Datadriven visualization of drug interactions Adverse Drug Events

![Adverse Drug Events • Almost 1 million deaths/injuries each year in the US[1] • Adverse Drug Events • Almost 1 million deaths/injuries each year in the US[1] •](https://slidetodoc.com/presentation_image/02a0b7903334c7c021c6a35a9044ceaa/image-2.jpg)



- Slides: 12

Data-driven visualization of drug interactions
![Adverse Drug Events Almost 1 million deathsinjuries each year in the US1 Adverse Drug Events • Almost 1 million deaths/injuries each year in the US[1] •](https://slidetodoc.com/presentation_image/02a0b7903334c7c021c6a35a9044ceaa/image-2.jpg)
Adverse Drug Events • Almost 1 million deaths/injuries each year in the US[1] • Some fraction of ADEs are caused by previously unknown drug-drug interactions • Clinical trials aren’t large enough to detect many potential interactions • FDA, WHO, pharmaceutical companies maintain databases of reported[2] ADEs • You can download a sample of the FDA data from the Adverse Event Reporting System website[3] • We can analyze the reported data to identify suspicious drug interactions Copyright 2011 Cloudera Inc. All rights reserved

Challenges in Analyzing Adverse Drug Events • Biased Sample • Adverse event reporting is voluntary • We don’t see events from patients who took the drugs and nothing happened • Correlation != Causation • No controlled trials, some correlations are coincidences • Requires Advanced Statistical Modeling Skills • Multi-item Gamma Poisson Shrinkage Estimator is used to score the significance of a drug interactions • The model is too complex to solve directly, we use Expectation Maximization (EM) to estimate its parameters Copyright 2011 Cloudera Inc. All rights reserved

The Hard Problem: Counting • It is a “small” data problem… • 250, 000+ events reported to the FDA annually • …that explodes when we consider: • Multi-drug, multi-symptom interactions • Analyzed by strata (e. g. , month of report, patient age, patient gender, etc. ) • ~1 million reports => ~360 million buckets • Analysts typically filter the data to only consider a few adverse reactions at a time… • …but that is not the way of the data scientist Copyright 2011 Cloudera Inc. All rights reserved

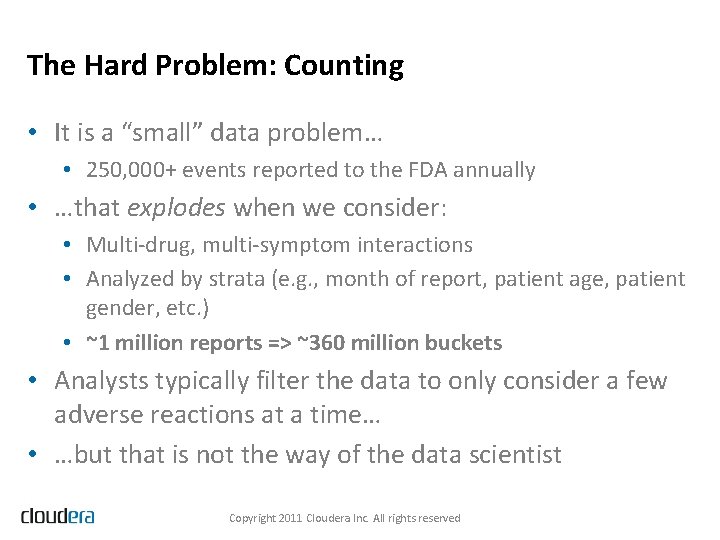
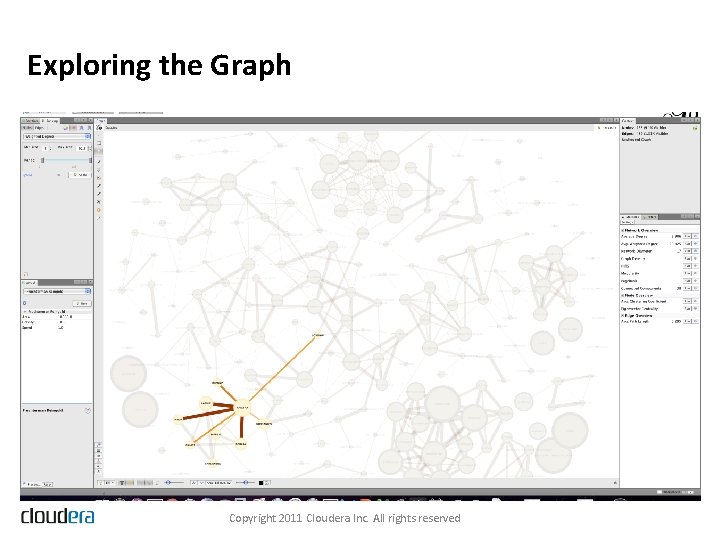
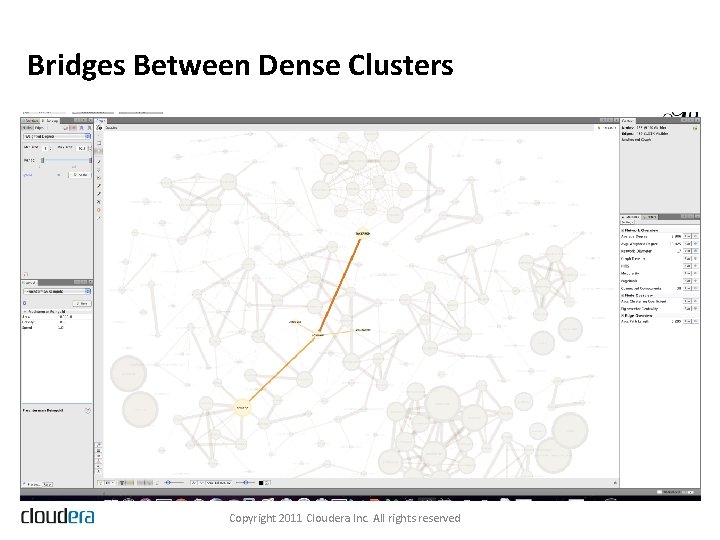
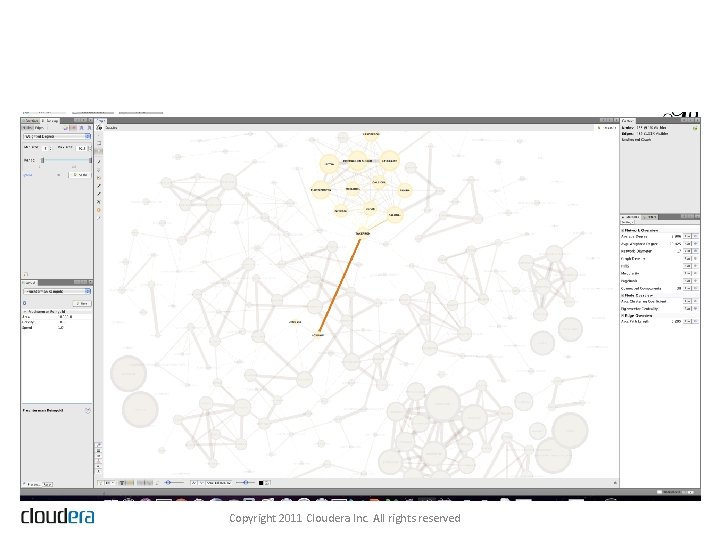
Solving the Hard Problem • Map. Reduce on Hadoop • • 20 Map. Reduce jobs Filter, aggregate, join, aggregate again Model the resulting data in R Use Map. Reduce to apply the model parameters to the data, score each drug-drug interaction, and then filter the data to obtain the highest scoring interactions • Visualizing the Results • Even applying a restrictive filter on the scores, we end up with 20, 000+ statistically significant drug-reaction triples Copyright 2011 Cloudera Inc. All rights reserved

The Drug-Drug Interaction Graph Copyright 2011 Cloudera Inc. All rights reserved

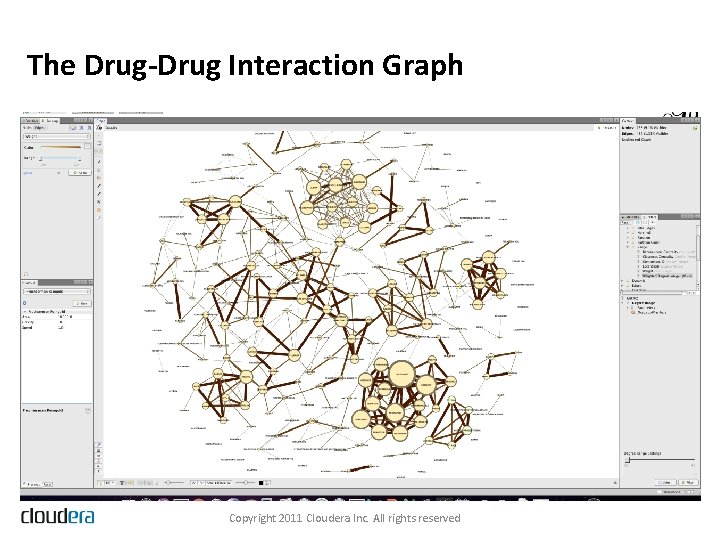
HIV Medications Copyright 2011 Cloudera Inc. All rights reserved

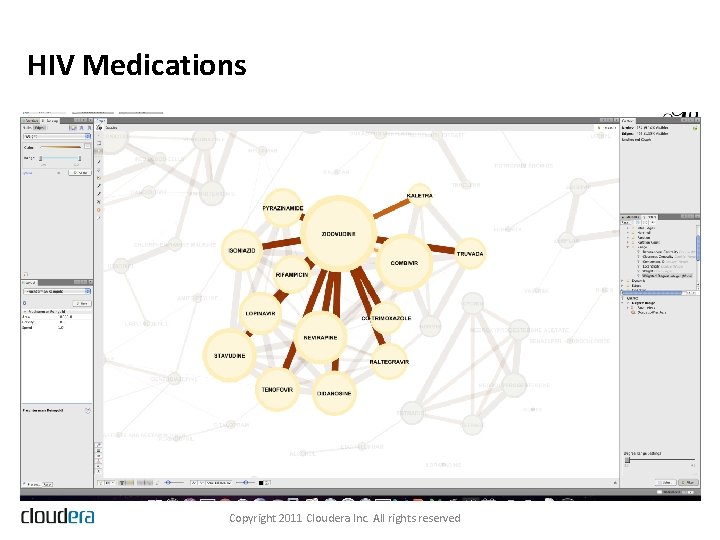
Cancer Medications Copyright 2011 Cloudera Inc. All rights reserved

Exploring the Graph Copyright 2011 Cloudera Inc. All rights reserved

Bridges Between Dense Clusters Copyright 2011 Cloudera Inc. All rights reserved

Copyright 2011 Cloudera Inc. All rights reserved

Acknowledgments and References • Thanks to Josh Wills, Director of Data Science at Cloudera, for the data collection and analysis shown here. • References: • [1] ADE instances/year: http: //www. ahrq. gov/qual/aderia. htm • [2] AERS reporting site: http: //www. ahrq. gov/qual/aderia. htm • [3] Download ADE instance data: http: //www. fda. gov/Drugs/Guidance. Compliance. Regulatory. Inform ation/Surveillance/Adverse. Drug. Effects/ucm 082193. htm • Other resources: • http: //www. cloudera. com/blog • http: //wiki. cloudera. com/ Copyright 2011 Cloudera Inc. All rights reserved 12
 Datadriven marketing
Datadriven marketing Adverse events in hospital
Adverse events in hospital Adverse events in hospital
Adverse events in hospital Adverse events following immunization (aefi) course answers
Adverse events following immunization (aefi) course answers Adverse events in hospital
Adverse events in hospital Azalastyna
Azalastyna Vitamin e main function
Vitamin e main function Felodapine
Felodapine Crpc
Crpc Ppi omeprazole
Ppi omeprazole Diazepam cyp450
Diazepam cyp450 Google.visualization.events.addlistener
Google.visualization.events.addlistener Mutually exclusive vs non mutually exclusive
Mutually exclusive vs non mutually exclusive























