //www. youtube. com/watch? v=e. OSRn 0 j. Pb. Tk The Rate Constant 6: 52 (a bit more in depth starting about halfway through the video) If you’re going to skip a video, this one would be it! Brightstorm videos: https: //www. youtube. com/watch? v=F-Cu_Ao. WOK 4 – 3: 04 Reaction rates factors https: //www. youtube. com/watch? v=t 8 S 1 h. Ka. TY_w - 9: 38 Reaction rate problems. Excellent! Look at primarily the second problem (which is quite involved)