Empirical Formulas Empirical Formula Lowest whole ratio H






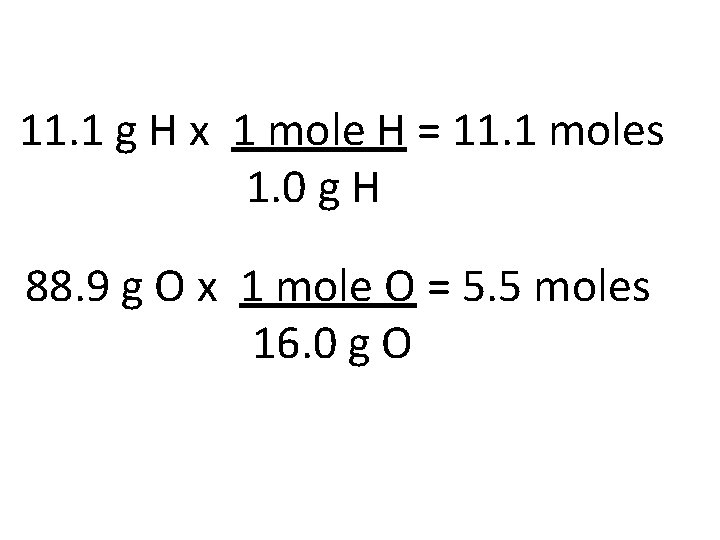




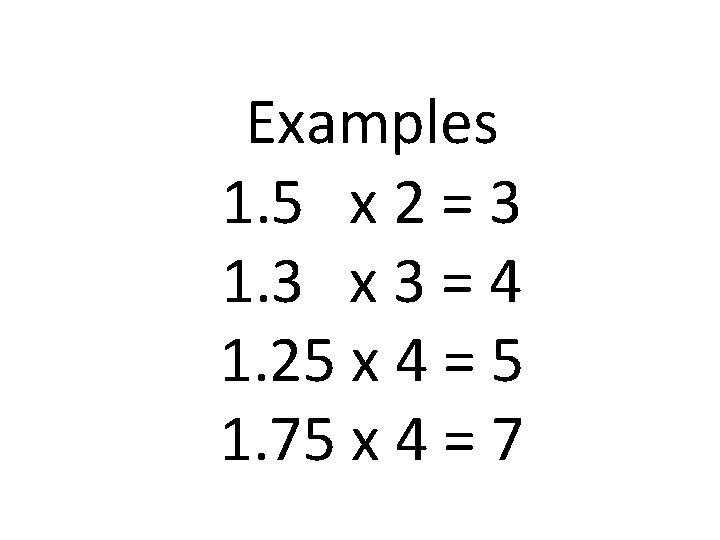
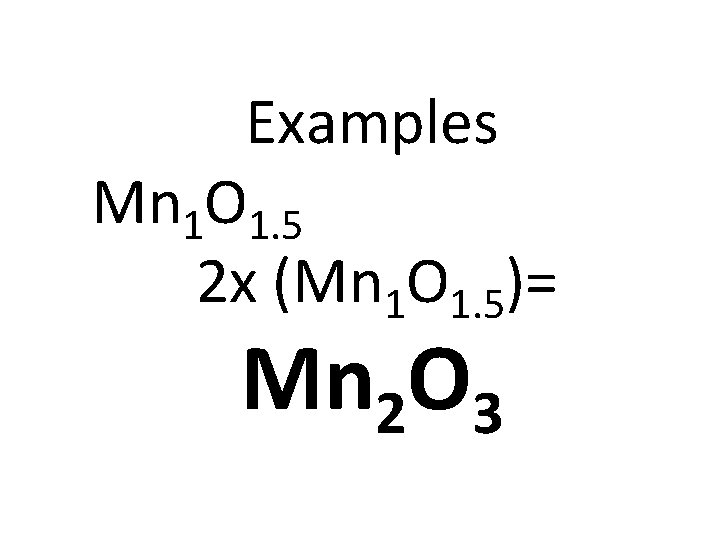




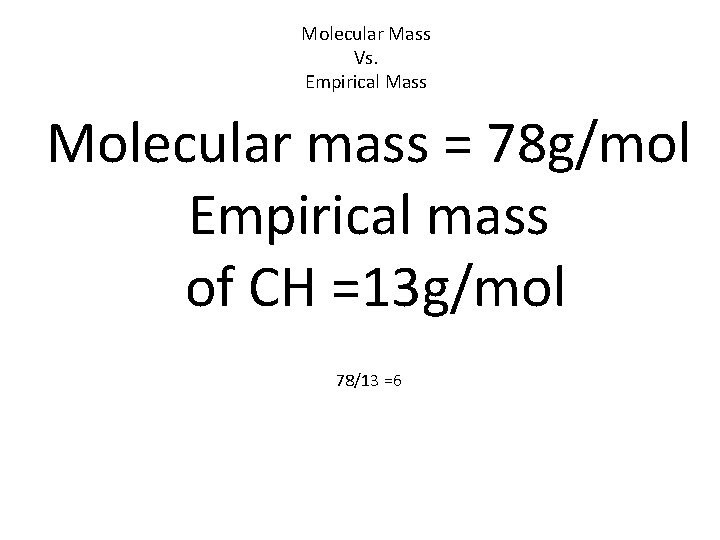
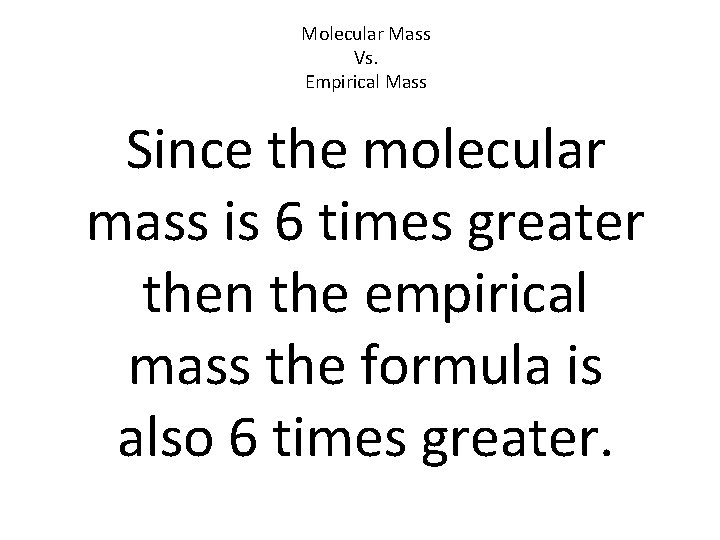
- Slides: 22

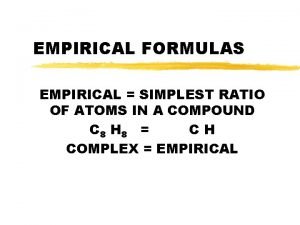
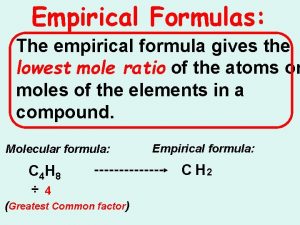
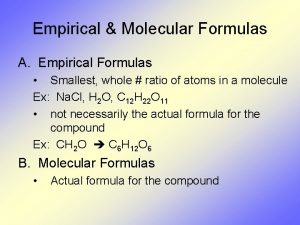
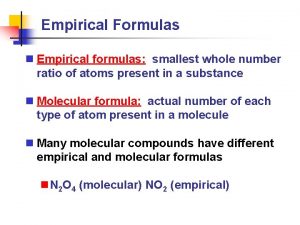
Empirical Formulas

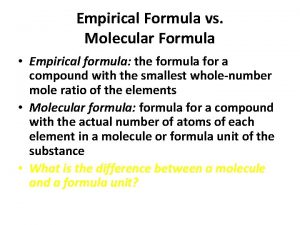
Empirical Formula: Lowest whole # ratio H 2 O 2 (hydrogen peroxide) is it a empirical Formula? No, you can reduce it to HO

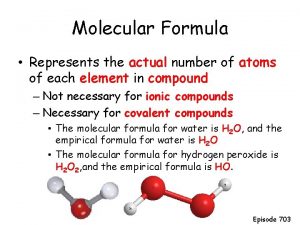
H 2 O 2 is the molecular formula Molecular formula shows the way the molecule is actually found in nature.

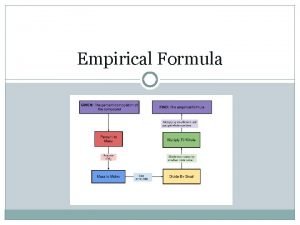
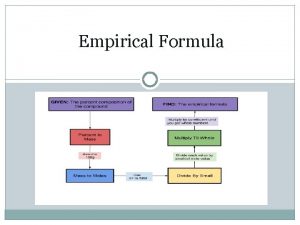
How do we write empirical formulas? ? ? 1. Take the % compositions and convert the % to grams.

11. 1 % H 88. 9% O changes to 11. 1 g H 88. 9 g O

1. Take the % compositions and convert the % to grams. 2. Convert grams to moles
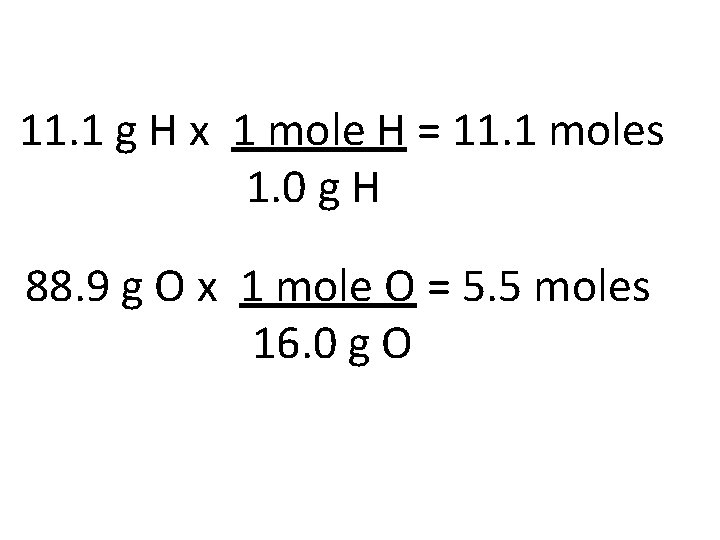
11. 1 g H x 1 mole H = 11. 1 moles 1. 0 g H 88. 9 g O x 1 mole O = 5. 5 moles 16. 0 g O

1. Take the % compositions and convert the % to grams. 2. Convert grams to moles 3. Divide by the smallest # of moles

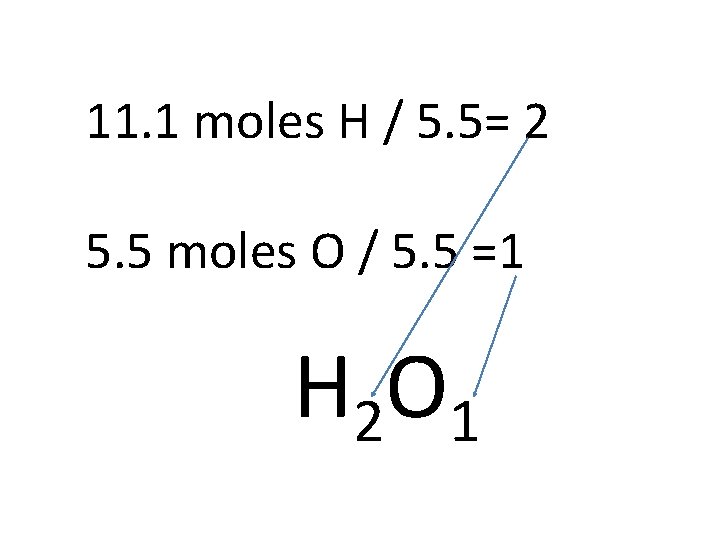
11. 1 moles H / 5. 5= 2 5. 5 moles O / 5. 5 =1

1. Take the % compositions and convert the % to 4. Plug the whole #’s into grams. the empirical formula 2. Convert grams to moles 3. Divide by the smallest # of moles

11. 1 moles H / 5. 5= 2 5. 5 moles O / 5. 5 =1 H 2 O 1

1. 2. 3. 4. Take the % compositions and convert the % to grams. Convert grams to moles Divide by the smallest # of moles Plug the whole #’s into the empirical formula 5. If you do not have whole #’s after dividing you must multiply through to make them all whole #’s
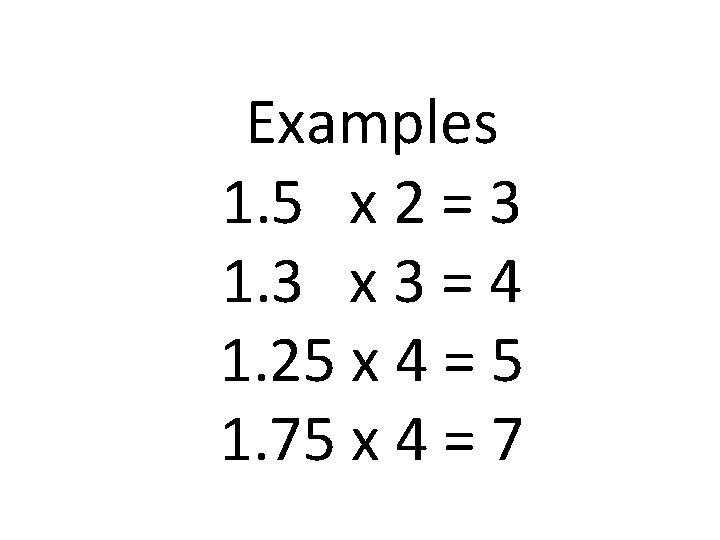
Examples 1. 5 x 2 = 3 1. 3 x 3 = 4 1. 25 x 4 = 5 1. 75 x 4 = 7
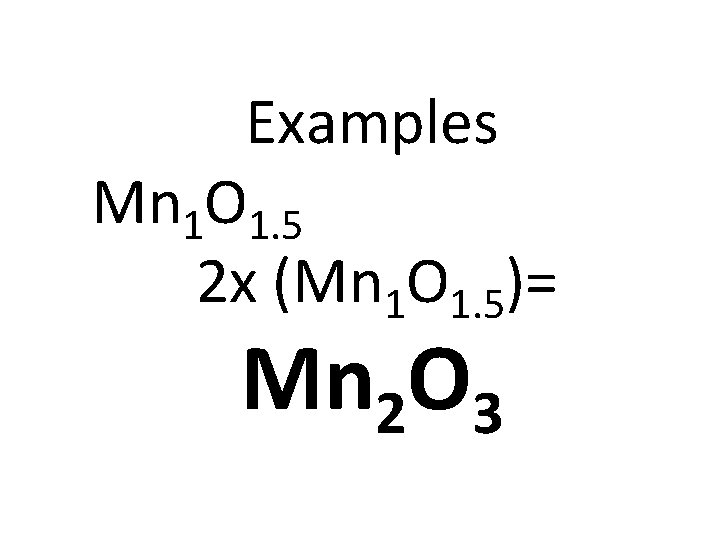
Examples Mn 1 O 1. 5 2 x (Mn 1 O 1. 5)= Mn 2 O 3
Molecular Mass Vs. Empirical Mass

Molecular Mass Is found experimentally

Empirical Mass Is found using the molar mass from the periodic table.

Molecular Mass Vs. Empirical Mass If they are the same GREAT the Molecular Formula and Empirical Formula are the same

Molecular Mass Vs. Empirical Mass If they are not the same you must divide the Molecular mass by the Empirical mass to see how many time greater it is.
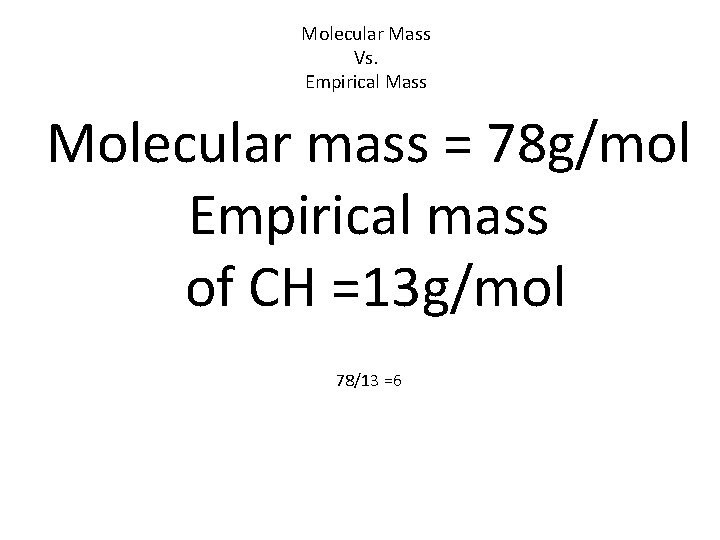
Molecular Mass Vs. Empirical Mass Molecular mass = 78 g/mol Empirical mass of CH =13 g/mol 78/13 =6
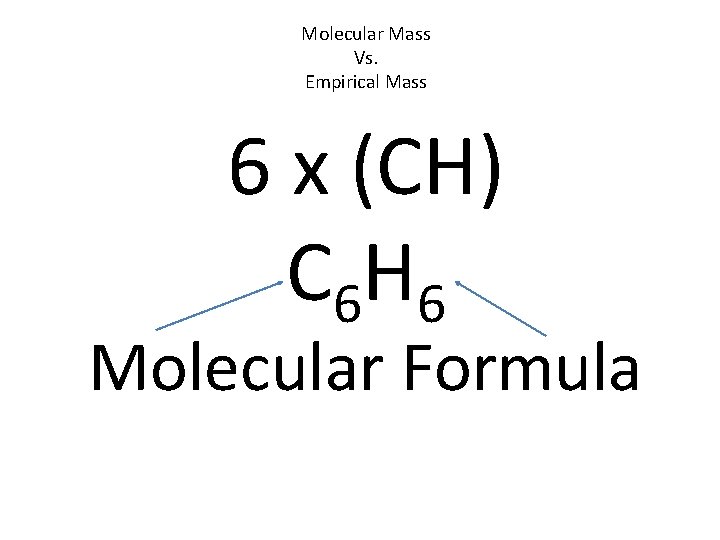
Molecular Mass Vs. Empirical Mass Since the molecular mass is 6 times greater then the empirical mass the formula is also 6 times greater.

Molecular Mass Vs. Empirical Mass 6 x (CH) C 6 H 6 Molecular Formula
 Lowest whole number
Lowest whole number Whole school whole community whole child model
Whole school whole community whole child model Simplest ratio empirical formula
Simplest ratio empirical formula Simplest ratio empirical formula
Simplest ratio empirical formula Whole part whole practice
Whole part whole practice Empirical and molecular formula worksheet doc
Empirical and molecular formula worksheet doc Ibuprofen percent composition
Ibuprofen percent composition Cho empirical formula
Cho empirical formula Empirical formula
Empirical formula How to do empirical formula
How to do empirical formula Empirical formula of haemoglobin
Empirical formula of haemoglobin Percentage composition
Percentage composition Define molecular formula
Define molecular formula Molecular formula and empirical formula
Molecular formula and empirical formula Molecular formula = n × empirical formula
Molecular formula = n × empirical formula Empirical formula and molecular formula pogil
Empirical formula and molecular formula pogil Empirical formula rhyme
Empirical formula rhyme Empirical formula of c5h12
Empirical formula of c5h12 Empirical formula
Empirical formula Glyceraldehyde empirical formula
Glyceraldehyde empirical formula In a one ratio compares a part to a whole
In a one ratio compares a part to a whole Dupont formulas
Dupont formulas Ratio analysis formulas
Ratio analysis formulas