Empirical Formula The simplest ratio of atoms For



- Slides: 9

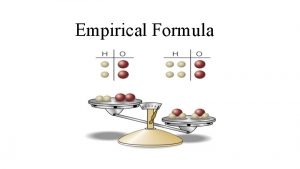
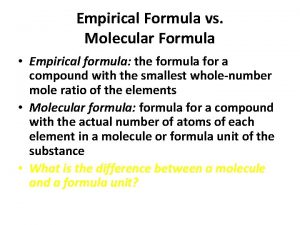
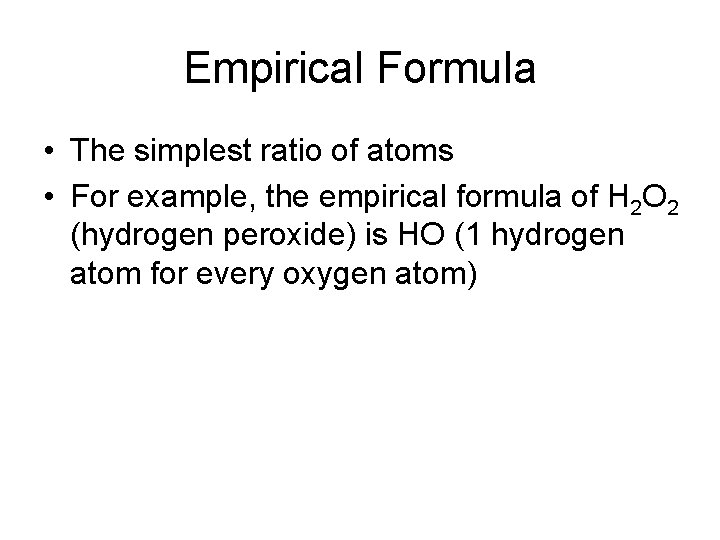
Empirical Formula • The simplest ratio of atoms • For example, the empirical formula of H 2 O 2 (hydrogen peroxide) is HO (1 hydrogen atom for every oxygen atom)

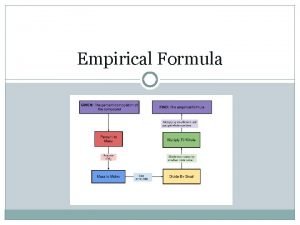
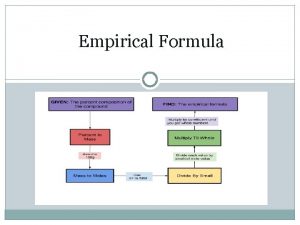
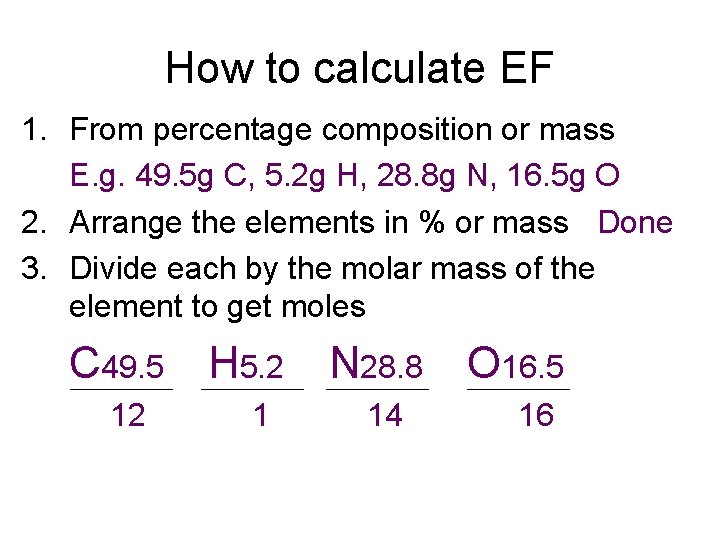
How to calculate EF 1. From percentage composition or mass E. g. 49. 5 g C, 5. 2 g H, 28. 8 g N, 16. 5 g O 2. Arrange the elements in % or mass Done 3. Divide each by the molar mass of the element to get moles C 49. 5 12 H 5. 2 1 N 28. 8 14 O 16. 5 16

C 4. 121 H 5. 159 N 2. 056 O 1. 031 4. Divide everything by the SMALLEST NUMBER 4. 121 5. 159 2. 056 1. 031 = 4 : 5 : 2 : 1 5. Write the formula with the simplest whole number ratio C 4 H 5 N 2 O ANSWER

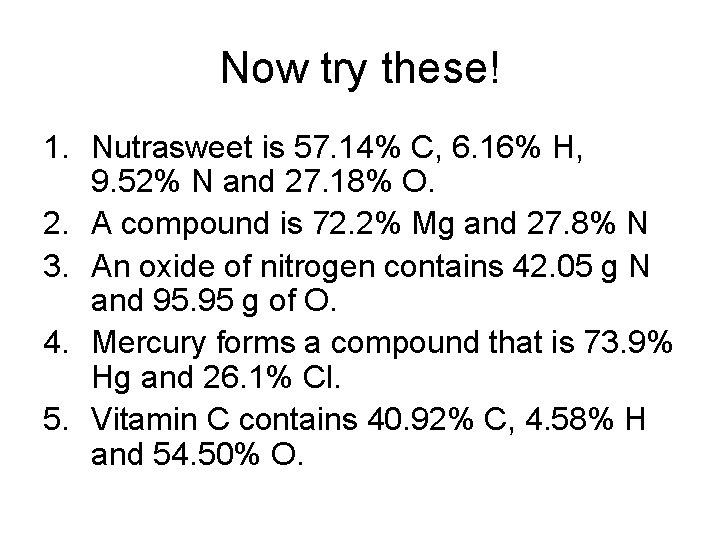
Now try these! 1. Nutrasweet is 57. 14% C, 6. 16% H, 9. 52% N and 27. 18% O. 2. A compound is 72. 2% Mg and 27. 8% N 3. An oxide of nitrogen contains 42. 05 g N and 95. 95 g of O. 4. Mercury forms a compound that is 73. 9% Hg and 26. 1% Cl. 5. Vitamin C contains 40. 92% C, 4. 58% H and 54. 50% O.

Now try these - ANSWERS 1. 2. 3. 4. 5. C 14 H 18 N 2 O 5 Mg 3 N 2 NO 2 Hg. Cl 2 C 3 H 4 O 3

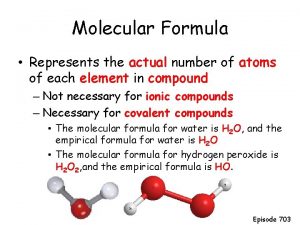
From EF to molecular formula • The molecular formula is the ACTUAL NUMBER OF EACH ATOM in the compound HO Multiplication factor x 2 H 2 O 2 (hydrogen peroxide) CH 2 O X 6 x 2 C 6 H 12 O 6 (glucose) CH 3 COOH (ethanoic acid) EF MF

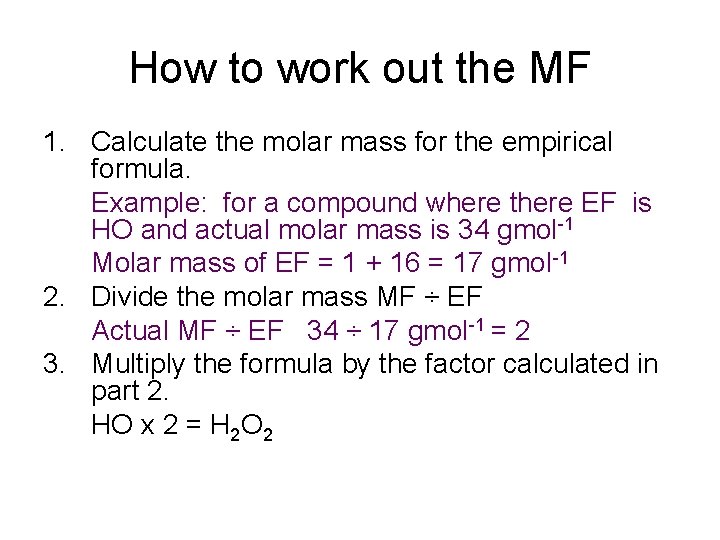
How to work out the MF 1. Calculate the molar mass for the empirical formula. Example: for a compound where there EF is HO and actual molar mass is 34 gmol-1 Molar mass of EF = 1 + 16 = 17 gmol-1 2. Divide the molar mass MF ÷ EF Actual MF ÷ EF 34 ÷ 17 gmol-1 = 2 3. Multiply the formula by the factor calculated in part 2. HO x 2 = H 2 O 2

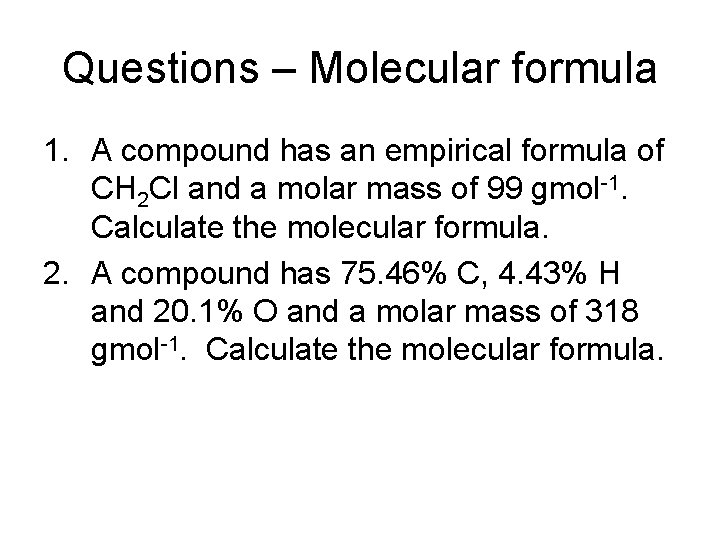
Questions – Molecular formula 1. A compound has an empirical formula of CH 2 Cl and a molar mass of 99 gmol-1. Calculate the molecular formula. 2. A compound has 75. 46% C, 4. 43% H and 20. 1% O and a molar mass of 318 gmol-1. Calculate the molecular formula.

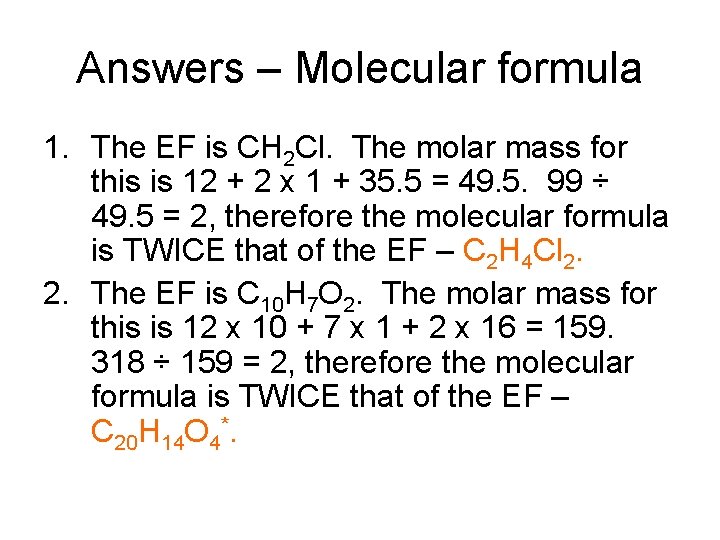
Answers – Molecular formula 1. The EF is CH 2 Cl. The molar mass for this is 12 + 2 x 1 + 35. 5 = 49. 5. 99 ÷ 49. 5 = 2, therefore the molecular formula is TWICE that of the EF – C 2 H 4 Cl 2. 2. The EF is C 10 H 7 O 2. The molar mass for this is 12 x 10 + 7 x 1 + 2 x 16 = 159. 318 ÷ 159 = 2, therefore the molecular formula is TWICE that of the EF – C 20 H 14 O 4*.