ADVERSE REACTION TRACKING Adverse Reaction Tracking ART The









![Subj: REACTION ENTERED IN ERROR [#20942] 30 Sep 03 17 Lines From: POSTMASTER (DSD-RPMS) Subj: REACTION ENTERED IN ERROR [#20942] 30 Sep 03 17 Lines From: POSTMASTER (DSD-RPMS)](https://slidetodoc.com/presentation_image/f710c7226d01fd8daff2cbfeb0e60013/image-13.jpg)
![ART Menus • 1) Adverse Reaction Tracking [GMRAMGR] - The top level menu. Given ART Menus • 1) Adverse Reaction Tracking [GMRAMGR] - The top level menu. Given](https://slidetodoc.com/presentation_image/f710c7226d01fd8daff2cbfeb0e60013/image-14.jpg)













































- Slides: 68

ADVERSE REACTION TRACKING

Adverse Reaction Tracking (ART) • The objective of the software is to track and report patient allergy and adverse reaction data. • Different menus for different types of users • Can set up site parameters based on how your facility plans to use the package • Drug interactions will be interactive based on VA drug class in Outpatient Pharmacy v 7. 0 and Inpatient v 5. 0 • Establish mail groups and bulletins based on site parameters

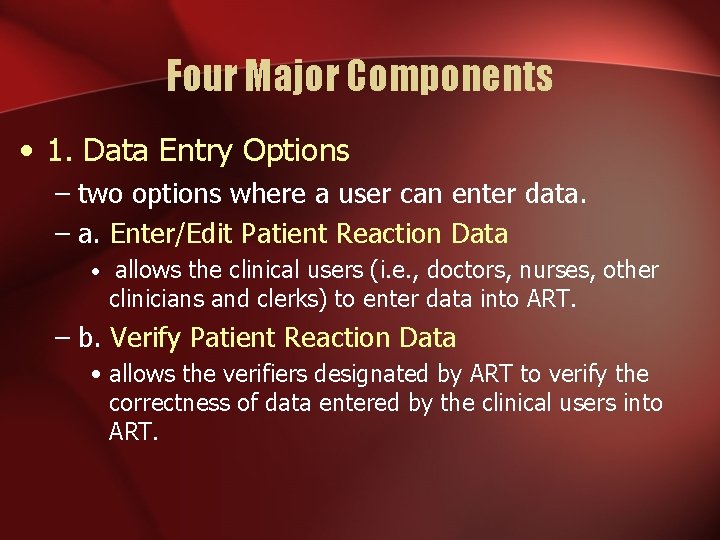
Four Major Components • 1. Data Entry Options – two options where a user can enter data. – a. Enter/Edit Patient Reaction Data • allows the clinical users (i. e. , doctors, nurses, other clinicians and clerks) to enter data into ART. – b. Verify Patient Reaction Data • allows the verifiers designated by ART to verify the correctness of data entered by the clinical users into ART.

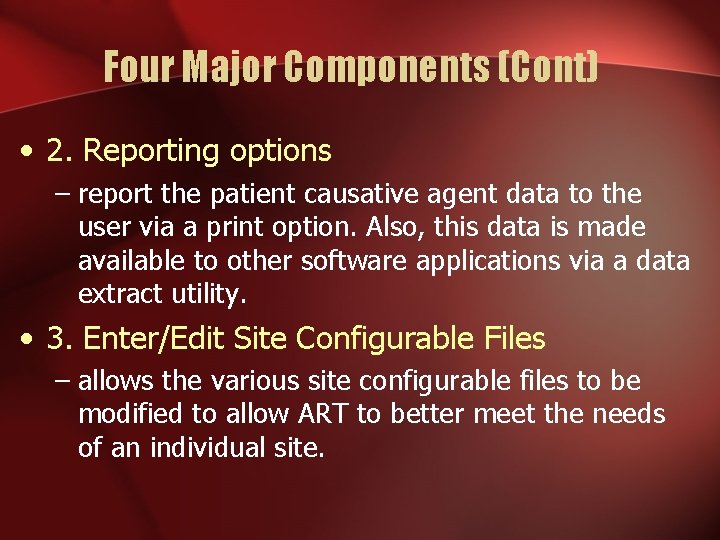
Four Major Components (Cont) • 2. Reporting options – report the patient causative agent data to the user via a print option. Also, this data is made available to other software applications via a data extract utility. • 3. Enter/Edit Site Configurable Files – allows the various site configurable files to be modified to allow ART to better meet the needs of an individual site.

Four Major Components (Cont) • 4. Adverse Drug Reaction (ADR) options – allows for the evaluation of a suspected ADR by a qualified individual (e. g. , clinical pharmacist, clinical pharmacologist) other than the attending physician. – generates the reports needed by the FDA.

Four Major Users • 1. Clinical users – are the doctors, nurses, other clinicians and clerks entering the data into ART. – if the reaction was observed at the site, data pertaining to any possible legal action could be tracked. Data is made available to users of any service utilizing the Reporting options, avoiding errors in care. – two other data elements that are tracked are: 1. date/time that the patient chart was marked, 2. date/time that the patient ID band was marked. – automated mail bulletins sent to the appropriate users when the date/time patient chart marked data field has not been recorded.

Four Major Users (Cont. ) • 2. Verifiers – designated users by the site who verify the correctness of the data in ART. – Need GMRA-ALLERGY VERIFY security key and have the ART Verifier Menu. – May be clinical pharmacists, dietitians, and other clinical personnel. Automated mail bulletins sent to the ART verifiers when an allergy/adverse reaction has been entered and signed (completed) by a user. Verification - important in observed instances of adverse drug reactions where a Quality Assurance (QA) investigation may be conducted.

Four Major Users (Cont. ) • 3. Pharmacy and Therapeutics (P&T) Committee users – members of the hospital's P&T Committee and are assigned the P&T Committee Menu option. They review ADRs in the facility. – Classify ADR’s as significant reactions and determine whether they are related to particular drugs. – Depending on the severity of the ADR, may report it further to the FDA. A printed copy of the form can be generated by ART. – Automated mail bulletins sent to the P&T Committee users when an observed drug reaction is entered into the system.

Four Major Users (Cont. ) • 4. Software developers – Utilize the data extract utility (GMRADPT routine) to gather ART data for display within their specific DHCP application.

Security Keys GMRA ALLERGY VERIFY • needed to verify allergy/adverse reactions. GMRA SUPERVISOR • given only to those users who have the authority to override the software’s security in order to edit data.

Mail Groups Automatic mail bulletins sent to specific users: • 1) GMRA MARK CHART - A list of users who will need to mark a patient’s chart to record an allergy/adverse reaction. • 2) GMRA VERIFY DRUG ALLERGY - A list of all verifiers who will need to be sent drug reaction information. • 3) GMRA VERIFY FOOD ALLERGY - A list of all verifiers who will need to be sent food reaction information. • 4) GMRA VERIFY OTHER ALLERGY - A list of all verifiers who will need to be sent other types of reaction information (i. e. , not drug or food). • 5) GMRA P&T COMMITTEE FDA - A list of the members of the Pharmacy and Therapeutic (P&T) Committee.

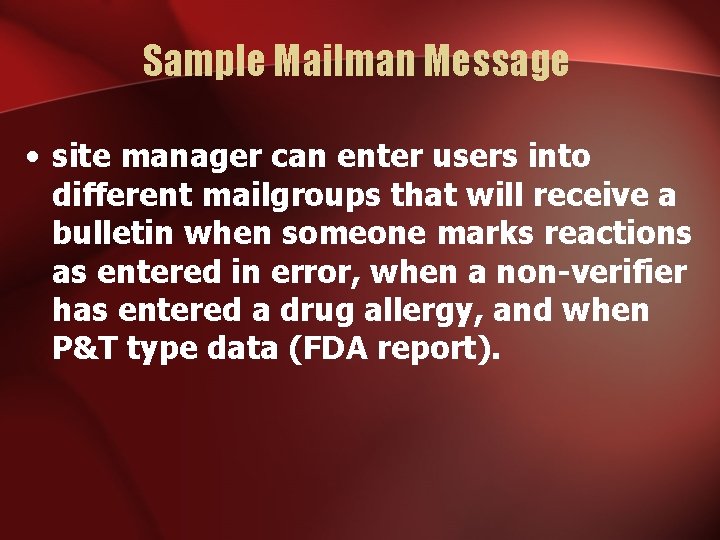
Sample Mailman Message • site manager can enter users into different mailgroups that will receive a bulletin when someone marks reactions as entered in error, when a non-verifier has entered a drug allergy, and when P&T type data (FDA report).
![Subj REACTION ENTERED IN ERROR 20942 30 Sep 03 17 Lines From POSTMASTER DSDRPMS Subj: REACTION ENTERED IN ERROR [#20942] 30 Sep 03 17 Lines From: POSTMASTER (DSD-RPMS)](https://slidetodoc.com/presentation_image/f710c7226d01fd8daff2cbfeb0e60013/image-13.jpg)
Subj: REACTION ENTERED IN ERROR [#20942] 30 Sep 03 17 Lines From: POSTMASTER (DSD-RPMS) (Sender: STARR, JANICE) Page 1 ---------------------------------------The following reaction has been ENTERED IN ERROR. Please ensure that the patient's Chart/ID Band are updated to reflect this change. Patient: LOPEZ, BYRON SCOTT SSN: 10 -95 -68 Reaction: CAPTOPRIL Location: 3 EAST Originator: STARR, JANICE Entered in Error by: STARR, JANICE Entered in Error on: Sep 30, 2003@14: 47: 28 Comments: ENTERED IN ERROR Date: Sep 30, 2003@14: 47: 28 User: STARR, JANICE Title: Patient denies reaction, is taking Captopril with no problems. Select MESSAGE Action: IGNORE (in WASTE basket)// D Deleted !!
![ART Menus 1 Adverse Reaction Tracking GMRAMGR The top level menu Given ART Menus • 1) Adverse Reaction Tracking [GMRAMGR] - The top level menu. Given](https://slidetodoc.com/presentation_image/f710c7226d01fd8daff2cbfeb0e60013/image-14.jpg)
ART Menus • 1) Adverse Reaction Tracking [GMRAMGR] - The top level menu. Given to the package’s support person. • 2) Adverse Reaction Tracking User Menu [GMRA USER MENU] - Assigned to clerks who will enter adverse reaction data. • 3) Adverse Reaction Tracking Clinician Menu [GMRA CLINICIAN MENU] - Assigned to clinicians who will use the package. • 4) Adverse Reaction Tracking Verifier Menu [GMRA VERIFIER MENU] – Assigned to users who will verify adverse reaction data. • 5) P&T Committee Menu [GMRA P&T MENU] - Given to Pharmacy and Therapeutic Committee members.

Enter/Edit Site CONFIGURABLE FILES MENU • 1. Edit Allergy File • 2. Enter/Edit Signs/Symptoms Data • 3. Enter/Edit Site Parameters • 4. Sign/Symptoms List • 5. Allergies File List Site can use to tailor ART to meet its needs. Get P&T consesus.

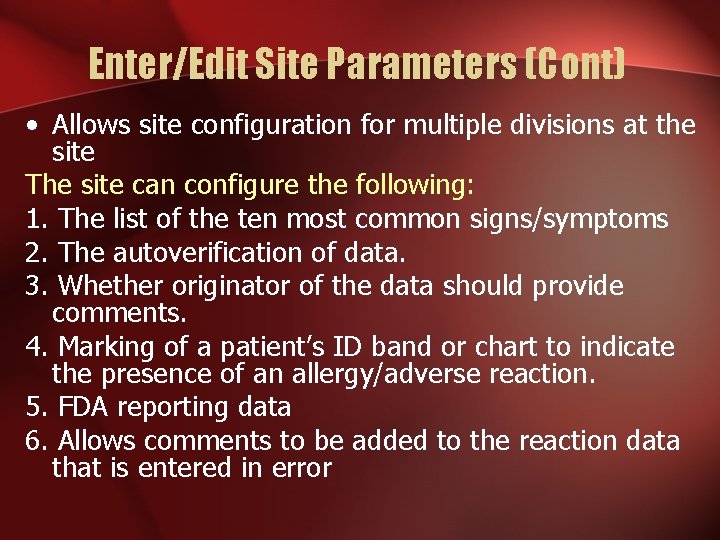
Enter/Edit Site Parameters (Cont) • Allows site configuration for multiple divisions at the site The site can configure the following: 1. The list of the ten most common signs/symptoms 2. The autoverification of data. 3. Whether originator of the data should provide comments. 4. Marking of a patient’s ID band or chart to indicate the presence of an allergy/adverse reaction. 5. FDA reporting data 6. Allows comments to be added to the reaction data that is entered in error

Entering Site Parameters - Example • Entering site parameters for a site that allows autoverfication of FOOD or OTHER allergies • BUT requires verification of DRUG allergies

Select Enter/Edit Site Configurable Files Option: 3 Enter/Edit Site Parameters Select GMR ALLERGY SITE PARAMETERS NAME: HOSPITAL Everyone has to enter HOSPITAL here NAME: HOSPITAL// (No editing) Select DIVISION: NAME OF YOUR INSTITUTION The following are the ten most common signs/symptoms: 1. ANXIETY 6. DIARRHEA 2. ITCHING, WATERING EYES 7. HIVES 3. HYPOTENSION 8. DRY MOUTH 4. DROWSINESS 9. ANAPHYLAXIS 5. NAUSEA, VOMITING 10. RASH Enter the number of the sign/symptom that you would like to edit: 3 REACTION: HYPOTENSION// DIZZINESS Note – there are 200 national sign/symptoms. You can enter ? ? to see your choices. The following are the ten most common signs/symptoms: 1. ANXIETY 6. DIARRHEA 2. ITCHING, WATERING EYES 7. HIVES 3. DIZZINESS 8. DRY MOUTH 4. DROWSINESS 9. ANAPHYLAXIS 5. NAUSEA, VOMITING 10. RASH

Enter the number of the sign/symptom that you would like to edit: ENTER AUTOVERIFY FOOD/DRUG/OTHER: AUTOVERIFY FOOD/OTHER AUTOVERIFY OBSERVED/HISTORICAL: AUTOVERIFY BOTH AUTOVERIFY LOGICAL OPERATOR: AND REQUIRE ORIGINATOR COMMENTS: NO MARK ID BAND FLAG: NO METHOD OF NOTIFICATION: BULLETIN ALERT ID BAND/CHART MARK: NO SEND CHART MARK BULLETIN FOR NEW ADMISSIONS: NO FDA DATA REQUIRED: NO ENABLE COMMENTS FIELD FOR REACTIONS THAT ARE ENTERED IN ERROR: YES REPORTER NAME: JONES, ALBERT ADDRESS: RED LAKE INDIAN HOSPITAL, P. O. BOX 497 CITY: RED LAKE STATE: MINNESOTA ZIP: 56671 PHONE: 999 -9999 OCCUPATION: PHARM Do you want to edit Reporter Information shown above? No// ENTER (No)

REPORTER NAME: JONES, CHARLES ADDRESS: INDIAN HEALTH SERVICE HOSPITAL RT. 1, BOX 234 CITY: ANY WHERE STATE: NEW MEXICO ZIP: 87222 PHONE: 505. 848. 5834 OCCUPATION: PHARM NOTE: The “Reporter” data fields contain the site’s default values that will appear on the FDA adverse reaction reports. This information may be left blank. The user will be prompted for the reporter information when creating an FDA report.

Associating Drugs with Correct VA Drug Class • Should do this matching procedure for most common drugs causing allergies or reactions before start • Once you have matched even one patient to the drug and VA class, the next one that you do, will automatically attach the Class. If the VA Class has not been populated, then there will not be any matching by the program when you try to fill the prescription. Select Adverse Reaction Tracking Option: 1 Enter/Edit Site Configurable Files 1 Edit Allergy File 2 Enter/Edit Signs/Symptoms Data 3 Enter/Edit Site Parameters 4 Sign/Symptoms List 5 Allergies File List Select Enter/Edit Site Configurable Files Option: 1 Edit Allergy File Select a LOCAL ALLERGY/ADVERSE REACTION: CODEINE Are you adding 'CODEINE' as a new GMR ALLERGIES (the 117 TH)? No// Y (Yes) GMR ALLERGIES ALLERGY TYPE: DRUG NAME: CODEINE// <ENTER>

Associating Drug with Correct VA Drug Class (Cont) Select SYNONYM: <ENTER> 1 Drug 2 Food 3 Other Select the type(s) for this reaction: 1// <ENTER > Select DRUG INGREDIENT: CODEINE 1 CODEINE 2 CODEINE PHOSPHATE 3 CODEINE POLISTIREX 4 CODEINE SULFATE CHOOSE 1 -4: 1 CODEINE Are you adding 'CODEINE' as a new DRUG INGREDIENTS (the 1 ST for this GMR ALLERGIES)? No// Y (Yes) Select DRUG INGREDIENT: Select VA DRUG CLASSES: CN 101 OPIOID ANALGESICS Are you adding 'CN 101' as a new VA DRUG CLASSES (the 1 ST for this GMR ALLERGIES)? No// Y (Yes) Select VA DRUG CLASSES: <ENTER>

Entering a New Drug in ART File Select DRUG INGREDIENT: ? Answer with DRUG INGREDIENTS You may enter a new DRUG INGREDIENTS, if you wish Enter one of the drug ingredients that make up this allergy. Answer with DRUG INGREDIENTS NAME Do you want the entire 3585 -Entry DRUG INGREDIENTS List? N (No) Select DRUG INGREDIENT: ENTER NAME OF DRUG<RET> Select VA DRUG CLASSES: ? Answer with VA DRUG CLASSES You may enter a new VA DRUG CLASSES, if you wish Answer with VA DRUG CLASS CODE, or CLASSIFICATION Do you want the entire 494 -Entry VA DRUG CLASS List? N (No) Select VA DRUG CLASSES: AA 000<RET> Select a LOCAL ALLERGY/ADVERSE REACTION:

Edit Allergy File • enter allergies into the system. • Software is distributed with a list of entries that is categorized as NATIONAL allergies. • Can set autoverify ON for FOOD and OTHER allergies • Recommended that all DRUG allergies be verfied

Example of an allergy added Select Enter/Edit Site Configurable Files Option: 1 Edit Allergy File Select a LOCAL ALLERGY/ADVERSE REACTION: STINKWEED Are you adding ‘STINKWEED’ as a new GMR ALLERGIES (the 117 TH)? Y (Yes) GMR ALLERGIES ALLERGY TYPE: ? ? This field contains the type(s) for this allergy/adverse reaction. The user can enter the type(s) separated by commas, or the following codes: D=Drug, F=Food, O=Other. If codes are used, do not use commas to separate multiple codes. Examples of valid entries are: DRUG or DRUG, FOOD or DF or OTHER. GMR ALLERGIES ALLERGY TYPE: O NAME: STINKWEED// <RET> Select SYNONYM: WEED Are you adding ‘WEED’ as a new SYNONYM (the 1 ST for this GMR ALLERGIES)? Y (Yes) Select SYNONYM: <RET> 1 Drug 2 Food 3 Other Select the type(s) for this reaction: 3// <RET> Select DRUG INGREDIENT: ? Answer with DRUG INGREDIENTS You may enter a new DRUG INGREDIENTS, if you wish Enter one of the drug ingredients that make up this allergy. Answer with DRUG INGREDIENTS NAME Do you want the entire 3585 -Entry DRUG INGREDIENTS List? N (No)

Adding a Synonym to the National Allergy File Select Enter/Edit Site Configurable Files Option: 1 Edit Allergy File Select a LOCAL ALLERGY/ADVERSE REACTION: CAFFEINE NATIONAL ALLERGY CANNOT EDIT NAME FIELD OF A NATIONAL ALLERGY. Select SYNONYM: STIMULANT Are you adding ‘STIMULANT’ as a new SYNONYM (the 1 ST for this GMR ALLERGIES)? Y(Yes) Select SYNONYM: <RET> Select a Local Allergy/Adverse Reaction: <RET>

Enter/Edit Signs/Symptoms Data • Addition/editing of the site-specific allergy reactions. • Allows the site to add any data as appropriate. - The signs/symptoms list provided by ART may be inadequate or need revising.

Enter/Edit SIGNS/SYMPTOMS DATA Select Enter/Edit Site Configurable Files Option: 2 Enter/Edit Signs/Symptoms Data Select a LOCAL SIGN/SYMPTOM: HAIR LOSS NAME: HAIR LOSS// <RET> Select SYNONYM: BALD// <RET> Select a LOCAL SIGN/SYMPTOM: <RET>

Sign/Symptom List • Prints a list of entries in the Sign/Symptoms file Select Enter/Edit Site Configurable Files Option: 4 Sign/Symptoms List START WITH NAME: FIRST// <RET> DEVICE: RX 01<RET> RIGHT MARGIN: 80// <RET> SIGN/SYMPTOMS LIST FEB 2, 1996 08: 21 PAGE 1 NAME Nat'l/Local SYNONYM -------------------------------------AGITATION National AGRANULOCYTOSIS National ALOPECIA National ANAPHYLAXIS National

Allergies File List • Prints a captioned list of all entries in the GMR Allergies file START WITH NAME: FIRST// <RET> DEVICE: <RET> HOME RIGHT MARGIN: 80// <RET> GMR ALLERGIES LIST FEB 2, 1996 08: 21 PAGE 1 ---------------------------------------NAME: ADHESIVE TAPE ALLERGY TYPE: OTHER NATIONAL ALLERGY: NATIONAL ALLERGY NAME: ALCOHOL ALLERGY TYPE: DRUG, FOOD NATIONAL ALLERGY: NATIONAL ALLERGY DRUG INGREDIENT: ALCOHOL NAME: ANIMAL HAIR ALLERGY TYPE: OTHER NATIONAL ALLERGY: NATIONAL ALLERGY

Adverse Reaction Tracking User Menu • Assigned to all clerks of Adverse Reaction Tracking who are not clinicians, verifiers, or ADP coordinators. • Allows user to enter, edit and display allergy/adverse reaction data.

Enter/Edit Patient Reaction Data • allows entering and editing of patient allergies/adverse reactions. • enter the name of the agent that caused the reaction • whether the reaction was observed during the patient’s stay/visit at the facility • any signs/symptoms associated with the reaction • date and time the sign/symptom occurred • type of reaction (i. e. , mechanism), • any appropriate comments concerning the entry, • whether the patient’s chart is marked for this reaction.

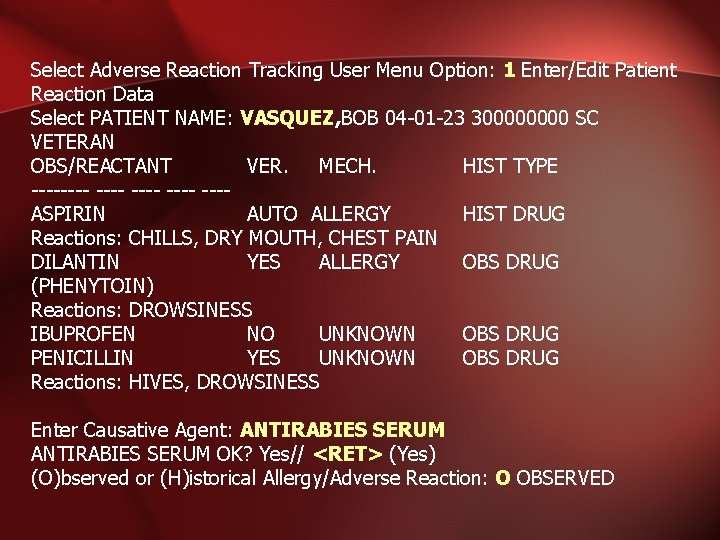
Select Adverse Reaction Tracking User Menu Option: 1 Enter/Edit Patient Reaction Data Select PATIENT NAME: VASQUEZ, BOB 04 -01 -23 30000 SC VETERAN OBS/REACTANT VER. MECH. HIST TYPE ---- ---- ---ASPIRIN AUTO ALLERGY HIST DRUG Reactions: CHILLS, DRY MOUTH, CHEST PAIN DILANTIN YES ALLERGY OBS DRUG (PHENYTOIN) Reactions: DROWSINESS IBUPROFEN NO UNKNOWN OBS DRUG PENICILLIN YES UNKNOWN OBS DRUG Reactions: HIVES, DROWSINESS Enter Causative Agent: ANTIRABIES SERUM OK? Yes// <RET> (Yes) (O)bserved or (H)istorical Allergy/Adverse Reaction: O OBSERVED

Select date reaction was OBSERVED (Time Optional): NOW (FEB 29, 1996@14: 01) Are you adding 'FEB 29, 1996@14: 01' as a new ADVERSE REACTION REPORTING? Y (Yes) No signs/symptoms have been specified. Please add some now. The following are the top ten most common signs/symptoms: 1. CONFUSION 7. HIVES 2. ITCHING, WATERING EYES 8. DRY MOUTH 3. HYPOTENSION 9. CHILLS 4. DROWSINESS 10. RASH 5. CHEST PAIN 11. OTHER SIGN/SYMPTOM 6. DIARRHEA Enter from the list above : 5 Date(Time Optional) of appearance of Sign/Symptom(s): Feb 29, 1996@14: 01//

The following is the list of reported signs/symptoms for this reaction: Signs/Symptoms Date Observed 1 CHEST PAIN Feb 29, 1996@14: 01 Select Action (A)DD, (D)ELETE OR <RET>: <RET> COMMENTS: 1>severe chest pains and breathing trouble EDIT Option: Enter another Causative Agent? YES// N NO Causative Agent Data edited this Session: ADVERSE REACTION ANTIRABIES SERUM Reactions: CHEST PAIN OBSERVED ORIGINATOR COMMENTS: Date: Feb 29, 1996@14: 01: 21 User: MEAD, ANN Title: NURSE severe chest pains and breathing trouble Is this correct? NO// Y YES This session you have CHOSEN: ANTIRABIES SERUM Have the Chart(s) been marked for this CAUSATIVE AGENT? Y (Yes) Select PATIENT NAME: <RET>

Active Listing of Patient Reactions • Gives a brief listing of the active (i. e. , data that is signed off and not entered in error) allergy/adverse reaction data for a selected patient. • If the patient has no known reactions, the body of the report will display that the patient has no known allergies. If the patient was never asked if he/she has any allergy/adverse reactions, the body of the report will display a message stating that there are no reactions on file.

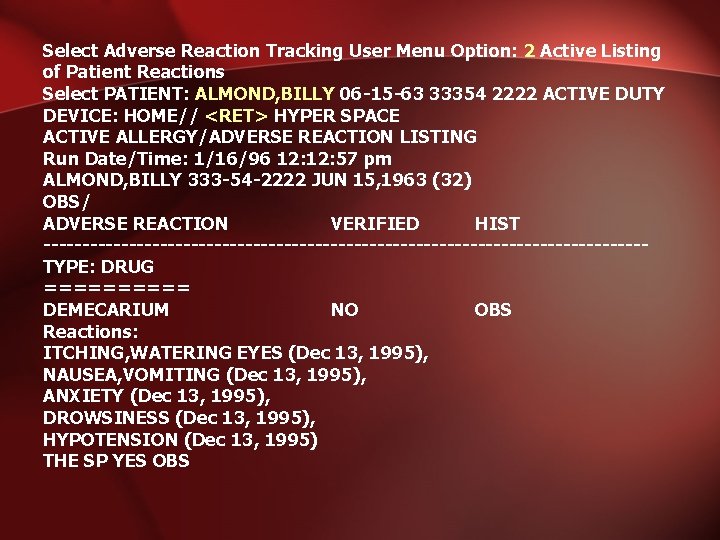
Select Adverse Reaction Tracking User Menu Option: 2 Active Listing of Patient Reactions Select PATIENT: ALMOND, BILLY 06 -15 -63 33354 2222 ACTIVE DUTY DEVICE: HOME// <RET> HYPER SPACE ACTIVE ALLERGY/ADVERSE REACTION LISTING Run Date/Time: 1/16/96 12: 57 pm ALMOND, BILLY 333 -54 -2222 JUN 15, 1963 (32) OBS/ ADVERSE REACTION VERIFIED HIST ---------------------------------------TYPE: DRUG ===== DEMECARIUM NO OBS Reactions: ITCHING, WATERING EYES (Dec 13, 1995), NAUSEA, VOMITING (Dec 13, 1995), ANXIETY (Dec 13, 1995), DROWSINESS (Dec 13, 1995), HYPOTENSION (Dec 13, 1995) THE SP YES OBS

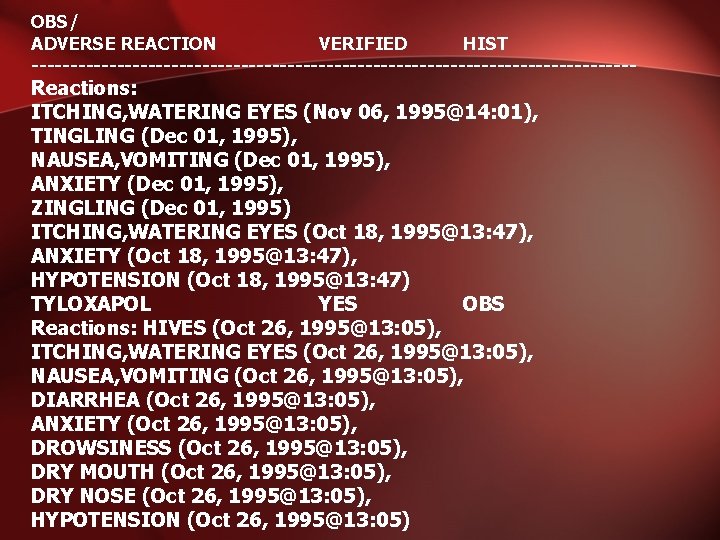
OBS/ ADVERSE REACTION VERIFIED HIST --------------------------------------- Reactions: ITCHING, WATERING EYES (Nov 06, 1995@14: 01), TINGLING (Dec 01, 1995), NAUSEA, VOMITING (Dec 01, 1995), ANXIETY (Dec 01, 1995), ZINGLING (Dec 01, 1995) ITCHING, WATERING EYES (Oct 18, 1995@13: 47), ANXIETY (Oct 18, 1995@13: 47), HYPOTENSION (Oct 18, 1995@13: 47) TYLOXAPOL YES OBS Reactions: HIVES (Oct 26, 1995@13: 05), ITCHING, WATERING EYES (Oct 26, 1995@13: 05), NAUSEA, VOMITING (Oct 26, 1995@13: 05), DIARRHEA (Oct 26, 1995@13: 05), ANXIETY (Oct 26, 1995@13: 05), DROWSINESS (Oct 26, 1995@13: 05), DRY MOUTH (Oct 26, 1995@13: 05), DRY NOSE (Oct 26, 1995@13: 05), HYPOTENSION (Oct 26, 1995@13: 05)

Adverse Reaction Tracking - Clinician Menu • assigned to all clinicians of Adverse Reaction Tracking who are not verifiers or ADP coordinators. MENU 1. Enter/Edit Patient Reaction Data 2. FDA Enter/Edit Menu. . . 3. Reports Menu. . . 4. Edit Chart and ID Band 5. Online Reference Card

Adverse Reaction Tracking - Verifier Menu • given to the verifiers of the Adverse Reaction Tracking data. • allows the user to edit/verify/print the data. MENU 1. Enter/Edit Patient Reaction Data 2. Verify Patient Reaction Data 3. Reports Menu. . . 4. Edit Chart and ID Band 5. FDA Enter/Edit Menu. . . 6. Online Reference Card

Enter/Edit Patient Reaction Data • Enter/Edit Patient Reaction Data – enter and edit patient allergies/adverse reactions – Data Fields: Select a Causative Agent, Whether was an Observed vs. Historical Reaction, Signs/Symptoms, Mechanism, Comments, FDA Data, Verification of Data, Generating Progress Notes, Mark Patient Chart and ID Band, Signing Off on an Entry

Example of Patients with no Reactions Select Adverse Reaction Tracking Verifier Menu Option: 1 Enter/Edit Patient Reaction Data Select PATIENT NAME: 109549 JONES, DENISE RENAE F 06 -11 -1961 394026110 109549 Does this patient have any known allergies or adverse reactions? : No

Patient with Historical Reaction Select PATIENT NAME: 109620 TAFOYA, NICOLE LYNN F 10 -27 -1954 551513961 109620 Does this patient have any known allergies or adverse reactions? : Yes This patient has no allergy/adverse reaction data. Enter Causative Agent: CODEINE. . . OK? Yes// <ENTER> (Yes) (O)bserved or (H)istorical Allergy/Adverse Reaction: H HISTORICAL No signs/symptoms have been specified. Please add some now. The following are the top ten most common signs/symptoms: 1. ANXIETY 7. HIVES 2. ITCHING, WATERING EYES 8. DRY MOUTH 3. DIZZINESS 9. ANAPHYLAXIS 4. DROWSINESS 10. RASH 5. NAUSEA, VOMITING 11. OTHER SIGN/SYMPTOM 6. DIARRHEA Enter from the list above : 5

Date(Time Optional) of appearance of Sign/Symptom(s): 1999 (1999) The following is the list of reported signs/symptoms for this reaction: Signs/Symptoms Date Observed -------------------------------------1 NAUSEA, VOMITING 1999 Select Action (A)DD, (D)ELETE OR <RET>: <ENTER> Choose one of the following: A - ALLERGY P - PHARMACOLOGICAL U - UNKNOWN MECHANISM: UNKNOWN// P PHARMACOLOGIC COMMENTS: No existing text Edit? NO// <ENTER> Currently you have verifier access. Would you like to verify this Causative Agent now? Yes// <ENTER> (Yes)

CAUSATIVE AGENT: CODEINE TYPE: DRUG INGREDIENTS: CODEINE VA DRUG CLASSES: This is important. Drug-Allergy alerts in v 7 and Inpatient v 5. 0 require this. OBS/HIST: HISTORICAL SIGNS/SYMPTOMS: NAUSEA, VOMITING (1999) MECHANISM: PHARMACOLOGIC Would you like to edit any of this data? Y (Yes) CAUSATIVE AGENT: CODEINE// (Uneditable) 1 Drug 2 Food 3 Other Select the type(s) for this reaction: 1// <ENTER> Select DRUG INGREDIENT: CODEINE// <ENTER> Select VA DRUG CLASS: CN 101 OPIOID ANALGESICS VA DRUG CLASS: OPIOID ANALGESICS// <ENTER> Select VA DRUG CLASS: <ENTER> (O)bserved or (H)istorical Allergy/Adverse Reaction: HISTORICAL//

The following is the list of reported signs/symptoms for this reaction: Signs/Symptoms Date Observed -------------------------------------1 NAUSEA, VOMITING 1999 Select Action (A)DD, (D)ELETE OR <RET>: <ENTER> Choose one of the following: A - ALLERGY P - PHARMACOLOGICAL U - UNKNOWN MECHANISM: PHARMACOLOGIC// <ENTER> CAUSATIVE AGENT: CODEINE TYPE: DRUG INGREDIENTS: CODEINE VA DRUG CLASSES: CN 101 - OPIOID ANALGESICS OBS/HIST: HISTORICAL SIGNS/SYMPTOMS: NAUSEA, VOMITING (1999) MECHANISM: PHARMACOLOGIC Would you like to edit any of this data? N (No)

PATIENT: TAFOYA, NICOLE LYNN CAUSATIVE AGENT: CODEINE INGREDIENTS: CODEINE VA DRUG CLASSES: OPIOID ANALGESICS ORIGINATOR: STARR, JANICE ORIGINATED: AUG 07, 2003@11: 35 SIGN OFF: NO OBS/HIST: HISTORICAL ID BAND MARKED: CHART MARKED: SIGNS/SYMPTOMS: NAUSEA, VOMITING (1999) Change status of this allergy/adverse reaction to verified? Y (Yes) Enter another Causative Agent? YES// NO This session you have CHOSEN: CODEINE Have the Chart(s) been marked for this CAUSATIVE AGENT? Y (Yes)

Patient with Observed Reaction Select PATIENT NAME: 109746 SALAZAR, JEREMIAH JAY M 07 -17 -1961 590395167 109476 Does this patient have any known allergies or adverse reactions? : Yes This patient has no allergy/adverse reaction data. Enter Causative Agent: PENICILLIN OK? Yes// <ENTER> (Yes) (O)bserved or (H)istorical Allergy/Adverse Reaction: O OBSERVED Select date reaction was OBSERVED (Time Optional): N (SEP 30, 2003@14: 35) SE P 30, 2003@14: 35 (SEP 30, 2003) Are you adding 'SEP 30, 2003' as a new ADVERSE REACTION REPORTING? No// Y (Yes) No signs/symptoms have been specified. Please add some now. The following are the top ten most common signs/symptoms: 1. ANXIETY 7. HIVES 2. ITCHING, WATERING EYES 8. DRY MOUTH 3. DIZZINESS 9. ANAPHYLAXIS 4. DROWSINESS 10. RASH 5. NAUSEA, VOMITING 11. OTHER SIGN/SYMPTOM 6. DIARRHEA Enter from the list above : 9

Date(Time Optional) of appearance of Sign/Symptom(s): Sep 30, 2003// (SEP 30, 2003) The following is the list of reported signs/symptoms for this reaction: Signs/Symptoms Date Observed -------------------------------------1 ANAPHYLAXIS Sep 30, 2003@14: 35 Select Action (A)DD, (D)ELETE OR <RET>: <ENTER> Choose one of the following: A - ALLERGY P - PHARMACOLOGICAL U - UNKNOWN MECHANISM: UNKNOWN// A ALLERGY COMMENTS: No existing text Edit? NO// <ENTER> Complete the observed reaction report? Yes// <ENTER> (Yes) DATE/TIME OF EVENT: SEP 30, 2003// <ENTER> OBSERVER: STARR, JANICE// <ENTER> JS

SEVERITY: ? MILD - Requires minimal therapeutic interventions and does not prolong length of stay. MODERATE - Requires therapeutic intervention and/or prolongs hospitalization by at least one day. SEVERE - Life threatening or contributed to death or permanently disabling; recovery takes > 15 days. Choose from: 1 MILD 2 MODERATE 3 SEVERE SEVERITY: 3 SEVERE DATE MD NOTIFIED: Sep 30, 2003// <ENTER> (SEP 30, 2003 Complete the FDA data? Yes// N (No) Currently you have verifier access. Would you like to verify this Causative Agent now? Yes// <ENTER> (Yes) CAUSATIVE AGENT: PENICILLIN TYPE: DRUG INGREDIENTS: PENICILLIN VA DRUG CLASSES: AM 050 - PENICILLINS OBS/HIST: OBSERVED SIGNS/SYMPTOMS: ANAPHYLAXIS (Sep 30, 2003) MECHANISM: ALLERGY Would you like to edit any of this data? N (No)

PATIENT: SALAZAR, JEREMIAH JAY CAUSATIVE AGENT: PENICILLIN INGREDIENTS: PENICILLIN VA DRUG CLASSES: PENICILLINS ORIGINATOR: STARR, JANICE ORIGINATED: SEP 30, 2003 SIGN OFF: NO OBS/HIST: OBSERVED ID BAND MARKED: CHART MARKED: SIGNS/SYMPTOMS: ANAPHYLAXIS (Sep 30, 2003@14: 35) Change status of this allergy/adverse reaction to verified? Y (Yes) Enter another Causative Agent? YES// NO This session you have CHOSEN: PENICILLIN Have the Chart(s) been marked for this CAUSATIVE AGENT? Y (Yes)

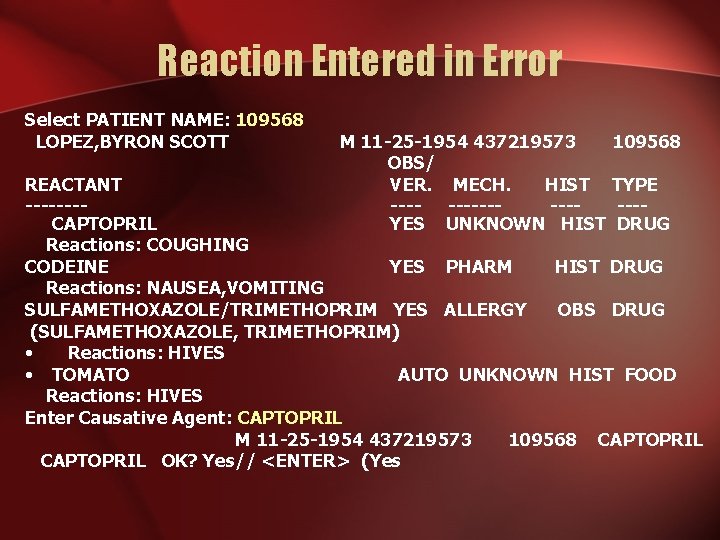
Reaction Entered in Error Select PATIENT NAME: 109568 LOPEZ, BYRON SCOTT M 11 -25 -1954 437219573 109568 OBS/ VER. MECH. HIST TYPE --------YES UNKNOWN HIST DRUG REACTANT -------CAPTOPRIL Reactions: COUGHING CODEINE YES PHARM HIST DRUG Reactions: NAUSEA, VOMITING SULFAMETHOXAZOLE/TRIMETHOPRIM YES ALLERGY OBS DRUG (SULFAMETHOXAZOLE, TRIMETHOPRIM) • Reactions: HIVES • TOMATO AUTO UNKNOWN HIST FOOD Reactions: HIVES Enter Causative Agent: CAPTOPRIL M 11 -25 -1954 437219573 109568 CAPTOPRIL OK? Yes// <ENTER> (Yes

CAPTOPRIL OK? Yes// <ENTER> (Yes) PATIENT: LOPEZ, BYRON SCOTT CAUSATIVE AGENT: CAPTOPRIL INGREDIENTS: CAPTOPRIL VA DRUG CLASSES: ACE INHIBITORS ORIGINATOR: STARR, JANICE ORIGINATED: APR 05, 2002@17: 02 SIGN OFF: YES OBS/HIST: HISTORICAL ID BAND MARKED: CHART MARKED: APR 05, 2002@17: 04: 55 SIGNS/SYMPTOMS: COUGHING (1997) MECHANISM: UNKNOWN VERIFIER: STARR, JANICE VERIFIED: APR 05, 2002@17: 04: 08 Is the reaction information correct? Yes// N (No) Mark this reaction as 'Entered-in-Error'? YES COMMENTS: No existing text Edit? NO// YES [ WRAP ]==[ INSERT ]=======< COMMENTS >===[ <PF 1>H=Help Patient denies reaction, is taking Captopril with no problems. <=======T=======T=======T>=== Hit the <F 1> key (or <PF 1> on some dumb terminals) followed by the <E> key to save the information and exit the text field. Enter another Causative Agent? YES// NO

Verify Patient Reaction Data • allows designated verifiers to verify the correctness of data entered by the clinical users. • may select a single patient’s data to verify or a list or range (e. g. , 1, 3, 7 or 1 -10) of patients to verify. • may select to view/verifydrug reactions only, non-drug reactions only, or drug and non-drug reactions.

Verify Patient Reaction Data (Cont. ) • answers YES to the “change status of this allergy/adverse reaction to verified” prompt, the reaction will be marked as verified. If the verifier answers NO to that prompt, the reaction is marked as entered in error. • If no hospital location is associated with the patient, the verifier will be prompted to enter a location. • A progress note is created. The verifier may electronically sign, edit or delete the progress note. The verifier may print the progress note, too.

Reports Menu - Verifier • • • • 1. Active Listing of Patient Reactions 2. Print Patient Reaction Data 3. Print an FDA report for a Patient 4. Print all FDA events within D/T range 5. Print Patient FDA Exception Data 6. Print all FDA Exceptions within a D/T range 7. List by Location of Unmarked ID Bands/Charts 8. Patient Allergies Not Signed Off 9. List by Location of Undocumented Allergies 10. List Autoverified Reaction Data 11. List by Location Not Verified Reactions 12. List by Location and Date all Sign Reactions 13. List FDA Data by Report Date

Active Listing of Patient Reactions gives a brief listing of the active (data that is signed off and not entered in error) allergy/adverse reaction data for a particular patient. the report divides the data by reaction type (e. g. , Drug) and lists the causative agent, the signs/symptoms and when they were observed or if they were historical, and whether it was verified. Print Patient Reaction Data allows the user to get a captioned data display of all of the patient's allergy/adverse reaction data. can select the types of reactions to include in the report. Any combination of types can be selected (i. e. , FOOD and DRUG) Any combination can be selected (i. e. , ACTIVE and ENTERED IN ERROR). Print an FDA Report for a Patient allows the user to print an individual FDA report for a patient. produces a listing of allergy/adverse reactions that are awaiting sign off by the person entering the data into the system. Print all FDA Events Within D/T Range prints all the FDA reports over a given date range, entered by the user. may choose to print Complete FDA Adverse Event Reports or an abbreviated listing of reports.

List by Location of Unmarked ID Bands/Charts -produces a list of all patients by ward/clinic who have not had their chart or ID bands marked. -report functions like the List of Patients Not Asked About Allergies option Patient Allergies Not Signed Off -prints allergy/adverse reactions for patients that have not been signed off (completed) by the user entering data. -Users who have the GMRA-ALLERGY VERIFY key will see all reactions that are not signed off. Users who do not have that key will see just the entries that they created. List by Location of Undocumented Allergies -report is used to list all patients in the patient database who have never been asked if they have any known allergies. List Autoverified Reaction Data -lists autoverified reaction data by date/time range, location and mechanism. List by Location Not Verified Reactions -prints a list of patient reactions that have not been verified. -data is sorted by hospital location, patient and reaction. -report can be scheduled to automatically run at a regular interval (e. g. , daily) option name to schedule this report to automatically run is: GMRA TASK A/AR NV

List by Location of Undocumented Allergies -report is used to list all patients in the patient database who have never been asked if they have any known allergies. List Autoverified Reaction Data -lists autoverified reaction data by date/time range, location and mechanism. List by Location Not Verified Reactions -prints a list of patient reactions that have not been verified. -data is sorted by hospital location, patient and reaction. -report can be scheduled to automatically run at a regular interval (e. g. , daily) option name to schedule this report to automatically run is: GMRA TASK A/AR NV. List by Location and Date all Signed Reactions -prints a list of all patient reactions that have been signed off (completed) for a user supplied date range. -data is sorted by location and date range. Edit Chart and ID Band -allows the user to enter if the patient ID band or the chart has been marked. -used by the personnel charged with the responsibility of making sure that the patient's paper chart has been marked to indicate that an allergy/adverse reaction is present.

FDA Enter/Edit Menu - Verifier • given to people responsible for the FDA portion of Adverse Reaction Tracking as designated by the site. • allows the user to edit the FDA data. MENU 1. Enter/Edit FDA Report Data 2. Enter/Edit P&T Committee Data

Enter/Edit FDA Report Data • allows users to enter and edit FDA related data concerning an adverse reaction. • Five sections to the FDA Report 1. Fields for Reaction Information 2. Suspect Drug(s) Information 3. Concomitant Drugs and History 4. Manufacturer Information 5. Initial Reporter

Enter/Edit P&T Committee Data • allows the user to edit P&T data. • allows for the evaluation of a suspected Drug Reaction (ADR) by a qualified individual (e. g. , clinical pharmacist, clinical pharmacologist), other than the attending physician. • can also track a report to see if it has been sent to the FDA or manufacturer.

P&T Committee Menu • should be given to the P&T Committee members • allows the user to edit P&T data and print FDA data. • allows for the evaluation of a suspected ADR by a qualified individual (e. g. , clinical pharmacist, clinical pharmacologist) other than the attending physician MENU 1. Enter/Edit P&T Committee Data 2. Enter/Edit FDA Report Data 3. Reports Menu

Reports Menu - P&T Committee • • • 1. Print an FDA Report for a Patient 2. Print all FDA Events within D/T range 3. Print Patient FDA Exception Data 4. Print all FDA Exceptions within a D/T range 5. Patient Allergies Not Signed Off 6. Print Patient Reaction Data 7. Active Listing of Patient Reactions 8. List by Location of Undocumented Allergies 9. List Autoverified Reaction Data 10. List by Location Not Verified Reactions

Reports Menu - P&T (cont) • • • 11. List by Location and Date all Sign Reactions 12. List FDA Data by Report Date 13. List of Fatal Reaction Over a Date Range 14. Print Summary of Outcomes 15. Frequency Distribution of Causative Agents 16. Frequency Distribution of Drug Classes 17. Total Reported Reactions Over a Date Range 18. P&T Committee ADR Outcome Report 19. P&T Committee ADR Report

List of Fatal Reaction Over a Date Range -lists all fatal adverse drug reactions over a date range Print Summary of Outcomes -prints a summary report of patient outcomes for a date range Frequency Distribution of Causative Agents -options prints a report of the frequency distribution of causative agents for a date range selected Frequency Distribution of Drug Classes -prints a report of the frequency distribution of drug classes for a date range selected Total Reported Reactions Over a Date Range -prints a report of the total number of reported reactions for a date range selected

P&T Committee ADR Outcome Report -displays a list of Adverse Drug Reactions (ADRs) over a date range and a summary of the listed outcomes for those ADRs. P&T Committee ADR Report -displays a list of Adverse Drug Reactions (ADRs) over a date range. The Sign/Symptoms, Mechanism, Severity and Comments are displayed for each ADR.

Patient Allergies Not Signed Off -prints allergy/adverse reactions for patients that have not been signed off (completed) by the user entering data. Users who have the GMRA-ALLERGY VERIFY key will see all reactions that are not signed off. List by Location of Undocumented Allergies -used to list all patients in the patient database who have never been asked if they have any known allergies. Print Patient Reaction Data -allows the user to get a captioned data display of all of the patient's allergy/adverse reaction data. Online Reference Card -provides the user with an online reference guide to the ART software
 Adverse reaction definition
Adverse reaction definition Adverse reaction definition
Adverse reaction definition Adverse reaction definition
Adverse reaction definition Adr adverse drug reaction
Adr adverse drug reaction Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Ng-html
Ng-html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Chó sói
Chó sói Tư thế worms-breton
Tư thế worms-breton Bài hát chúa yêu trần thế alleluia
Bài hát chúa yêu trần thế alleluia Kể tên các môn thể thao
Kể tên các môn thể thao Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tính độ biến thiên đông lượng
Công thức tính độ biến thiên đông lượng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Cách giải mật thư tọa độ
Cách giải mật thư tọa độ Phép trừ bù
Phép trừ bù độ dài liên kết
độ dài liên kết Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng bé xinh thế chỉ nói điều hay thôi
Cái miệng bé xinh thế chỉ nói điều hay thôi Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Biện pháp chống mỏi cơ
Biện pháp chống mỏi cơ đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Thế nào là giọng cùng tên? *
Thế nào là giọng cùng tên? * Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế điện thế nghỉ
điện thế nghỉ Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Dạng đột biến một nhiễm là
Dạng đột biến một nhiễm là Bảng số nguyên tố
Bảng số nguyên tố Tư thế ngồi viết
Tư thế ngồi viết Lời thề hippocrates
Lời thề hippocrates Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan ưu thế lai là gì
ưu thế lai là gì Hổ sinh sản vào mùa nào
Hổ sinh sản vào mùa nào Khi nào hổ con có thể sống độc lập
Khi nào hổ con có thể sống độc lập Sơ đồ cơ thể người
Sơ đồ cơ thể người Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Rate law
Rate law E1cb elimination reaction
E1cb elimination reaction Leukoerythroblastic reaction vs leukemoid reaction
Leukoerythroblastic reaction vs leukemoid reaction Bomb power
Bomb power What does sentinel event mean
What does sentinel event mean Adverse selection
Adverse selection Adverse selection
Adverse selection Adverse events in hospital
Adverse events in hospital Ir adverse event
Ir adverse event Puerperal sepsis
Puerperal sepsis Adverse selektion
Adverse selektion Effects of paracetamol
Effects of paracetamol Loop diuretics adverse effects
Loop diuretics adverse effects Analgesic mechanism
Analgesic mechanism Dpp4 inhibitor adverse effects
Dpp4 inhibitor adverse effects Adverse selection
Adverse selection Furosemide side effects
Furosemide side effects Diuretic side effects
Diuretic side effects Hayanil side effects
Hayanil side effects Adverse treatment
Adverse treatment Driving in adverse conditions
Driving in adverse conditions Driving in adverse conditions chapter 12
Driving in adverse conditions chapter 12 Adverse selection
Adverse selection