Discovering Novel Adverse Drug Events Using Natural Language

















- Slides: 40

Discovering Novel Adverse Drug Events Using Natural Language Processing and Mining of Electronic Health Records Carol Friedman, Ph. D Department of Biomedical Informatics Columbia University July 21 - AIME 2009

Motivation: Severity of Problem • Clinical trials do not test a broad population • Adverse Drug Events (ADEs) world-wide problem • *Expense from ADEs is $5. 6 billion annually • *Estimated that over 2 million patients hospitalized due to ADEs • *ADEs are fourth leading cause of death *In US alone July 21 - AIME 2009

Motivation: Limitations of Approaches • Manual review of case reports (Venulet J 1988) • Spontaneous reporting to designated agency (Evans JM 2001; Eland IA 1999; Wysowski DK 2005) – – – Serious ADEs reported less than 1 -10% of time Reporting is voluntary for physicians/patients Recognition of ADEs is highly subjective Difficult to determine cause of ADE Biased by length of time on market and other factors – Cannot determine number of patients on drug or percent at risk • Drug prescribing/claims data (Hershman D 2007; Ray WA 2009) July 21 - AIME 2009

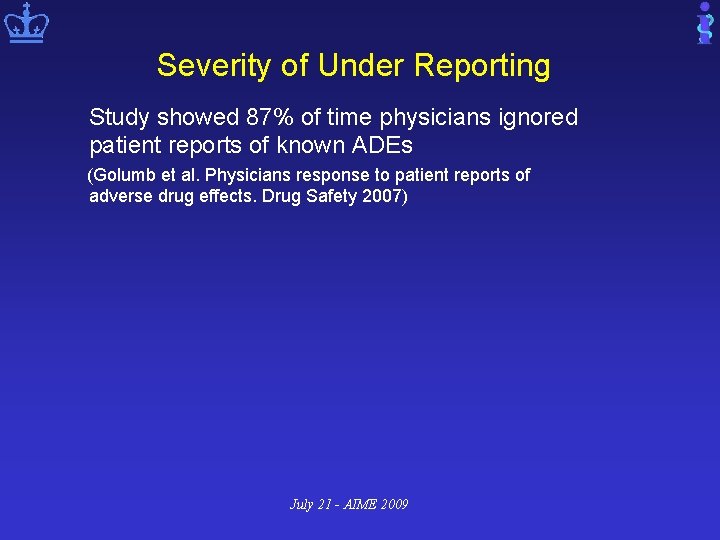
Severity of Under Reporting Study showed 87% of time physicians ignored patient reports of known ADEs (Golumb et al. Physicians response to patient reports of adverse drug effects. Drug Safety 2007) July 21 - AIME 2009

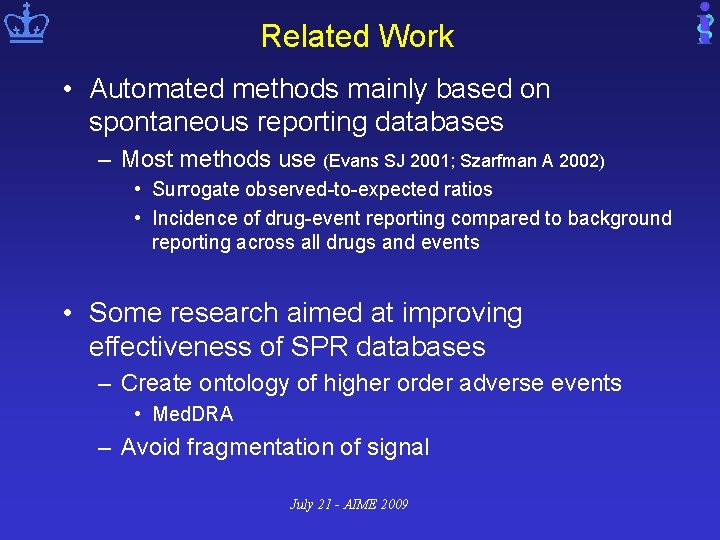
Related Work • Automated methods mainly based on spontaneous reporting databases – Most methods use (Evans SJ 2001; Szarfman A 2002) • Surrogate observed-to-expected ratios • Incidence of drug-event reporting compared to background reporting across all drugs and events • Some research aimed at improving effectiveness of SPR databases – Create ontology of higher order adverse events • Med. DRA – Avoid fragmentation of signal July 21 - AIME 2009

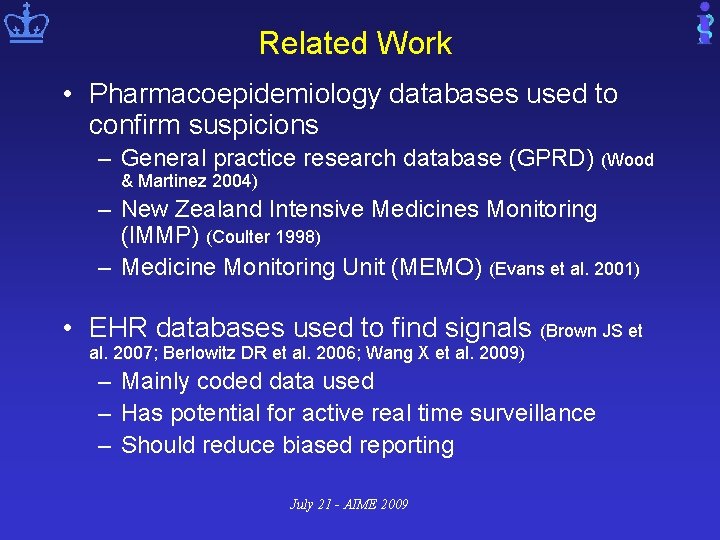
Related Work • Pharmacoepidemiology databases used to confirm suspicions – General practice research database (GPRD) (Wood & Martinez 2004) – New Zealand Intensive Medicines Monitoring (IMMP) (Coulter 1998) – Medicine Monitoring Unit (MEMO) (Evans et al. 2001) • EHR databases used to find signals (Brown JS et al. 2007; Berlowitz DR et al. 2006; Wang X et al. 2009) – Mainly coded data used – Has potential for active real time surveillance – Should reduce biased reporting July 21 - AIME 2009

Related Work • Consortiums involving multiple EHRs – EU-ADR project (http: //www. alert-project. org/) – e. Health initiative (http: //www. ehealthinitiative. org/drug. Safety/) • Related work using EHR to detect known ADEs – not aimed at discovering novel ADEs (Bates DW 2003; Hongman B 2001) July 21 - AIME 2009

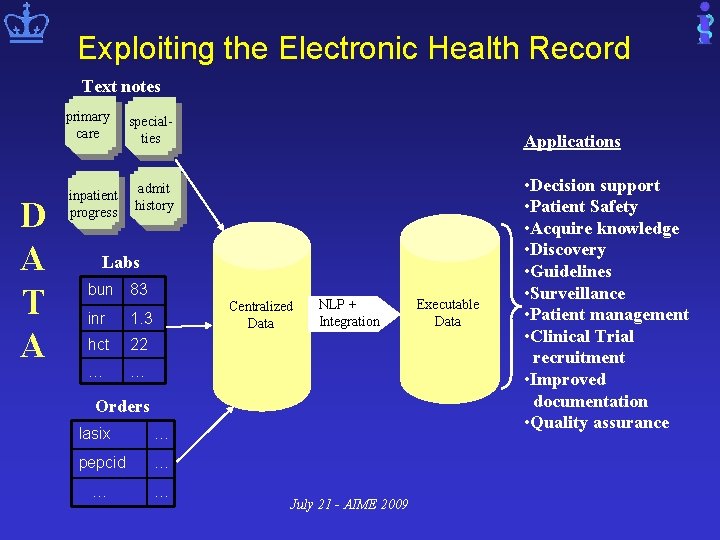
Exploiting the Electronic Health Record Text notes primary care D A T A inpatient progress specialties Applications admit history Labs bun 83 inr 1. 3 hct 22 … … Centralized Data NLP + Integration Orders lasix … pepcid … … … July 21 - AIME 2009 Executable Data • Decision support • Patient Safety • Acquire knowledge • Discovery • Guidelines • Surveillance • Patient management • Clinical Trial recruitment • Improved documentation • Quality assurance

The Electronic Health Record (EHR) • Rich source of patient information • Mostly untapped • Primary use for EHR – Documenting care in multi-provider environment – Manual review by providers • More complete than coded ICD-9 codes – Symptoms – Clinical conditions not beneficial for billing • Fragmented • Heterogeneous • Noisy July 21 - AIME 2009

Research Opportunities: NLP Issues • Occurrence of clinical events in natural language – Drugs, diseases, symptoms – Temporal information is critical • Irregularity of reports – Section headings important but abbreviated/missing – Use of indentation, lists, run on sentences – Tables & semi-structured data in reports • Abbreviations – 2/2 meaning secondary to – co meaning cardiac output or complaining of • Mapping terms in text to an ontology/controlled vocabulary – infiltrate in chest x-ray means chest infiltrate Julylimited 21 - AIMEthan 2009 language – ontology terms more

Research Opportunities: Statistical Issues • Find associations between drug, symptoms, and diseases – Not explicit in EHR • Large volumes of data – Statistical significance vs. clinical significance • Statistical associations – not relationships – Drug treats condition / Drug causes condition • Integrating time sequences is important – For treats: condition must precede drug event – For causes: drug event must precede condition July 21 - AIME 2009

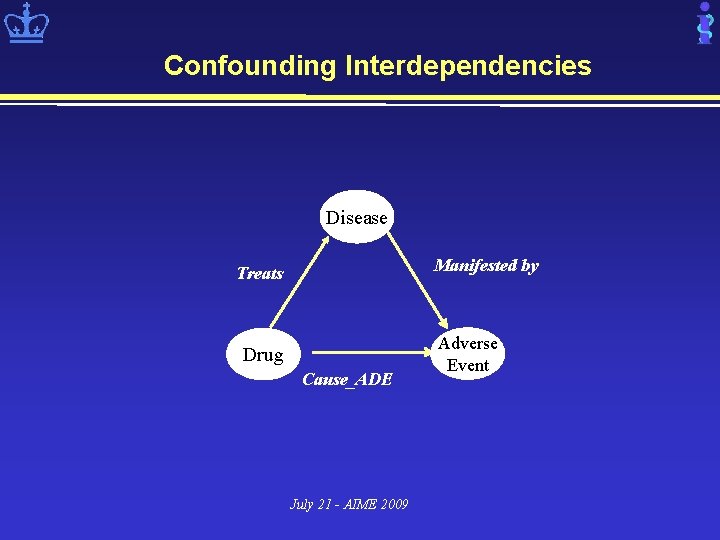
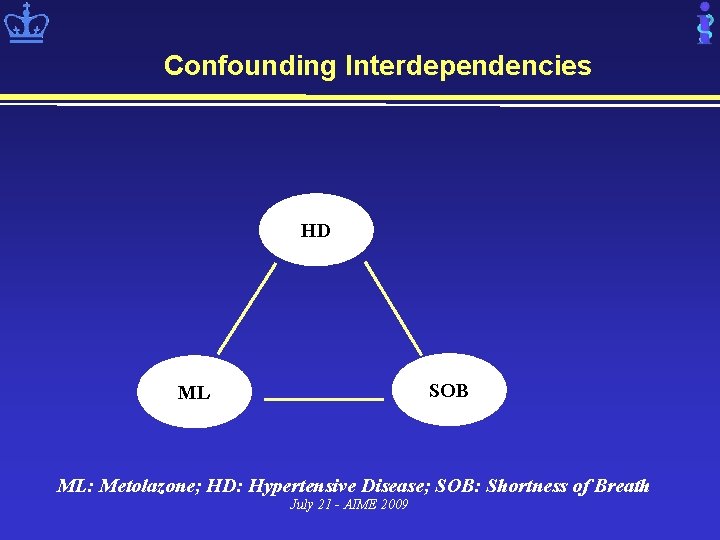
Research Opportunities: Statistical Issues • Confounding (indirect associations) – Metolazone treats heart failure (HF) – HF is manifested by shortness of breath (SOB) – Metolazone and SOB indirectly related • Higher order associations – Drug interactions: Drug 1, drug 2, condition – Drug-contraindications: Drug, disease, condition • Rare ADEs July 21 - AIME 2009

Other Research Opportunities: Knowledge Acquisition • Structured Knowledge bases – UMLS relations (may_be_treated_by) – Proprietary ones – usually unavailable • Text/Semi-Structured Knowledge (need NLP) – Spontaneous reporting databases: indications, drugs, adverse events – Literature (Medline) – Web sites (Web. MD, Micromedix) – Online medical textbooks – Claims Data (Health IT payors) July 21 - AIME 2009

Text Mining for Knowledge Acquisition • Statistical methods: co-occurrences – Discovered associations between diseases and diets from literature (Weeber M 2002) – Identified disease candidate genes ( Hristovski D 2005) • NLP systems – Trends in medications based on the literature and narrative clinical reports (Chen ES 2007, 2008) – Semantic relations in the literature (Hristovski D 2006) July 21 - AIME 2009

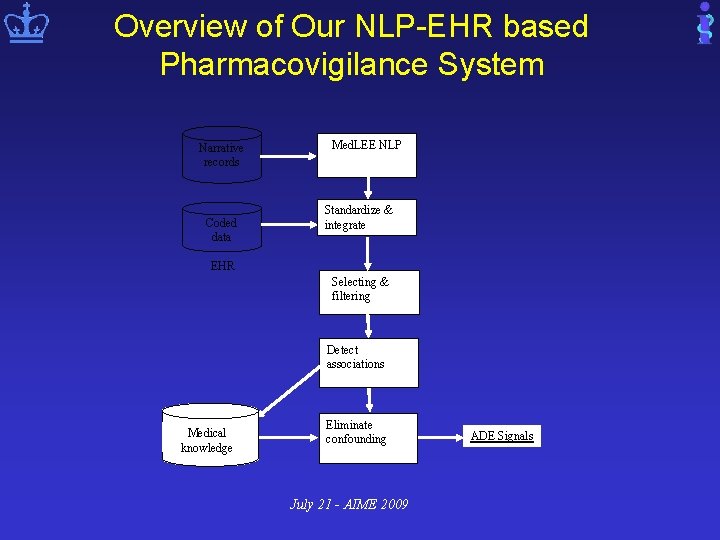
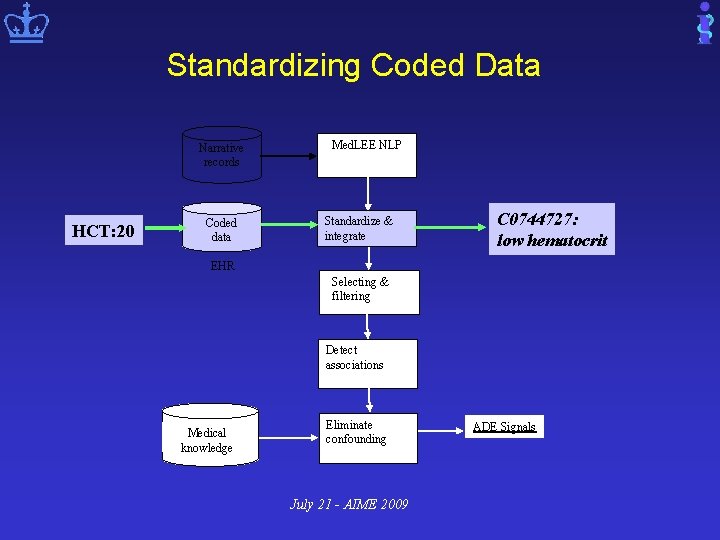
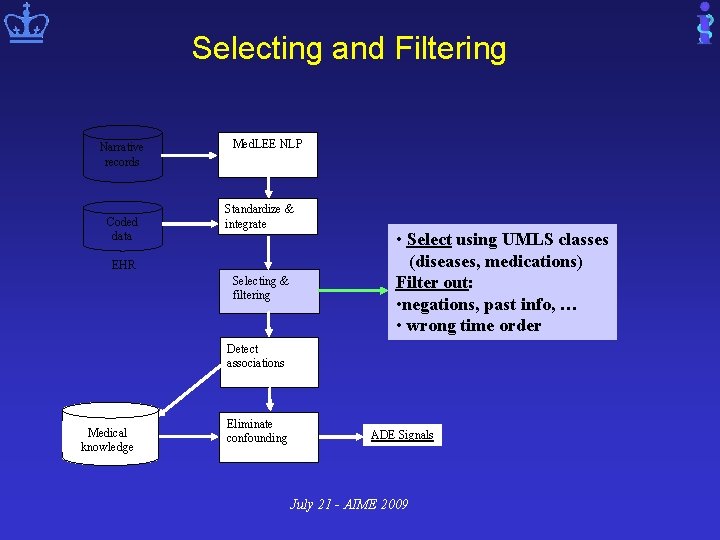
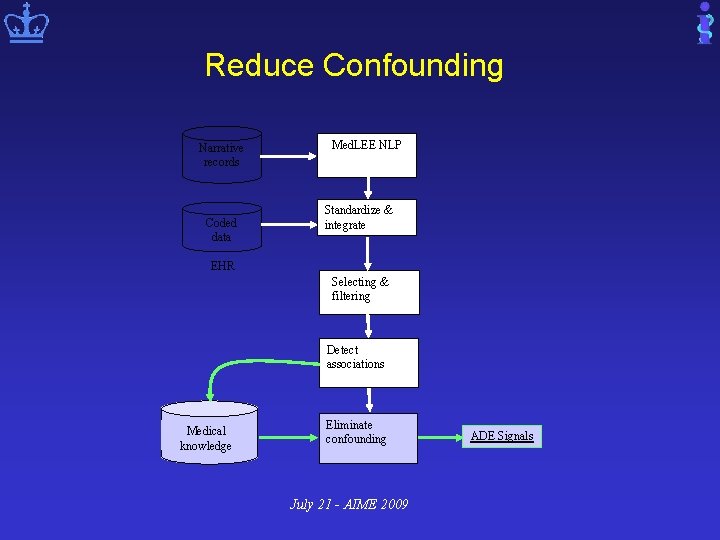
Overview of Our NLP-EHR based Pharmacovigilance System Narrative records Coded data Med. LEE NLP Standardize & integrate EHR Selecting & filtering Detect associations Medical knowledge Eliminate confounding July 21 - AIME 2009 ADE Signals

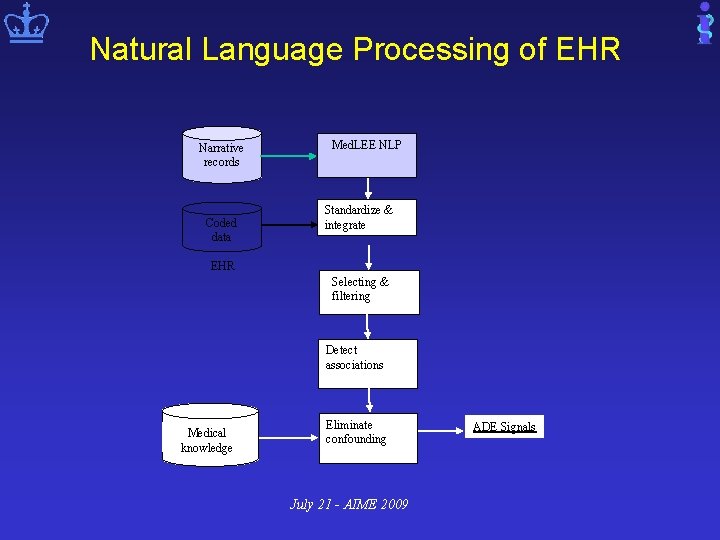
Natural Language Processing of EHR Narrative records Coded data Med. LEE NLP Standardize & integrate EHR Selecting & filtering Detect associations Medical knowledge Eliminate confounding July 21 - AIME 2009 ADE Signals

Meds: Tegretol xr Zocor All: Several sz meds PMHx: sz d/o - well controlled on tegretol high chol - on zocor CAD - 60% lesion in LADM by cath MR - secondary to mitral prolapse PSHx: rib fx in 2001, shoulder fx secondary to trauma Vitals: 130/80 12 80 A/P: 54 y/o m with mult med problems, all relatively well controlled. Pt sz free, not anemic as of 2/2003. Concerned of MR and its possible long term effects. July 21 - AIME 2009

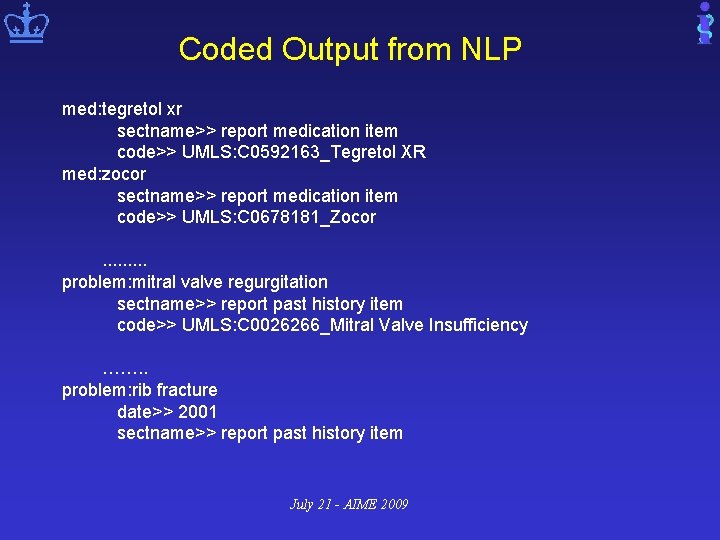
Coded Output from NLP med: tegretol xr sectname>> report medication item code>> UMLS: C 0592163_Tegretol XR med: zocor sectname>> report medication item code>> UMLS: C 0678181_Zocor. . problem: mitral valve regurgitation sectname>> report past history item code>> UMLS: C 0026266_Mitral Valve Insufficiency ……. . problem: rib fracture date>> 2001 sectname>> report past history item July 21 - AIME 2009

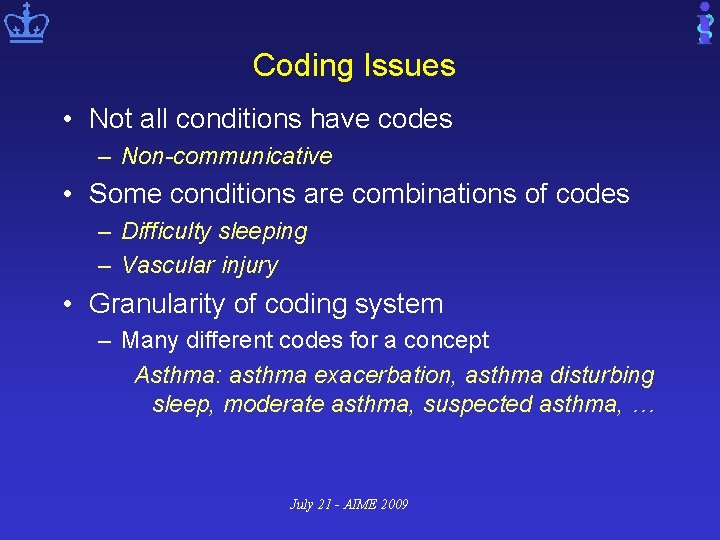
Coding Issues • Not all conditions have codes – Non-communicative • Some conditions are combinations of codes – Difficulty sleeping – Vascular injury • Granularity of coding system – Many different codes for a concept Asthma: asthma exacerbation, asthma disturbing sleep, moderate asthma, suspected asthma, … July 21 - AIME 2009

Standardizing Coded Data Narrative records HCT: 20 Coded data Med. LEE NLP Standardize & integrate C 0744727: low hematocrit EHR Selecting & filtering Detect associations Medical knowledge Eliminate confounding July 21 - AIME 2009 ADE Signals

Standardizing Coded EHR Data: Laboratory Tests and Medications • Lab values denoting normal/abnormal vary – Abnormal range may depend on age, sex, ethnicity, weight – Change in lab values and duration must be considered • Standardizing medications is complex & requires additional knowledge – Tradename to generic (Avandia rosaglitazone) – Handling of combination medications • 1. 5% Lidocaine with 1: 200, 000 Epinephrine – Handling of dose & Route • Diazepam 2 MG Oral Tablet July 21 - AIME 2009

Selecting and Filtering Narrative records Coded data Med. LEE NLP Standardize & integrate EHR Selecting & filtering • Select using UMLS classes (diseases, medications) Filter out: • negations, past info, … • wrong time order Detect associations Medical knowledge Eliminate confounding ADE Signals July 21 - AIME 2009

Selecting and Filtering • Dependence on accuracy of semantic classification – UMLS classification errors - Finding: birth history, cardiac output, divorce + Finding: cardiomegaly, fever • Temporal information difficult to obtain – An adverse drug event should only follow drug event – Processing of explicit time information is complex and vague • Yesterday, last admission, 2/5 – Information typically occur in reports without dates July 21 - AIME 2009

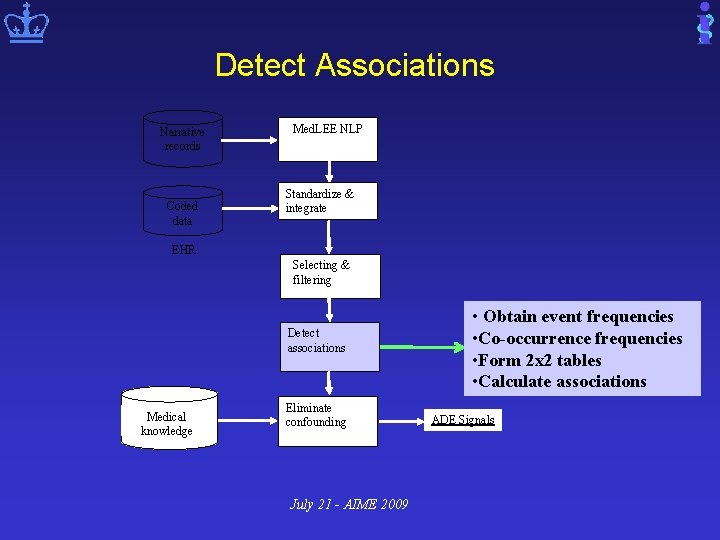
Detect Associations Narrative records Coded data Med. LEE NLP Standardize & integrate EHR Selecting & filtering Detect associations Medical knowledge Eliminate confounding July 21 - AIME 2009 • Obtain event frequencies • Co-occurrence frequencies • Form 2 x 2 tables • Calculate associations ADE Signals

Detect Associations • Correct temporal sequence is critical – Drug event should precede adverse event – Dates are not usually stated along with events – Section of reports helpful surrogate • Statistical associations correspond to different clinical relations – For pharmacovigilance: • Want drug causes adverse event • Confounding caused by dependencies in data July 21 - AIME 2009

Confounding Interdependencies Disease Manifested by Treats Drug Cause_ADE July 21 - AIME 2009 Adverse Event

Confounding Interdependencies HD SOB ML ML: Metolazone; HD: Hypertensive Disease; SOB: Shortness of Breath July 21 - AIME 2009

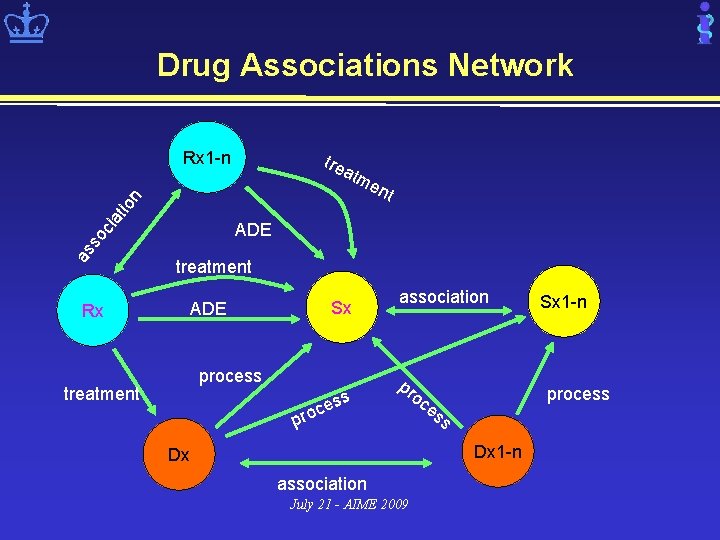
Drug Associations Network Rx 1 -n atm as so cia tio n tre Rx en t ADE treatment Sx ADE process treatment s ces association pr pro oc process es s Dx 1 -n Dx association July 21 - AIME 2009 Sx 1 -n

Reduce Confounding Narrative records Coded data Med. LEE NLP Standardize & integrate EHR Selecting & filtering Detect associations Medical knowledge Eliminate confounding July 21 - AIME 2009 ADE Signals

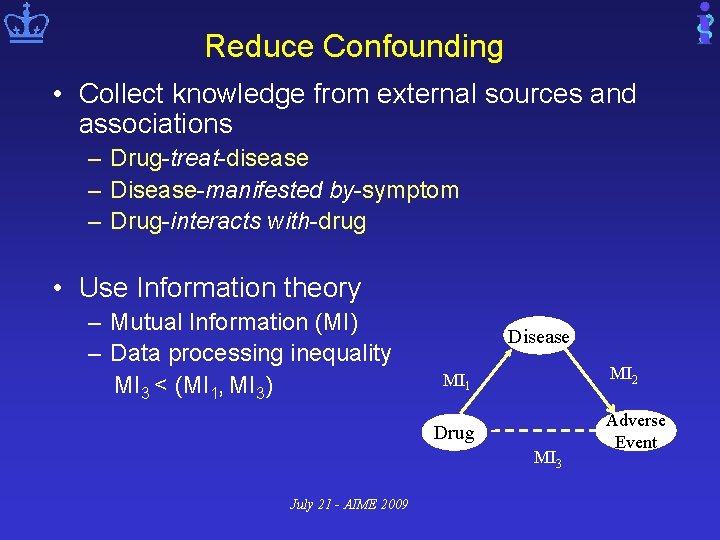
Reduce Confounding • Collect knowledge from external sources and associations – Drug-treat-disease – Disease-manifested by-symptom – Drug-interacts with-drug • Use Information theory – Mutual Information (MI) – Data processing inequality MI 3 < (MI 1, MI 3) Disease MI 2 MI 1 Drug MI 3 July 21 - AIME 2009 Adverse Event

Initial Study: Methods • 6 drugs chosen – – – • • • Ibuprofen, Morphine, Warfarin: longtime on market with known ADEs Bupropion, Paroxetine, Rosiglitazone: ADEs discovered after 2004 1 drug class: ACE inhibitors 25, 074 textual discharge summaries in 2004 from NYPH processed using Med. LEE NLP Reference standard created using expert knowledge sources Drug-potential ADE pairs determined Recall/precision calculated Qualitative analysis performed to classify drugpotential ADE pairs detected July 21 - AIME 2009

Initial Study: Results • Quantitative – recall (. 75), precision (. 30) • Qualitative analysis: potential drug-ADE pairs a. Known drug-ADEs: 30% b. Drug-indication pairs: 30% c. Remote drug-indication pair: 33% d. Unknown clinical associations: 6% July 21 - AIME 2009

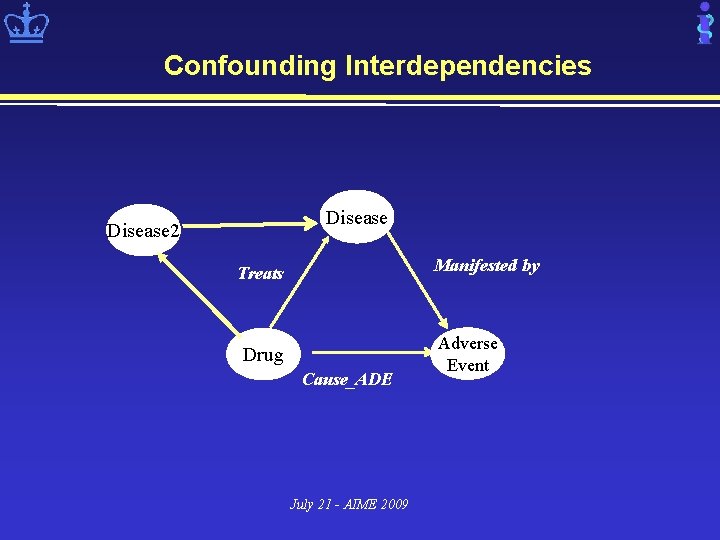
Confounding Interdependencies Disease 2 Manifested by Treats Drug Cause_ADE July 21 - AIME 2009 Adverse Event

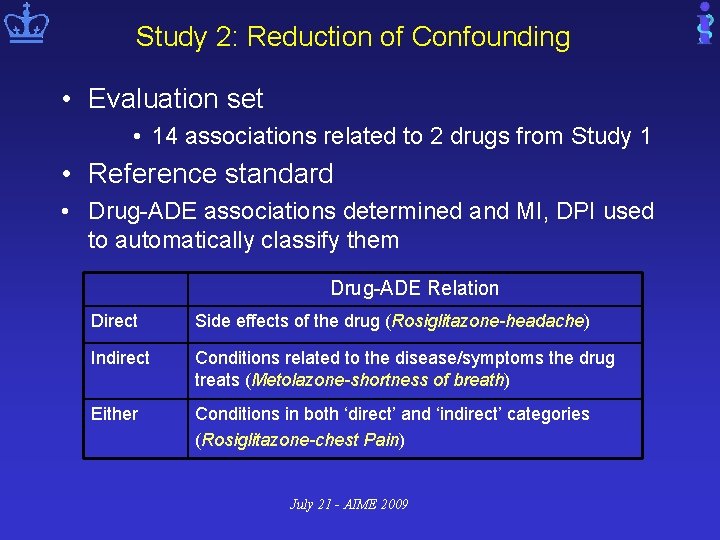
Study 2: Reduction of Confounding • Evaluation set • 14 associations related to 2 drugs from Study 1 • Reference standard • Drug-ADE associations determined and MI, DPI used to automatically classify them Drug-ADE Relation Direct Side effects of the drug (Rosiglitazone-headache) Indirect Conditions related to the disease/symptoms the drug treats (Metolazone-shortness of breath) Either Conditions in both ‘direct’ and ‘indirect’ categories (Rosiglitazone-chest Pain) July 21 - AIME 2009

Results • Precision • 0. 86 when handling confounding • 0. 31 when without handling confounding July 21 - AIME 2009

Discussion: Limitations & Future Directions • Mutual information only strategy to handle confounding – More complex MI strategy will be explored – Other statistical/knowledge based methods will be explored • Inpatient data only/sicker patient population – The same methods could be used for outpatient data as well possibly more noisy • Drug dosage, drug-drug and more complex interactions should be explored July 21 - AIME 2009

Discussion: Limitations & Future Directions • Small evaluation data set – More comprehensive evaluation • Limitations inherent from NLP, coding, association detection • Limitations due to fragmented/incomplete patient data July 21 - AIME 2009

Summary • Need for more pharmacovigilance research – Based on the EHR – Using available databases and text • Studies demonstrated promising results • Many interesting research opportunities – – – Natural language processing Statistical methods Integrating different sources of data Gathering knowledge from different sources Automated knowledge acquisition for evidence based medicine July 21 - AIME 2009

Acknowledgement • NLP Data Mining group at DBMI at Columbia – – – – George Hripcsak Marianthi Markatou Herb Chase Xiaoyan Wang David Albers Jung-wei Fan Lyudmila Shagina Noemie Elhadad • Grants – – R 01 LM 007659 from NLM R 01 LM 008635 from NLM R 01 LM 06910 from NLM 5 T 15 LM 007079 from NLM training grant July 21 - AIME 2009

QUESTIONS THANK YOU! July 21 - AIME 2009
 Azalastyna
Azalastyna Adverse events in hospital
Adverse events in hospital Ir adverse event
Ir adverse event Puerperal sepsis definition
Puerperal sepsis definition Adverse events following immunization (aefi) course answers
Adverse events following immunization (aefi) course answers Challenges of novel drug delivery system
Challenges of novel drug delivery system Novel clinical drug trial design
Novel clinical drug trial design An example of crude drug adulterated with exhausted drug
An example of crude drug adulterated with exhausted drug Mutually exclusive vs non mutually exclusive
Mutually exclusive vs non mutually exclusive Natural selection and drug resistance
Natural selection and drug resistance Natural selection
Natural selection Eeo compliance in job analysis
Eeo compliance in job analysis Benjamin latrobe
Benjamin latrobe Discovering computers 2018 chapter 1 ppt
Discovering computers 2018 chapter 1 ppt Discovering computers 2016
Discovering computers 2016 Discovering computers
Discovering computers Self-motivation meaning
Self-motivation meaning Discovering science 7
Discovering science 7 P value
P value Discovering economic systems guided practice
Discovering economic systems guided practice Discovering computers 2011
Discovering computers 2011 Readwise token
Readwise token Discovering computers 2018 chapter 1
Discovering computers 2018 chapter 1 Rock vs mineral
Rock vs mineral Muhammad computer technology
Muhammad computer technology Discovering engineering
Discovering engineering Discovering american ideals in primary sources
Discovering american ideals in primary sources Teachers discovering computers
Teachers discovering computers Pioneer species
Pioneer species Grade 8 science unit 3
Grade 8 science unit 3 A r williams author of discovering tut
A r williams author of discovering tut Discovering personal genius
Discovering personal genius Tactile output device
Tactile output device Discovering computers 2012
Discovering computers 2012 Ethics discovering right and wrong
Ethics discovering right and wrong Chapter 7 lesson 1 discovering parts of an atom answer key
Chapter 7 lesson 1 discovering parts of an atom answer key Discovering the internet
Discovering the internet Discovering cells
Discovering cells Discovering our past ancient civilizations
Discovering our past ancient civilizations Adverse reaction definition
Adverse reaction definition Sentinel event بالعربي
Sentinel event بالعربي