Drug drug interactions highlighting P 450 Cytochromes Louis





































- Slides: 50

Drug drug interactions highlighting P 450 Cytochromes Louis Wu, PA-C Presented at VAPAA Annual Meeting March 2, 2017

Financial Disclosures and Conflicts I have no financial disclosures nor conflicts concerning this talk.

Learning Objectives n n Develop a basic understanding of pharmacodynamics and pharmcokinetics as it applies to drug interactions Conceptualize the cytochrome P 450 enzyme system; enzyme inhibition and enzyme induction Review potential hazards of drug interactions in older populations Be able to access drug interaction resources on the VA CPRS system as well as online such as FDA website and publications such as Medical Letter

Why doesn’t your patient respond to the medication? n n n n Adherence Age of the patient Disease State Drug-drug and food-drug interactions Gender Genetics Others Langman and Dasgupta, in Pharmacogenomics in Clinical Therapeutics, 2012, pp 1 -4

What is the problem with drug-drug interactions? n n Precipitant drug Object (or substrate) drug Drug interaction between the two that increases or decreases the concentration of the object drug If significant enough, then the patient develops a drug toxicity or an inadequate therapeutic response from the object drug Hansten, PD. in Levy et al, Metabolic Drug Interactions, 2000, pg 715

Mechanism of drug interaction 1. Pharmacodynamic – one drug affects the ability of another drug to associate with its therapeutic target or receptor Examples: Methotrexate and Trimethoprim/sulfamethoxazole Epinephrine and non selective beta-blockers Grattagliano, J Fam Prac, 2010 (59) 6: 322 -329

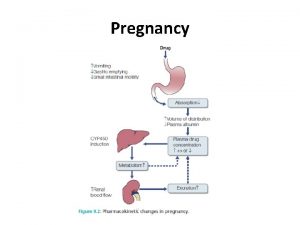
Mechanism of drug interaction 2. Pharmacokinetic: from drug absorption to elimination D - distribution A - absorption M – metabolism (P 450 Cytochrome system) E - elimination Grattagliano, J Fam Prac, 2010 (59) 6: 322 -329

Really fundamental concepts in drug biotransformation n n Lipid soluble drugs are poorly excreted in the urine. They tend to store in fat and/or circulate until they are converted (phase I biotransformation) to more water soluble metabolites or metabolites that conjugate (phase II biotransformation) with water soluble substances. Water soluble drugs are more readily excreted in the urine. They may be metabolized, but generally not by the CYP enzyme systems. https: //courses. washington. edu/chat 543/lectures/Power. Point. Slides/Biotransformation_07 ho. ppt

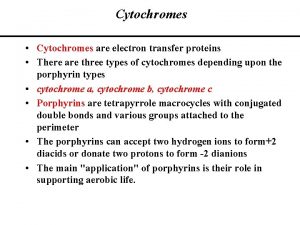
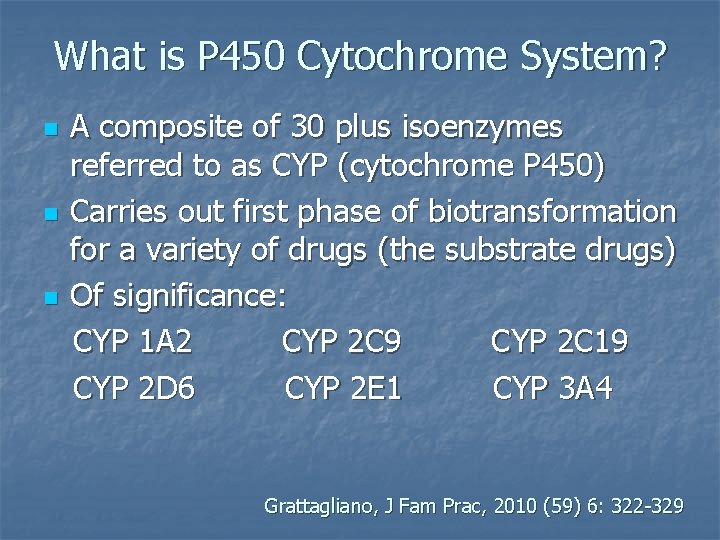
What is P 450 Cytochrome System? n n n A composite of 30 plus isoenzymes referred to as CYP (cytochrome P 450) Carries out first phase of biotransformation for a variety of drugs (the substrate drugs) Of significance: CYP 1 A 2 CYP 2 C 9 CYP 2 C 19 CYP 2 D 6 CYP 2 E 1 CYP 3 A 4 Grattagliano, J Fam Prac, 2010 (59) 6: 322 -329

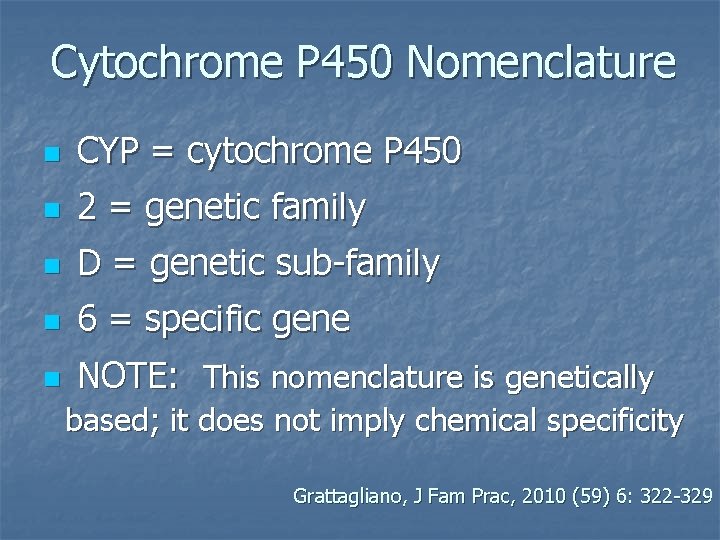
Cytochrome P 450 Nomenclature n CYP = cytochrome P 450 n 2 = genetic family n D = genetic sub-family n 6 = specific gene n NOTE: This nomenclature is genetically based; it does not imply chemical specificity Grattagliano, J Fam Prac, 2010 (59) 6: 322 -329

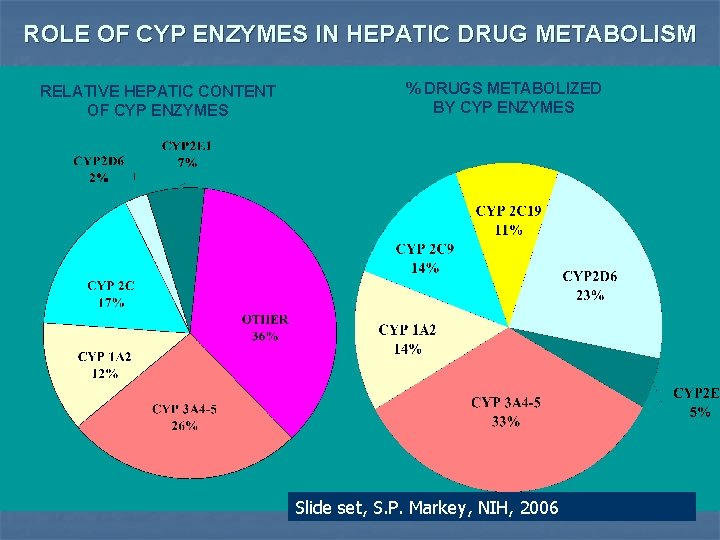
ROLE OF CYP ENZYMES IN HEPATIC DRUG METABOLISM RELATIVE HEPATIC CONTENT OF CYP ENZYMES % DRUGS METABOLIZED BY CYP ENZYMES Slide set, S. P. Markey, NIH, 2006

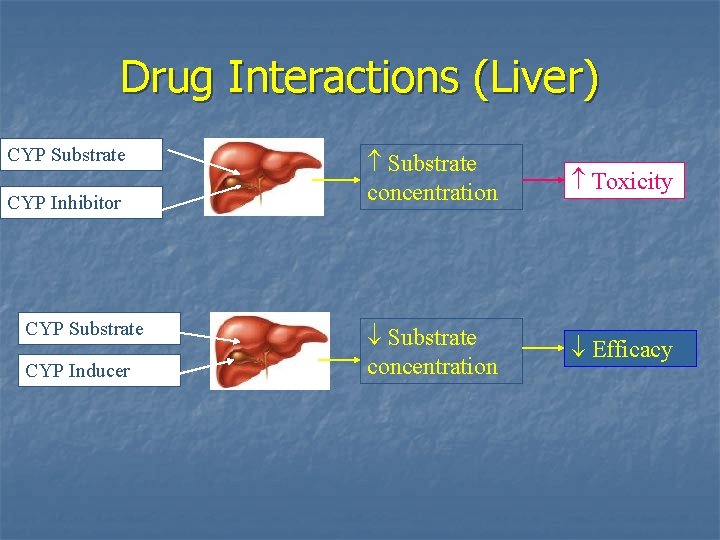
Drug Interactions (Liver) CYP Substrate CYP Inhibitor CYP Substrate CYP Inducer Substrate concentration Toxicity Substrate concentration Efficacy

Inhibitors of P-450 Results in decreased metabolism and hepatic clearance of the substrate drug n Example: Prolonged warfarin effect Warfarin as the substrate combined with Amiodarone as the inhibitor Lynch and Price, Am Fam Physician. 2007 Aug 1; 76(3): 391 -396.

Inducers of P-450 Results in increased metabolism and increased hepatic clearance of the substrate n Example: Decreased Oral Contraceptive effect ethinyl estardiol as the substrate combined with Tegretol as the inducer Lynch and Price, Am Fam Physician. 2007 Aug 1; 76(3): 391 -396.

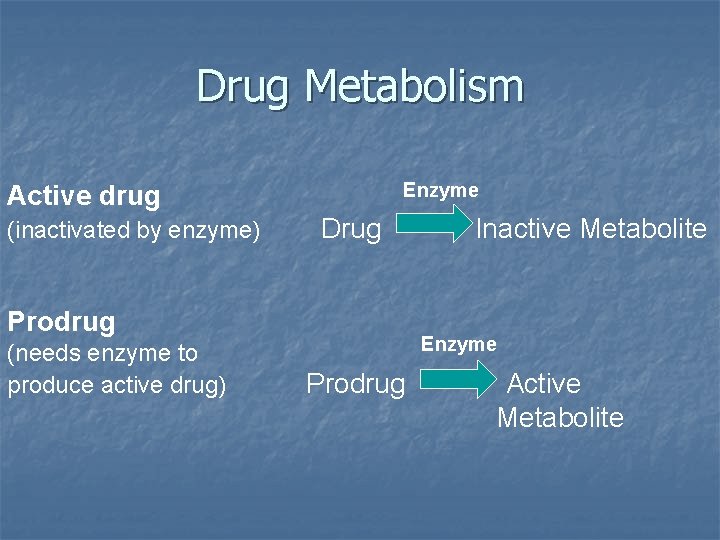
Drug Metabolism Enzyme Active drug (inactivated by enzyme) Drug Prodrug (needs enzyme to produce active drug) Inactive Metabolite Enzyme Prodrug Active Metabolite

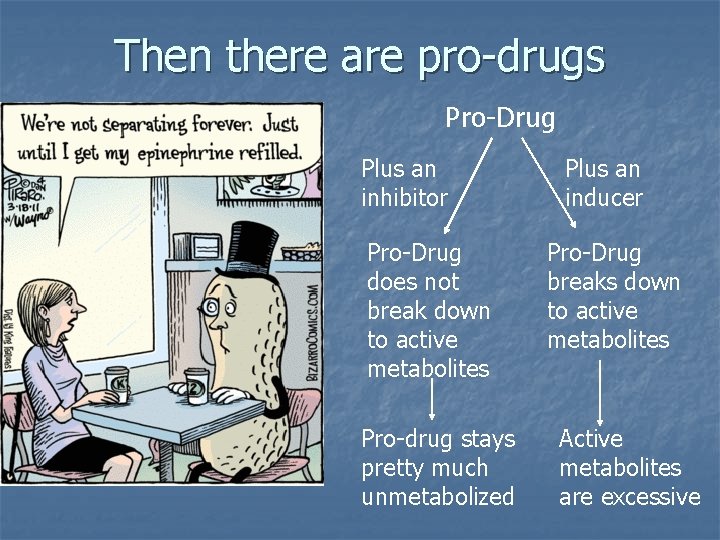
Then there are pro-drugs Pro-Drug Plus an inhibitor Pro-Drug does not break down to active metabolites Pro-drug stays pretty much unmetabolized Plus an inducer Pro-Drug breaks down to active metabolites Active metabolites are excessive

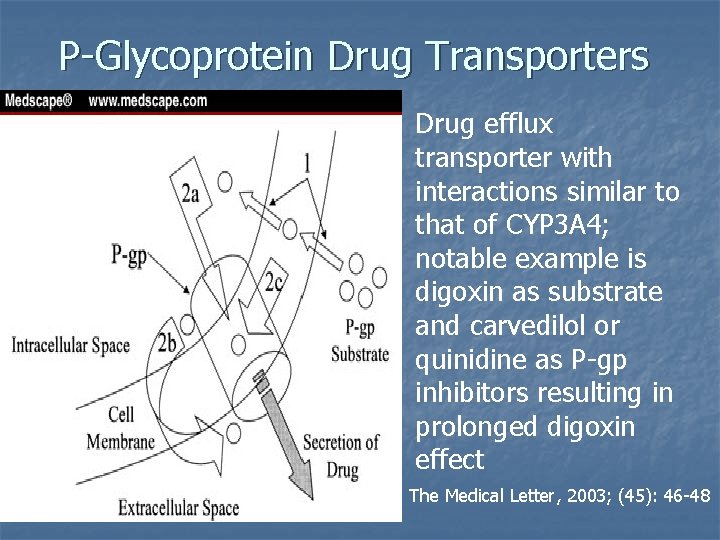
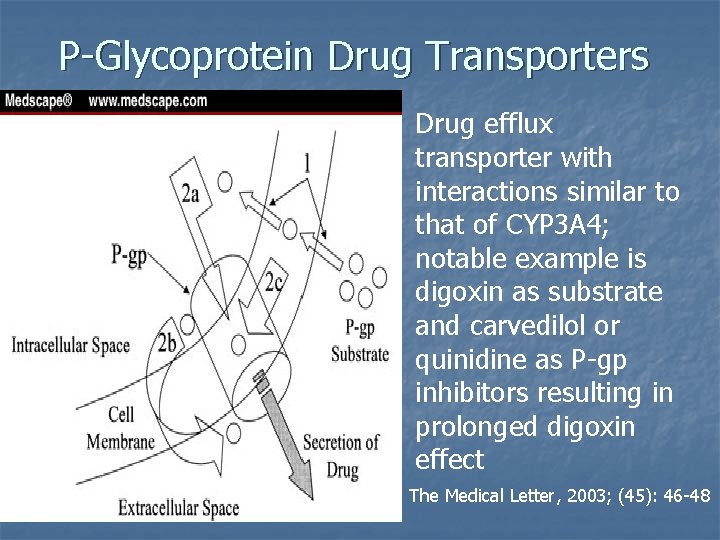
P-Glycoprotein Drug Transporters Drug efflux transporter with interactions similar to that of CYP 3 A 4; notable example is digoxin as substrate and carvedilol or quinidine as P-gp inhibitors resulting in prolonged digoxin effect The Medical Letter, 2003; (45): 46 -48

Case 1. Fluconazole and Warfarin EUROPEAN JOURNALOF EMERGENCYMEDICINE, 2002, 9, 175 -177 n n A 75 -year-old man on chronic anticoagulation with warfarin for atrial fibrillation and transient ischaemic attacks Found to have Candidasis on EGD specimen and Fluconazole given for 4 weeks. Five weeks later presented to the emergency department with complaints of back pain of three days’ duration radiating to both legs and leg weakness. His INR was found to be 40 MRI of the thoracic spine demonstrated a posterior epidural haematoma extending from T 3 to T 10 and an emergent laminectomy was performed.

CYP 2 C 9 and Warfarin If possible, fluconazole should not be used for patients anticoagulated with warfarin. Otherwise close monitoring is required.

dabigatran (Pradaxa) and rivaroxaban (Xarelto) n. Known as a direct thrombin inhibitor (dabigatran) or direct Factor Xa inhibitor (rivaroxaban) n. Both are approved to reduce the risk of stroke and blood clots (systemic embolism) in patients with non-valvular atrial fibrillation. n. XARELTO® is also indicated for the prophylaxis of deep vein thrombosis (DVT), which may lead to pulmonary embolism (PE) in patients undergoing knee or hip replacement surgery. n. Drug interactions with p-glycoproteins, CYP 34 A Prescriber's Letter 2011; 18(12): 271220

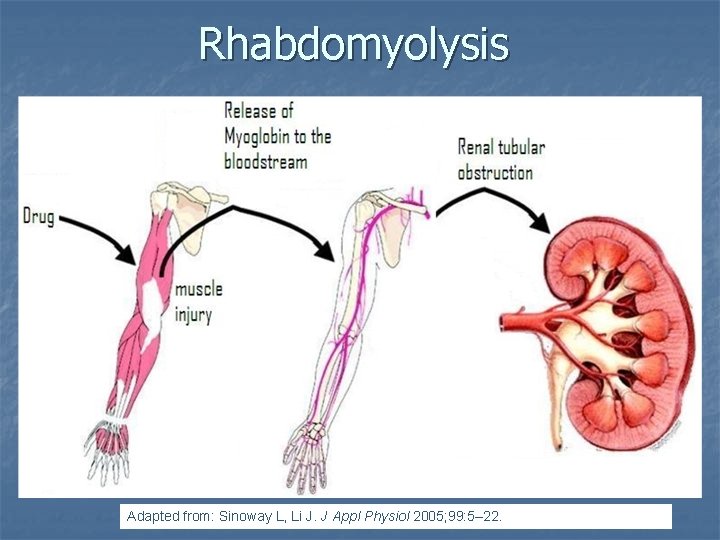
Case 2. Simvastatin and Amiodarone Ann Pharmacother 2006; 40: 753 -7. n n A 72 -year-old white man presented with thigh weakness, achiness, and dark urine for 7 days. Coronary artery bypass had been performed earlier and Amiodarone 200 mg/day was started and later simvastatin 80 mg/day was added Labs included creatine kinase (CK) 19 620 U/L (reference range 60– 224), blood urea nitrogen 50 mg/d. L, creatinine 2. 6 mg/d. L, (AST) 912 U/L (30– 60), (ALT) 748 U/L (30– 60), urine myoglobin 71100 μg/L (<50), and serum myoglobin 13 877 μg/L (<110).

Rhabdomyolysis Adapted from: Sinoway L, Li J. J Appl Physiol 2005; 99: 5– 22.

CYP 3 A 4 and Statins Simvastatin is metabolized primarily by CYP 3 A 4, and amiodarone is a recognized inhibitor of this enzyme

Facts about statins n n n A class of prescription drugs used together with diet and exercise to reduce blood levels of lowdensity lipoprotein (LDL) cholesterol Marketed as single-ingredient products, including Lipitor (atorvastatin), Lescol (fluvastatin), Mevacor (lovastatin), Altoprev (lovastatin extended-release), Livalo (pitavastatin), Pravachol (pravastatin), Crestor (rosuvastatin), and Zocor (simvastatin) Also marketed as combination products, including Advicor (lovastatin/niacin extended-release), Simcor (simvastatin/niacin extended-release), and Vytorin (simvastatin/ezetimibe) http: //www. fda. gov/Drugs/Drug. Safety/ucm 293101. htm

A word from the FDA Do not use simvastatin with these medications: n Itraconazole n Ketoconazole n Gemfibrozil n Cyclosporine n Danazol n Posaconazole n Erythromycin n Clarithromycin n Telithromycin n HIV protease inhibitors n Nefazodone http: //www. fda. gov/Drugs/Drug. Safety/ucm 256581. htm

A word from the FDA n n n Do not use more than 10 mg of simvastatin with these medications: Verapamil , Diltiazem (Note: These drugs are contraindicated with Simcor as Simcor is only available with 20 mg or 40 mg of simvastatin. ) Do not use more than 20 mg of simvastatin with these medications: Amlodipine, Amiodarone , Ranolazine Avoid large quantities of grapefruit juice (>1 quart daily)

Lovastatin Dose Limitations Contraindicated with lovastatin: n Itraconazole n Ketoconazole n Posaconazole n Erythromycin n Clarithromycin n Telithromycin n HIV protease inhibitors n Boceprevir n Telaprevir n Nefazodone http: //www. fda. gov/Drugs/Drug. Safety/ucm 293101. htm#hcp

Lovastatin Dose Limitations Avoid with lovastatin: n Cyclosporine n Gemfibrozil Do not exceed 20 mg lovastatin daily with: n Danazol n Diltiazem n Verapamil Do not exceed 40 mg lovastatin daily with: n Amiodarone Avoid large quantities of grapefruit juice (>1 quart daily)

Case 3. Tamoxifen and SSRI Am J Psychiatry 165: 10, October 2008 n n “Ms. B” is a 45 -year-old woman who was diagnosed with major depressive disorder treated with fluoxetine One year ago, she was diagnosed with breast cancer and underwent surgery, followed by chemotherapy, and then radiation therapy. Tamoxifen (a selective estrogen receptor modulator) added to prevent breast cancer recurrence. What potential problems do you forsee?

CYP 2 D 6 and Fluoxetine CYP 2 D 6 is necessary for conversion of the prodrug tamoxifen to its primary active metabolite endoxifen. Fluoxetine is a strong CYP 2 D 6 inhibitor, and therefore inhibits the conversion tamoxifen to endoxifen and compromise the efficacy of tamoxifen. Citalopram (Celexa®), sertraline (Zoloft®), and venlafaxine (Effexor®) do not significantly inhibit CYP 2 D 6 http: //www. azcert. org/medical-pros/education/index. cfm

Case 4. Plavix and PPIs Cardiology in Review • Volume 17, Number 4, July/August 2009 n n n A 52 -year-old man found to have 80% stenosis in the right coronary artery and a drug-eluting stent placed. On discharge, patient was on metoprolol 25 mg twice daily, lisinopril 5 mg once daily, pravastatin 40 mg once daily, aspirin 325 mg once daily, clopidogrel 75 mg once daily, and pantoprazole 40 mg once daily (for stress ulcer prophylaxis during coronary care unit stay). Should a PPI be continued for gastrointestinal (GI) ulcer prophylaxis? If so, is there a preferred PPI to use in this case?

CYP 2 C 19 and Plavix Clopidogrel (Plavix®) is a prodrug. Omeprazole (Prilosec®) is thought to inhibit the CYP 2 C 19 metabolism of clopidogrel to its active metabolite. Concomitant use of omeprazole and clopidogrel to be avoided. Unknown to what extent other PPIs may interact with clopidogrel. However, esomeprazole (Nexium®) also inhibits CYP 2 C 19 and should also be avoided. http: //www. azcert. org/medical-pros/education/index. cfm

Do you like controversies? n n FDA Advisory: “Concomitant use of clopidogrel and omeprazole should be avoided … may not get full protective anticlotting effect” The Medical Letter: “Patients at risk for upper GI bleeding who take clopidogrel should also take a PPI, but not Omeprazole… Pantoprazole would be reasonable choice” The Medical Letter, Nov 29, 2010 (52): 93

Do you like controversies? ACCF / ACG / AHA 2010 Expert Consensus: “Pharmacokinetic and pharmacodynamic studies… suggest that concomitant use of clopidogrel and a PPI reduces the antiplatelet effects of clopidogrel … It is not established that changes in these surrogate endpoints translate into clinically meaningful differences” n J. Am. Coll. Cardiology. 2010; (56): 2051 -2066

Case 5. Digoxin and Itraconazole Annals of Int Med, 1992; 116 (6): 525 n n 68 y/o man with chronic atrial fib treated with Digoxin for rate control Found to have elbow swelling with positive fungal culture on aspirate Treated with ketoconazole with no effect and switched to itraconazole Two weeks later, developed nausea and vomiting which resolved after itraconazole was stopped

P-Glycoprotein Drug Transporters Drug efflux transporter with interactions similar to that of CYP 3 A 4; notable example is digoxin as substrate and carvedilol or quinidine as P-gp inhibitors resulting in prolonged digoxin effect The Medical Letter, 2003; (45): 46 -48

Elderly and Polypharmcy By some estimates patients 65 and older average 8 different drugs daily n Frequently used: Nonnarcotic analgesic Calcium channel blocker Diuretics Antidepressants ACE inhibitors n Rathore et al, Fam Med (1998); 30: 733 -739

Why the elderly are more susceptible to adverse drug reactions n n Aging state: renal clearance diminished, hepatic drug metabolizing enzyme activity lessened, and drug sensitivity heightened Commonly seen: prolonged opioid, benzodiazepine and CNS depressant effects and bleeding from anticoagulation; paradoxically less responsive to beta adrenergic blockers and agonists Cooney and Pascuzzi, Clin Geriatr Med, 2009 (25): 221 -233

The Beers Criteria (age over 65) Medications or medication classes that generally should be avoided due to ineffectiveness or unnecessarily high risk when safer alternatives are available n Medications should not be used due to concurrent medical conditions n Fick et al, Arch Int Med 2003; (163): 2716 -24

Elderly and Adverse Drug Effects Four medications or medication classes were implicated alone or in combination in 67. 0% of emergency hospitalizations n Warfarin (33. 3%) n Insulins (13. 9%), n Oral antiplatelet agents (13. 3%), and n Oral hypoglycemic agents (10. 7%) n High-risk medications were implicated in only 1. 2% hospitalizations. n

Common presentation on admission n n Acute hemorrhages Hypoglycemia Neurologic symptoms (loss of consciousness, seizures, changes in mental status). Electrolyte or fluid-volume disturbances or nonspecific weakness NEJM, 2011; 365: 2002 -2012

3 drug interactions in elderly leading to hospitalizations n n n Population-based case control study of Canadian residents over age 66 Hospitalization with glyburide, digoxin, or ACE-I when combined with other drugs vs similar matched controls Glyburide + co-trimoxazole Digoxin + clarithyromycin ACE-I + potassium sparing diuretic Juurlink et al. , JAMA (2003), 289: 1652 -1657

Minimizing Drug Interaction Risks n n n L – List each drug’s name and dosage I – Indication and clearly defined therapeutic goal; discontinue if not S – Simple regimen as possible with once or twice a day dosing T – Treat multiple symptoms with single drug rather than multiple drugs E – Educate patients about adverse events and address all non prescriptions and supplements N – Narrow therapeutic window drugs, avoid Werder, J Fam Prac 2005 (4) 5

To Reduce Risk of Adverse Drug Interactions n n n Use of alternative noninteracting medications Adjust dose of object drug: when possible based on changes in response rather than prophylactically ( to avoid sub or supra dosing) Adjust dosing times (object drug two hours before or six hours after the binding drug) Monitor for altered response (lab monitoring) Computerized system to avoid adverse drug interactions (still patients may have multiple providers and multiple pharmacies)

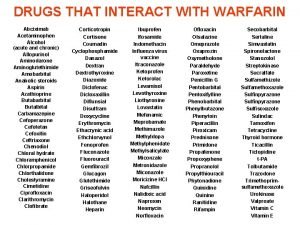
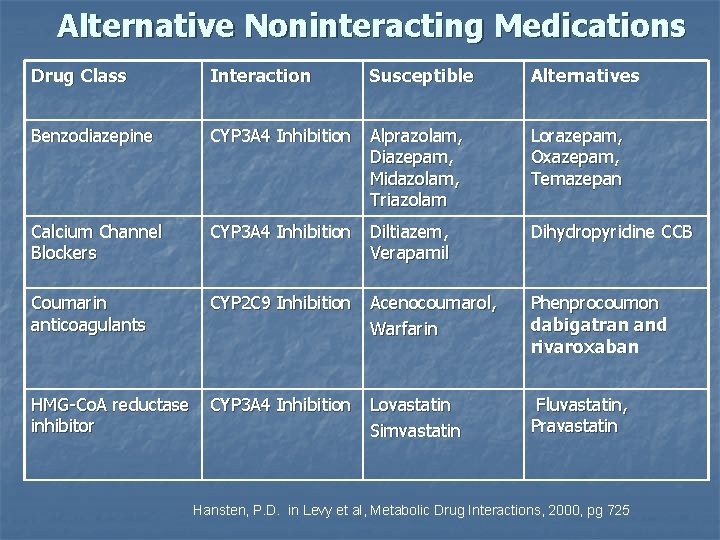
Alternative Noninteracting Medications Drug Class Interaction Susceptible Alternatives Benzodiazepine CYP 3 A 4 Inhibition Alprazolam, Diazepam, Midazolam, Triazolam Lorazepam, Oxazepam, Temazepan Calcium Channel Blockers CYP 3 A 4 Inhibition Diltiazem, Verapamil Dihydropyridine CCB Coumarin anticoagulants CYP 2 C 9 Inhibition Acenocoumarol, Warfarin Phenprocoumon dabigatran and rivaroxaban HMG-Co. A reductase inhibitor CYP 3 A 4 Inhibition Lovastatin Simvastatin Fluvastatin, Pravastatin Hansten, P. D. in Levy et al, Metabolic Drug Interactions, 2000, pg 725

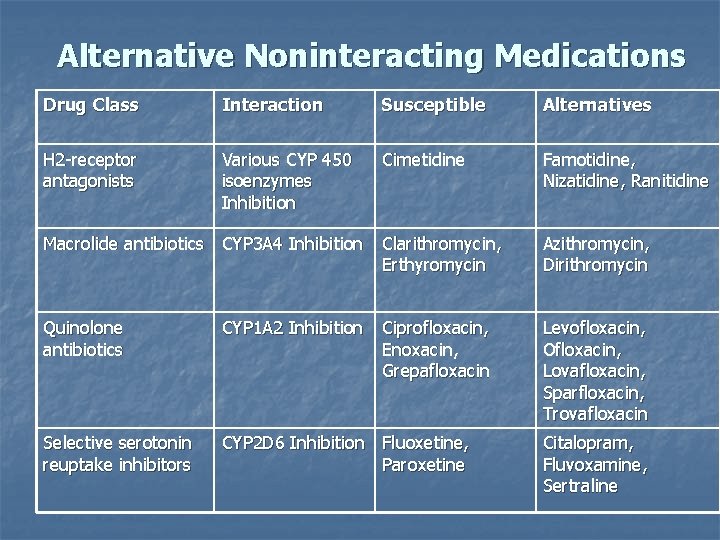
Alternative Noninteracting Medications Drug Class Interaction Susceptible Alternatives H 2 -receptor antagonists Various CYP 450 isoenzymes Inhibition Cimetidine Famotidine, Nizatidine, Ranitidine Macrolide antibiotics CYP 3 A 4 Inhibition Clarithromycin, Erthyromycin Azithromycin, Dirithromycin Quinolone antibiotics CYP 1 A 2 Inhibition Ciprofloxacin, Enoxacin, Grepafloxacin Levofloxacin, Ofloxacin, Lovafloxacin, Sparfloxacin, Trovafloxacin Selective serotonin reuptake inhibitors CYP 2 D 6 Inhibition Fluoxetine, Paroxetine Citalopram, Fluvoxamine, Sertraline

Avoiding Drug Interactions for Consumers http: //www. fda. gov/For. Consumers/Consumer. Updates/ucm 096386. htm

Reporting Adverse Drug Effects to Food and Drug Administration (FDA) http: //www. fda. gov/Safety/Med. Watch/How. To. Report/ default. htm

General References 1. Cozza, K. Drug Interactions. 2003, American Psychiatric Publishing Inc 2. www. hanstenandhorn. com (100 Top Drug Interactions) 3. www. drug-interactions. com (P 450 -mediated interactions) 4. http: //medicine. iupui. edu/clinpharm/ddis/ 5. http: //www. azcert. org/medical-pros/druginteractions. cfm 6. For Simvastatin product information http: //www. accessdata. fda. gov/drugsatfda_docs/label/2012/019766 s 085 lbl. pdf http: //www. medscape. com/viewarticle/750421 7. www. themedicalletter. com (pay site) 8. www. prescriberletter. com (pay site) 9. www. lexi. com (pay site and apps)

In Summary n n n Health care providers must pay attention to patients taking multiple drugs Significant drug interactions are clinically important if they retard or accentuate the normal effects of drugs Consult tables such as the P 450 cytochrome system or your electronic pharmacology software or call your pharmacist if there is a question about interactions Elderly patients require special attention Education of the patient and ongoing education of the clinician to drug interactions is essential to good care
 Gambar trigonometri
Gambar trigonometri Ppi drug interactions
Ppi drug interactions Ppi drug interactions
Ppi drug interactions Vitamin e drug interactions
Vitamin e drug interactions Felodapine
Felodapine Janssen carepath savings program
Janssen carepath savings program Vl louis vuitton
Vl louis vuitton Graphic highlighting in emails
Graphic highlighting in emails What is a preliminary strand test
What is a preliminary strand test Milady chapter 24 exam review
Milady chapter 24 exam review Highlighting strategy
Highlighting strategy Syntax highlighting
Syntax highlighting Types of adulteration of crude drugs
Types of adulteration of crude drugs Light interactions
Light interactions Interactions in the environment grade 7
Interactions in the environment grade 7 Cape buffalo and cattle egrets relationship
Cape buffalo and cattle egrets relationship Protein binding interactions
Protein binding interactions Chapter 14 interactions in ecosystems
Chapter 14 interactions in ecosystems Nutrient interactions
Nutrient interactions Noncovalent interactions
Noncovalent interactions Chapter 22 reaching out cross-cultural interactions
Chapter 22 reaching out cross-cultural interactions Different types of community interactions
Different types of community interactions Nervous interactions with other systems
Nervous interactions with other systems The mimosa plant displays thigmotropism
The mimosa plant displays thigmotropism Sertraline interactions
Sertraline interactions Type of mechanical waves
Type of mechanical waves Dicloxaxillin
Dicloxaxillin Chapter 22 reaching out cross-cultural interactions
Chapter 22 reaching out cross-cultural interactions Regional and transregional interactions
Regional and transregional interactions What is product architecture
What is product architecture Symbiosis and species interactions keystone webquest
Symbiosis and species interactions keystone webquest Interaction planning process
Interaction planning process Abiotic components
Abiotic components Species interaction
Species interaction Intramolecular forces
Intramolecular forces Commensalism in science definition
Commensalism in science definition Foss chemical interactions
Foss chemical interactions Interactions among branches of government
Interactions among branches of government Modular and integral architecture
Modular and integral architecture Regional and transregional interactions
Regional and transregional interactions Habitats niches and species interactions worksheet
Habitats niches and species interactions worksheet Interactions
Interactions Expansionary monetary policy effects
Expansionary monetary policy effects Insight therapies involve verbal interactions
Insight therapies involve verbal interactions Kassandra munoz
Kassandra munoz Chapter 5 evolution and community ecology
Chapter 5 evolution and community ecology Chapter 22 reaching out cross-cultural interactions
Chapter 22 reaching out cross-cultural interactions Functions of the digestive system
Functions of the digestive system Fundamental particles and interactions
Fundamental particles and interactions Wave interactions
Wave interactions Earth sphere interactions
Earth sphere interactions