Recognition and Management of ImmuneRelated Adverse Events ir


- Slides: 19

Recognition and Management of Immune-Related Adverse Events (ir. AEs) with Immune Checkpoint Inhibitor Therapy in SCLC Anna Farago, M. D. , Ph. D. October 11, 2019

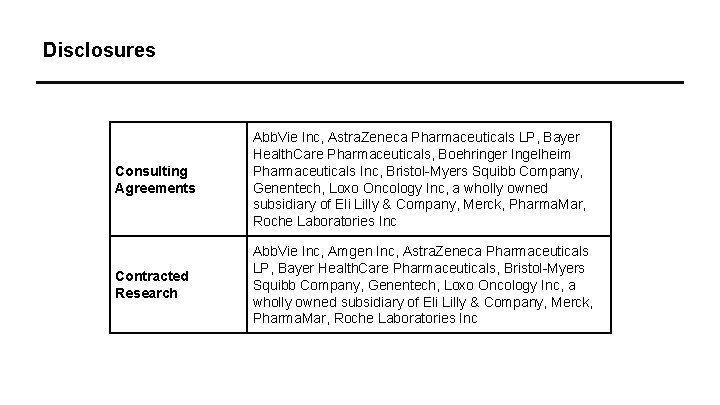
Disclosures Consulting Agreements Abb. Vie Inc, Astra. Zeneca Pharmaceuticals LP, Bayer Health. Care Pharmaceuticals, Boehringer Ingelheim Pharmaceuticals Inc, Bristol-Myers Squibb Company, Genentech, Loxo Oncology Inc, a wholly owned subsidiary of Eli Lilly & Company, Merck, Pharma. Mar, Roche Laboratories Inc Contracted Research Abb. Vie Inc, Amgen Inc, Astra. Zeneca Pharmaceuticals LP, Bayer Health. Care Pharmaceuticals, Bristol-Myers Squibb Company, Genentech, Loxo Oncology Inc, a wholly owned subsidiary of Eli Lilly & Company, Merck, Pharma. Mar, Roche Laboratories Inc

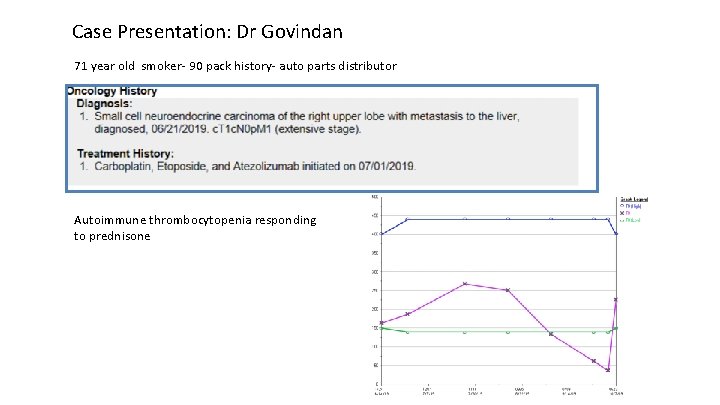
Case Presentation: Dr Govindan 71 year old smoker- 90 pack history- auto parts distributor Autoimmune thrombocytopenia responding to prednisone

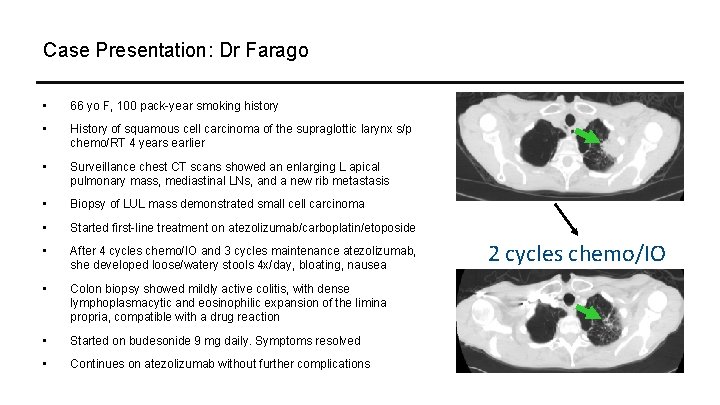
Case Presentation: Dr Farago • 66 yo F, 100 pack-year smoking history • History of squamous cell carcinoma of the supraglottic larynx s/p chemo/RT 4 years earlier • Surveillance chest CT scans showed an enlarging L apical pulmonary mass, mediastinal LNs, and a new rib metastasis • Biopsy of LUL mass demonstrated small cell carcinoma • Started first-line treatment on atezolizumab/carboplatin/etoposide • After 4 cycles chemo/IO and 3 cycles maintenance atezolizumab, she developed loose/watery stools 4 x/day, bloating, nausea • Colon biopsy showed mildly active colitis, with dense lymphoplasmacytic and eosinophilic expansion of the limina propria, compatible with a drug reaction • Started on budesonide 9 mg daily. Symptoms resolved • Continues on atezolizumab without further complications 2 cycles chemo/IO

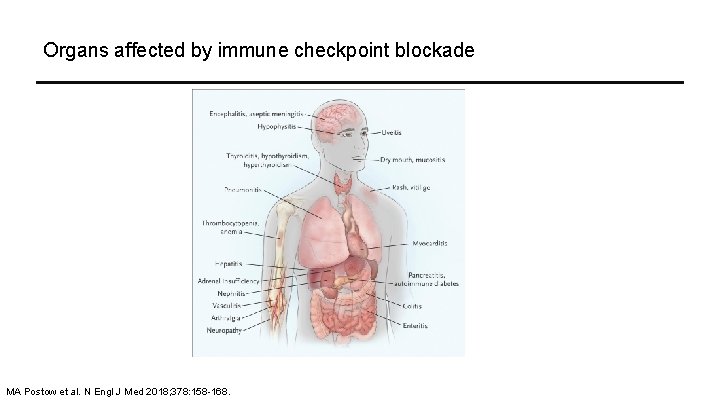
Organs affected by immune checkpoint blockade MA Postow et al. N Engl J Med 2018; 378: 158 -168.

Possible mechanisms underlying immune-related adverse events Immune-related AEs result from complex relationships between the immune system and the anti-tumor response. Goal of treating ir. AEs: Minimizing morbidity/mortality from toxicity without inhibiting antitumor immunity. MA Postow et al. N Engl J Med 2018; 378: 158 -168.

Paraneoplastic syndromes in SCLC: Cause for concern, or encouraging signal? • Ectopic hormone-associated syndromes – Hyponatremia (SIADH) – 15% – Cushing’s syndrome (ACTH) – 5% • Immune-mediated neurological syndromes – Lambert-Eaton myasthenia gravis (anti-voltage gated Ca channel) – 1% – Encephalomyelitis, sensory neuropathy, cerebellar degeneration and others (various antibodies) – < 1% – Immune-mediated neurological symptoms are associated with an earlier stage at presentation and a better overall survival

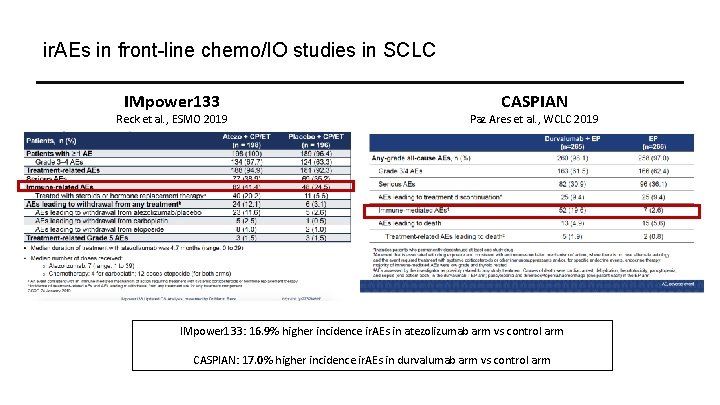
ir. AEs in front-line chemo/IO studies in SCLC IMpower 133 Reck et al. , ESMO 2019 CASPIAN Paz Ares et al. , WCLC 2019 IMpower 133: 16. 9% higher incidence ir. AEs in atezolizumab arm vs control arm CASPIAN: 17. 0% higher incidence ir. AEs in durvalumab arm vs control arm

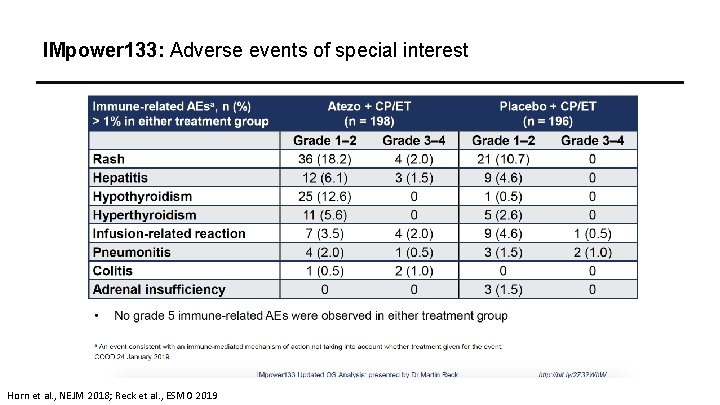
IMpower 133: Adverse events of special interest Horn et al. , NEJM 2018; Reck et al. , ESMO 2019

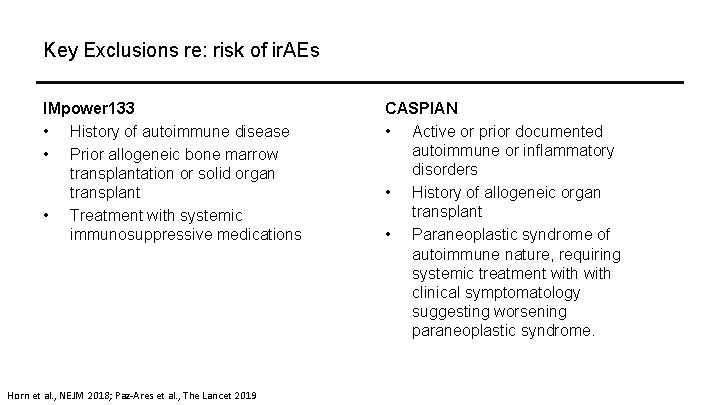
Key Exclusions re: risk of ir. AEs IMpower 133 • History of autoimmune disease • Prior allogeneic bone marrow transplantation or solid organ transplant • Treatment with systemic immunosuppressive medications Horn et al. , NEJM 2018; Paz-Ares et al. , The Lancet 2019 CASPIAN • Active or prior documented autoimmune or inflammatory disorders • History of allogeneic organ transplant • Paraneoplastic syndrome of autoimmune nature, requiring systemic treatment with clinical symptomatology suggesting worsening paraneoplastic syndrome.

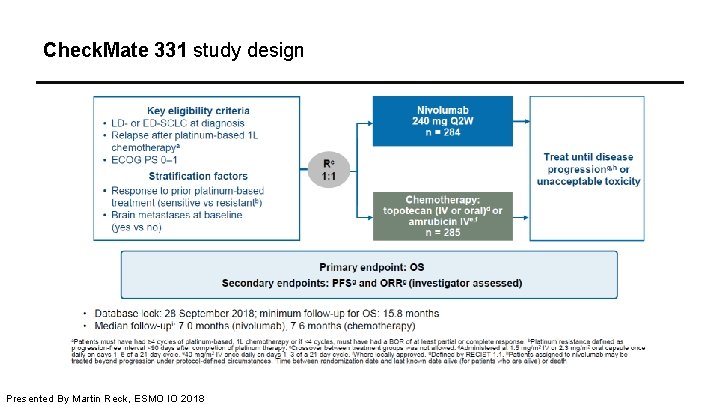
Check. Mate 331 study design Presented By Martin Reck, ESMO IO 2018

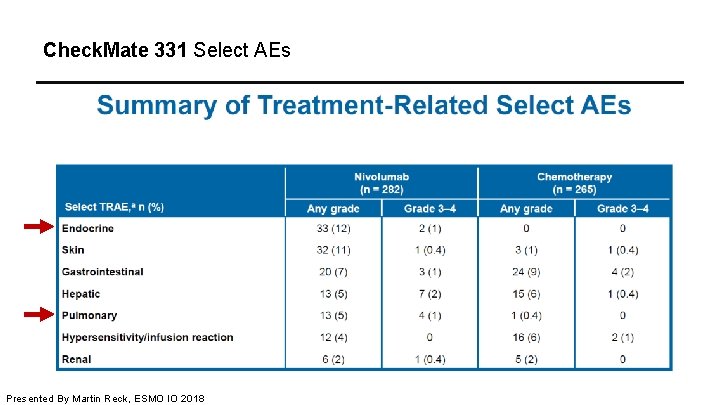
Check. Mate 331 Select AEs Presented By Martin Reck, ESMO IO 2018

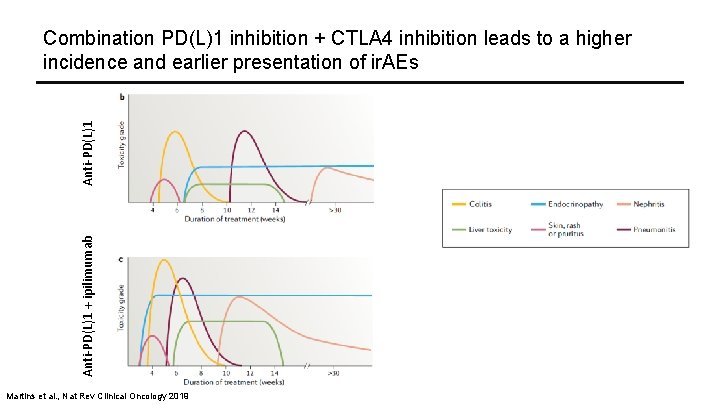
Anti-PD(L)1 + ipilimumab Anti-PD(L)1 Combination PD(L)1 inhibition + CTLA 4 inhibition leads to a higher incidence and earlier presentation of ir. AEs Martins et al. , Nat Rev Clinical Oncology 2019

Incidence of ir. AEs in Check. Mate 032 Presented By Matthew Hellmann at 2017 ASCO Annual Meeting

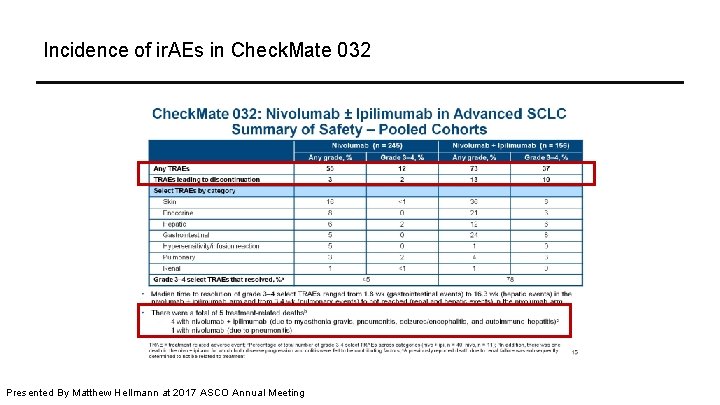
Incidence of ir. AEs in Check. Mate 032 Presented By Matthew Hellmann at 2017 ASCO Annual Meeting

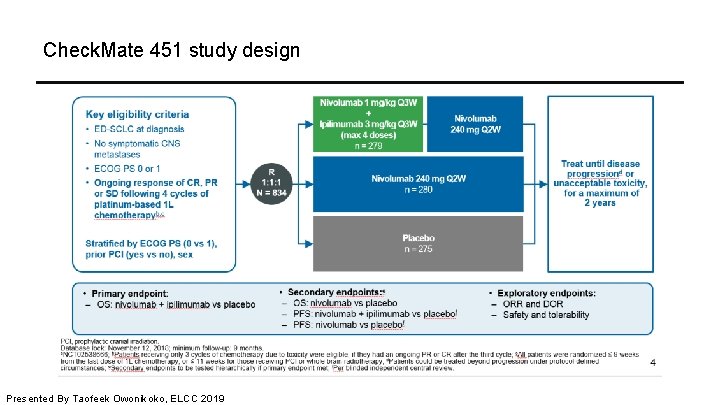
Check. Mate 451 study design Presented By Taofeek Owonikoko, ELCC 2019

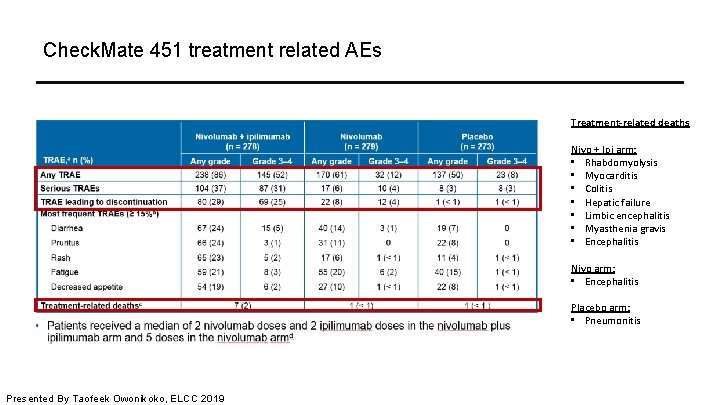
Check. Mate 451 treatment related AEs Treatment-related deaths Nivo + Ipi arm: • Rhabdomyolysis • Myocarditis • Colitis • Hepatic failure • Limbic encephalitis • Myasthenia gravis • Encephalitis Nivo arm: • Encephalitis Placebo arm: • Pneumonitis Presented By Taofeek Owonikoko, ELCC 2019

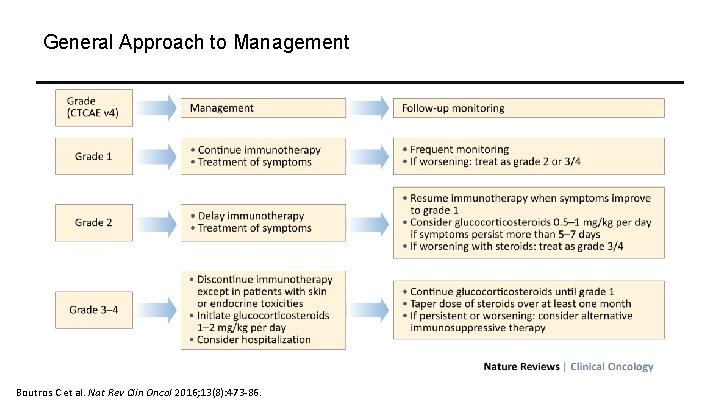
General Approach to Management Boutros C et al. Nat Rev Clin Oncol 2016; 13(8): 473 -86.

Summary and recommendations • ir. AEs in IMpower 133 and CASPIAN are generally consistent with the anti-PDL 1 monotherapy experience, and no significant new safety signals were seen. • Adding ipilimumab to nivolumab increases the incidence and severity of ir. AEs. • Unique considerations in SCLC include potential for increased risk of immune related paraneoplastic syndromes, though patients with histories of these were excluded from the trials. • For suspected ir. AEs, follow guidelines for management and involve a multidisciplinary team.
 Adverse events in hospital
Adverse events in hospital Adverse events in hospital
Adverse events in hospital Puerperal sepsis meaning
Puerperal sepsis meaning Adverse events following immunization (aefi) course answers
Adverse events following immunization (aefi) course answers Mutually exclusive events vs not mutually exclusive events
Mutually exclusive events vs not mutually exclusive events Adverse reaction definition
Adverse reaction definition Whats a sentinel event
Whats a sentinel event Adverse selection
Adverse selection Adverse selection
Adverse selection Adverse reaction definition
Adverse reaction definition Adverse reaction definition
Adverse reaction definition What is adverse selection
What is adverse selection Effects of paracetamol
Effects of paracetamol Loop diuretics adverse effects
Loop diuretics adverse effects Paracetamol dose child per kg
Paracetamol dose child per kg Sitaglimet
Sitaglimet Adverse selection
Adverse selection Adr adverse drug reaction
Adr adverse drug reaction Harley nadler
Harley nadler Classification of diuretics
Classification of diuretics