Weak Interactions NonCovalent Interactions Part of MATERIALS SCIENCE

- Slides: 9

Weak Interactions Non-Covalent Interactions Part of MATERIALS SCIENCE & A Learner’s Guide ENGINEERING AN INTRODUCTORY E-BOOK Anandh Subramaniam & Kantesh Balani Materials Science and Engineering (MSE) Indian Institute of Technology, Kanpur- 208016 Email: anandh@iitk. ac. in, URL: home. iitk. ac. in/~anandh http: //home. iitk. ac. in/~anandh/E-book. htm

Weak Interactions q We will discuss here about intermolecular weak interactions q The strong ‘bonds’ are: Covalent, Ionic and Metallic

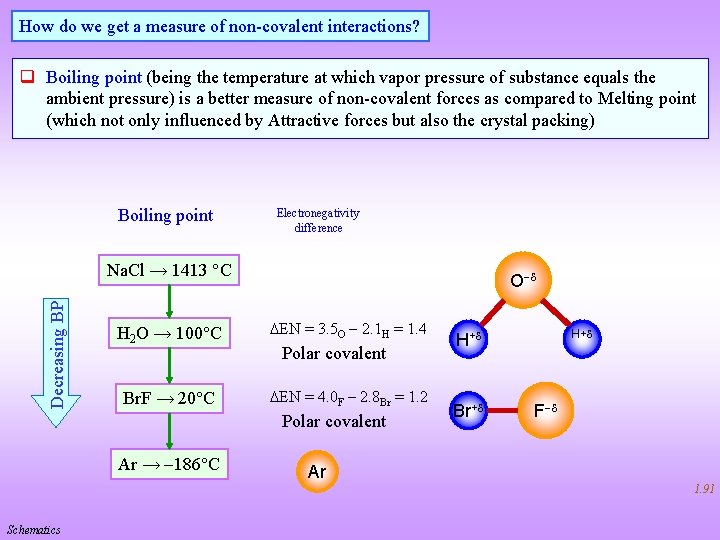
How do we get a measure of non-covalent interactions? q Boiling point (being the temperature at which vapor pressure of substance equals the ambient pressure) is a better measure of non-covalent forces as compared to Melting point (which not only influenced by Attractive forces but also the crystal packing) Boiling point Electronegativity difference Decreasing BP Na. Cl → 1413 C O H 2 O → 100 C DEN = 3. 5 O 2. 1 H = 1. 4 Br. F → 20 C DEN = 4. 0 F 2. 8 Br = 1. 2 Polar covalent Ar → 186 C H+ Br+ F Ar 1. 91 Schematics

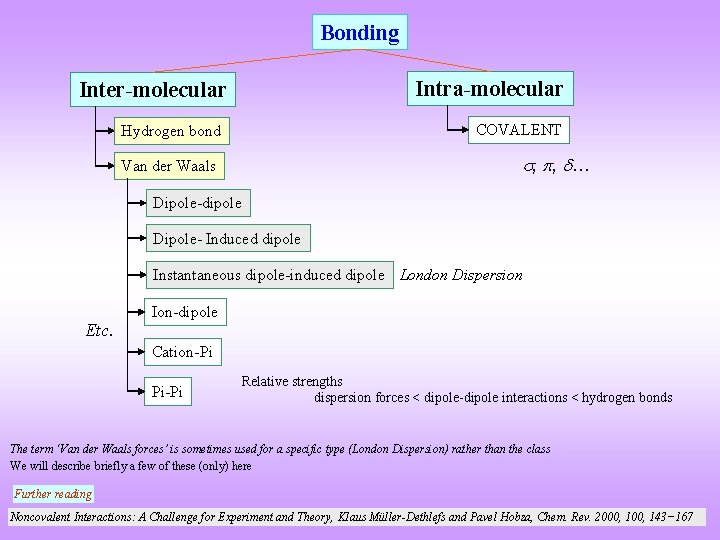
Bonding Intra-molecular Inter-molecular COVALENT Hydrogen bond , , … Van der Waals Dipole-dipole Dipole- Induced dipole Instantaneous dipole-induced dipole London Dispersion Etc. Ion-dipole Cation-Pi Pi-Pi Relative strengths dispersion forces < dipole-dipole interactions < hydrogen bonds The term ‘Van der Waals forces’ is sometimes used for a specific type (London Dispersion) rather than the class We will describe briefly a few of these (only) here Further reading Noncovalent Interactions: A Challenge for Experiment and Theory, Klaus Müller-Dethlefs and Pavel Hobza, Chem. Rev. 2000, 143− 167

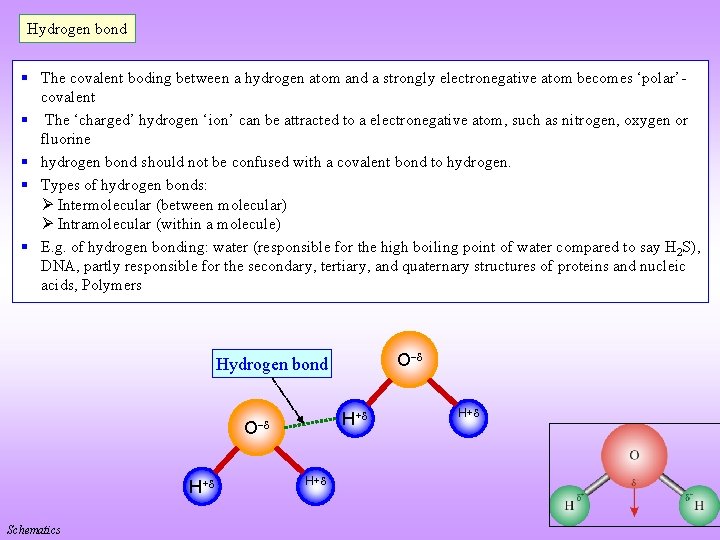
Hydrogen bond § The covalent boding between a hydrogen atom and a strongly electronegative atom becomes ‘polar’covalent § The ‘charged’ hydrogen ‘ion’ can be attracted to a electronegative atom, such as nitrogen, oxygen or fluorine § hydrogen bond should not be confused with a covalent bond to hydrogen. § Types of hydrogen bonds: Intermolecular (between molecular) Intramolecular (within a molecule) § E. g. of hydrogen bonding: water (responsible for the high boiling point of water compared to say H 2 S), DNA, partly responsible for the secondary, tertiary, and quaternary structures of proteins and nucleic acids, Polymers O Hydrogen bond H+ O H+ Schematics H+

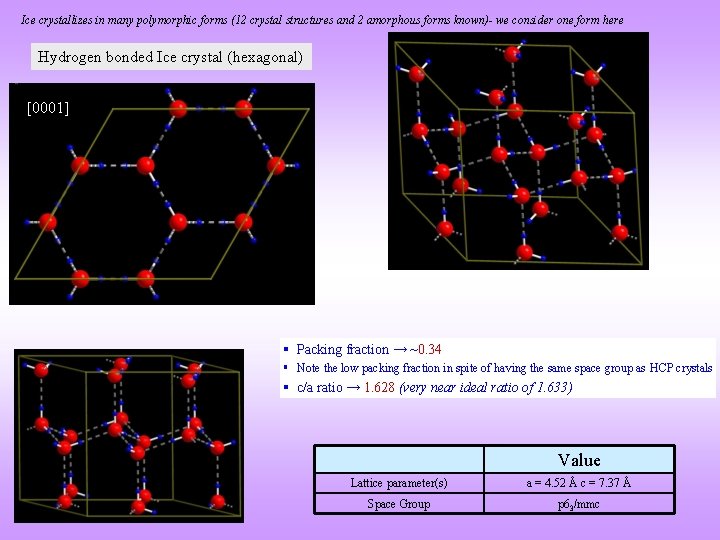
Ice crystallizes in many polymorphic forms (12 crystal structures and 2 amorphous forms known)- we consider one form here Hydrogen bonded Ice crystal (hexagonal) [0001] § Packing fraction → ~0. 34 § Note the low packing fraction in spite of having the same space group as HCP crystals § c/a ratio → 1. 628 (very near ideal ratio of 1. 633) Value Lattice parameter(s) a = 4. 52 Å c = 7. 37 Å Space Group p 63/mmc

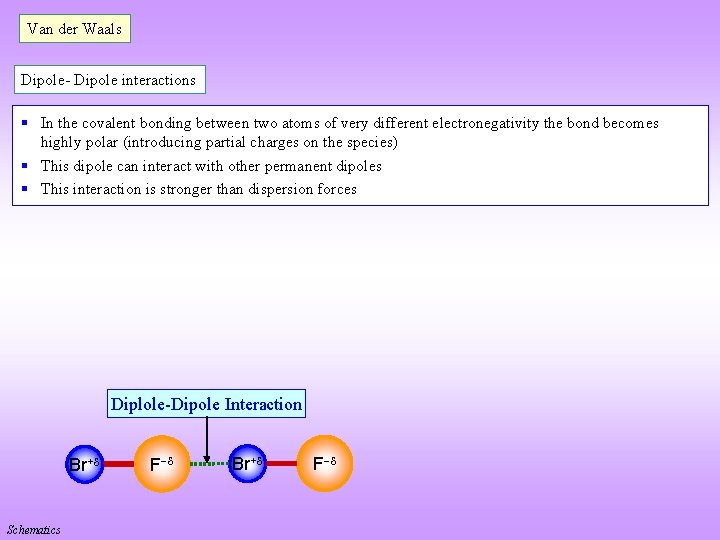
Van der Waals Dipole- Dipole interactions § In the covalent bonding between two atoms of very different electronegativity the bond becomes highly polar (introducing partial charges on the species) § This dipole can interact with other permanent dipoles § This interaction is stronger than dispersion forces Diplole-Dipole Interaction Br+ Schematics F Br+ F

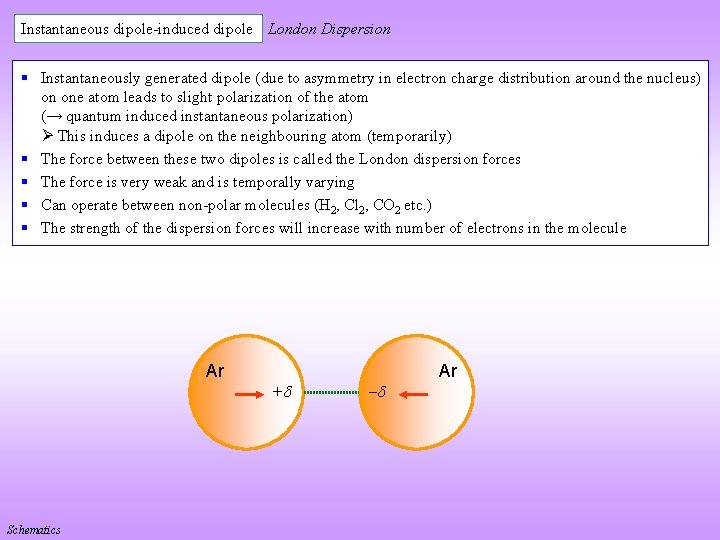
Instantaneous dipole-induced dipole London Dispersion § Instantaneously generated dipole (due to asymmetry in electron charge distribution around the nucleus) on one atom leads to slight polarization of the atom (→ quantum induced instantaneous polarization) This induces a dipole on the neighbouring atom (temporarily) § The force between these two dipoles is called the London dispersion forces § The force is very weak and is temporally varying § Can operate between non-polar molecules (H 2, Cl 2, CO 2 etc. ) § The strength of the dispersion forces will increase with number of electrons in the molecule Ar Schematics + Ar

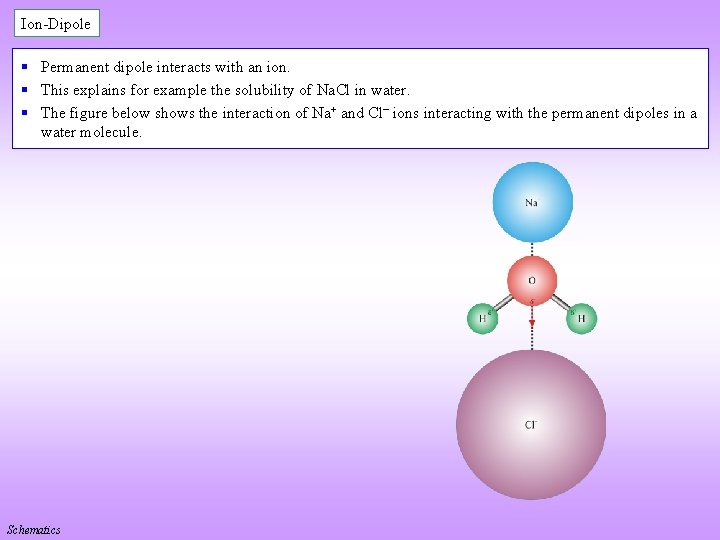
Ion-Dipole § Permanent dipole interacts with an ion. § This explains for example the solubility of Na. Cl in water. § The figure below shows the interaction of Na+ and Cl ions interacting with the permanent dipoles in a water molecule. Schematics