DIFFUSION IN POLYMERS CHARLES M HANSEN OUTLINE n








- Slides: 40

DIFFUSION IN POLYMERS CHARLES M. HANSEN

OUTLINE n n n n Laws of Diffusion Generalized Solutions to these Laws Concentration Dependent Coefficients Surface Condition can be significant Combine These - No Anomalies Predict Missing Data from Limited Results Control Solvent Retention

FICK’S FIRST AND SECOND LAWS Law 1: F = - D 0( c/ x) For Steady State Flux in the x Direction, and Law 2: c/ t = / x (D 0 c/ x) This is also called the Diffusion Equation

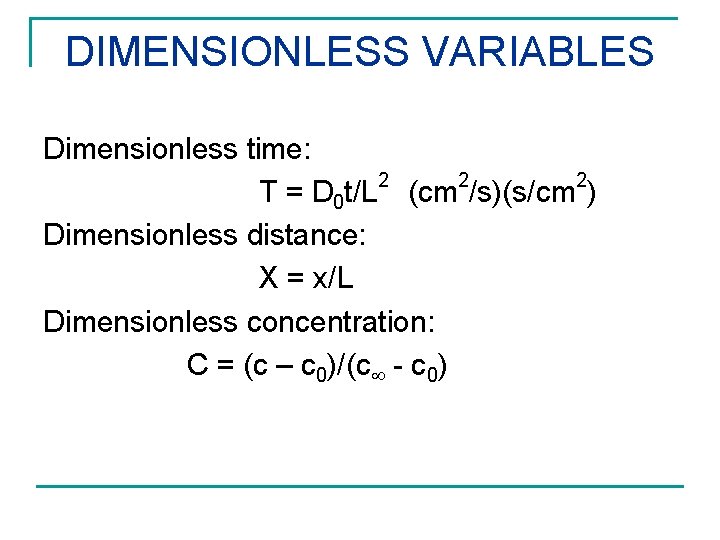
DIMENSIONLESS VARIABLES Dimensionless time: 2 2 2 T = D 0 t/L (cm /s)(s/cm ) Dimensionless distance: X = x/L Dimensionless concentration: C = (c – c 0)/(c - c 0)

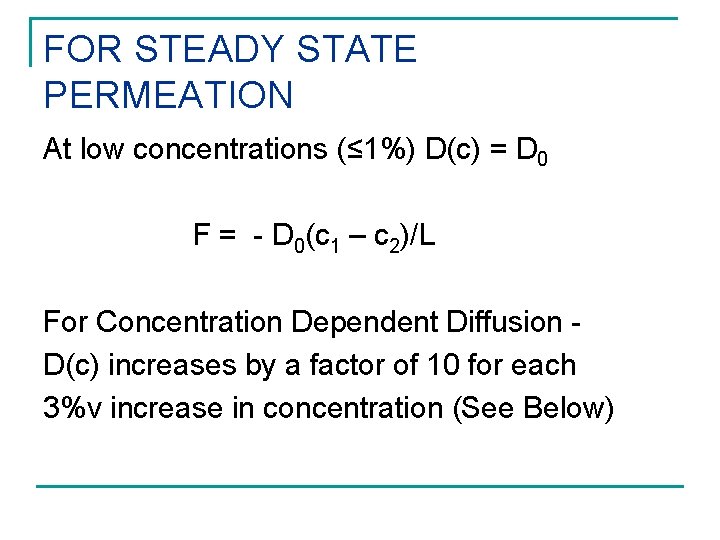
FOR STEADY STATE PERMEATION At low concentrations (≤ 1%) D(c) = D 0 F = - D 0(c 1 – c 2)/L For Concentration Dependent Diffusion D(c) increases by a factor of 10 for each 3%v increase in concentration (See Below)

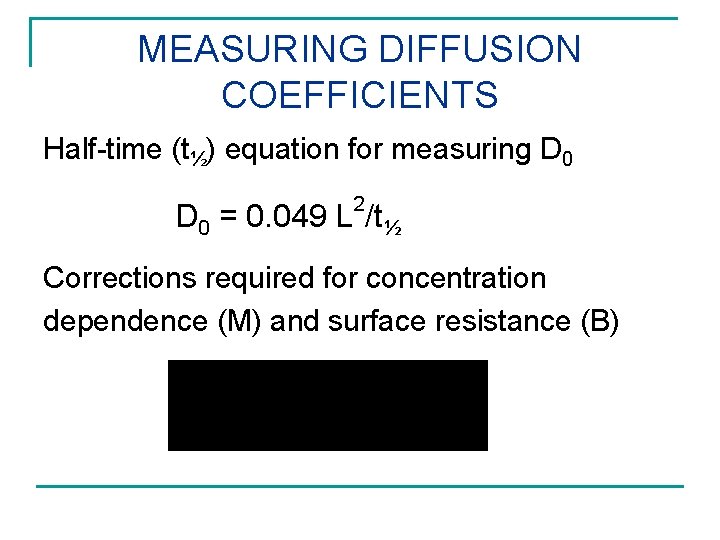
MEASURING DIFFUSION COEFFICIENTS Half-time (t½) equation for measuring D 0 2 D 0 = 0. 049 L /t½ Corrections required for concentration dependence (M) and surface resistance (B)

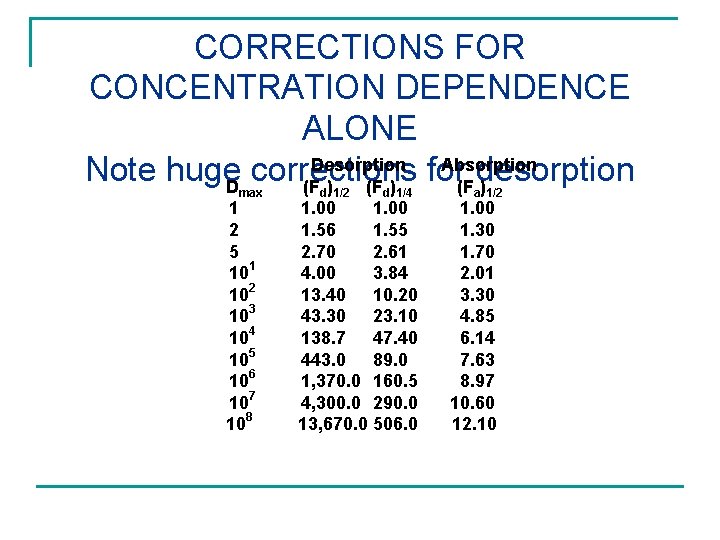
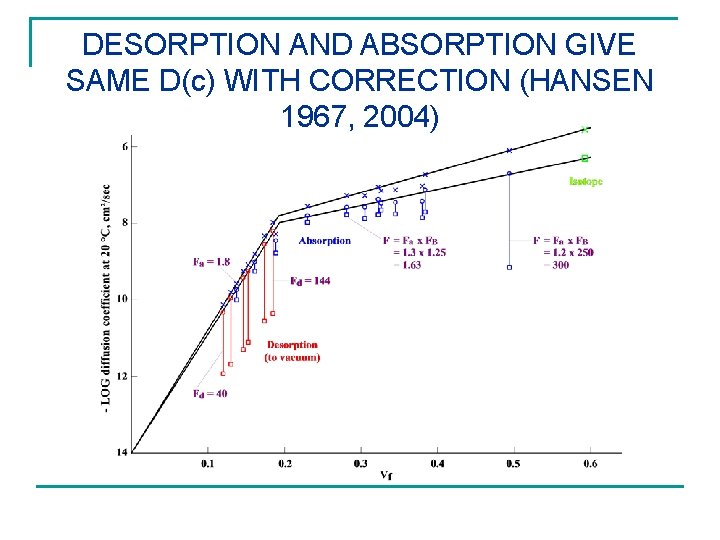
CORRECTIONS FOR CONCENTRATION DEPENDENCE ALONE Desorption Absorption Note huge. D corrections for desorption (F ) max 1 2 5 101 102 103 104 105 106 107 108 d 1/2 d 1/4 1. 00 1. 56 1. 55 2. 70 2. 61 4. 00 3. 84 13. 40 10. 20 43. 30 23. 10 138. 7 47. 40 443. 0 89. 0 1, 370. 0 160. 5 4, 300. 0 290. 0 13, 670. 0 506. 0 a 1/2 1. 00 1. 30 1. 70 2. 01 3. 30 4. 85 6. 14 7. 63 8. 97 10. 60 12. 10

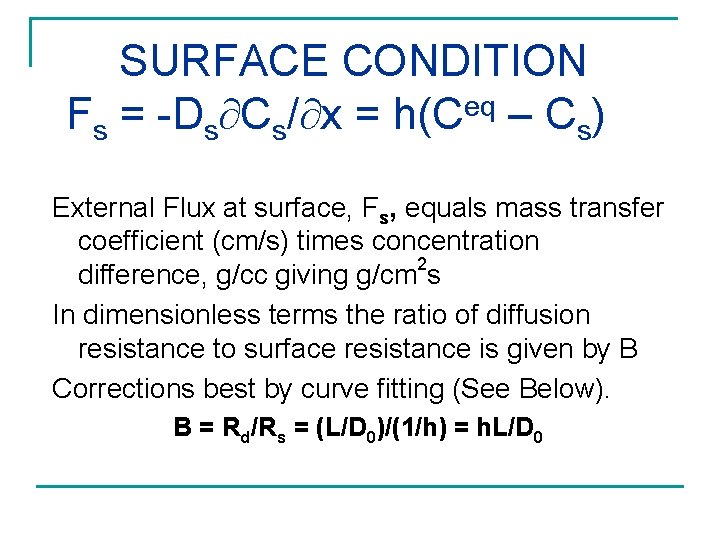
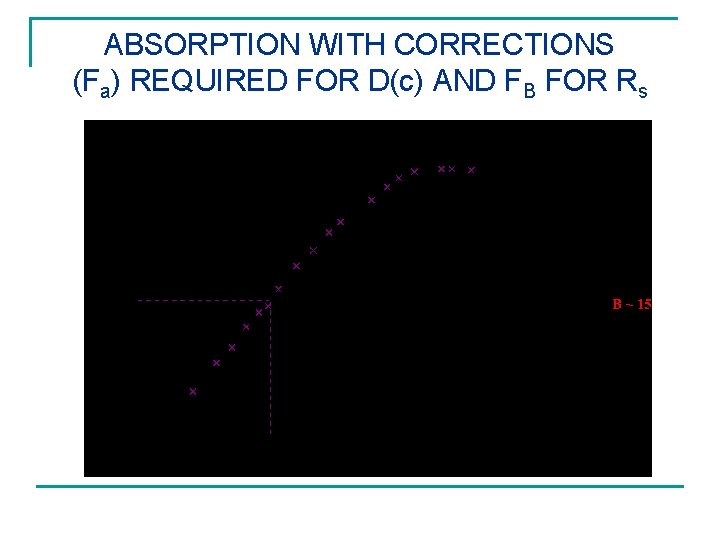
SURFACE CONDITION eq Fs = -Ds Cs/ x = h(C – Cs) External Flux at surface, Fs, equals mass transfer coefficient (cm/s) times concentration 2 difference, g/cc giving g/cm s In dimensionless terms the ratio of diffusion resistance to surface resistance is given by B Corrections best by curve fitting (See Below). B = Rd/Rs = (L/D 0)/(1/h) = h. L/D 0

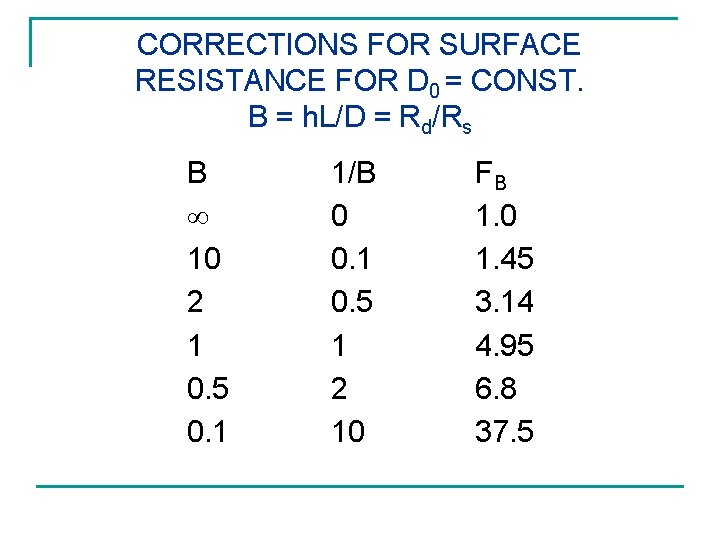
CORRECTIONS FOR SURFACE RESISTANCE FOR D 0 = CONST. B = h. L/D = Rd/Rs B 10 2 1 0. 5 0. 1 1/B 0 0. 1 0. 5 1 2 10 FB 1. 0 1. 45 3. 14 4. 95 6. 8 37. 5

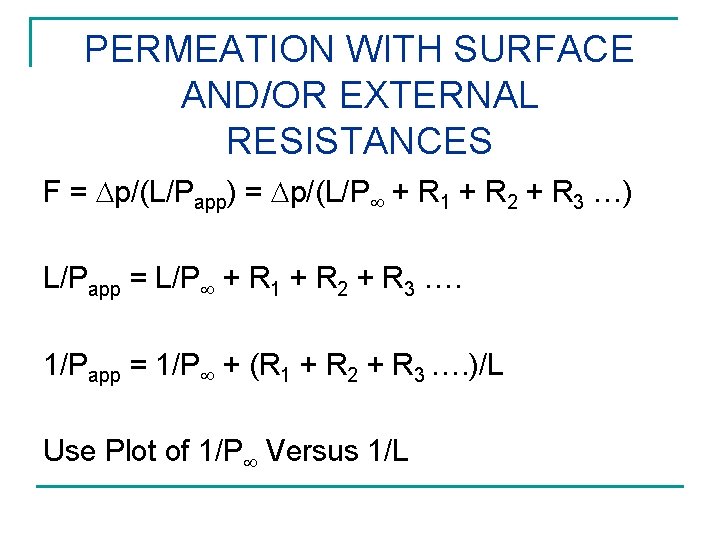
PERMEATION WITH SURFACE AND/OR EXTERNAL RESISTANCES F = p/(L/Papp) = p/(L/P + R 1 + R 2 + R 3 …) L/Papp = L/P + R 1 + R 2 + R 3 …. 1/Papp = 1/P + (R 1 + R 2 + R 3 …. )/L Use Plot of 1/P Versus 1/L

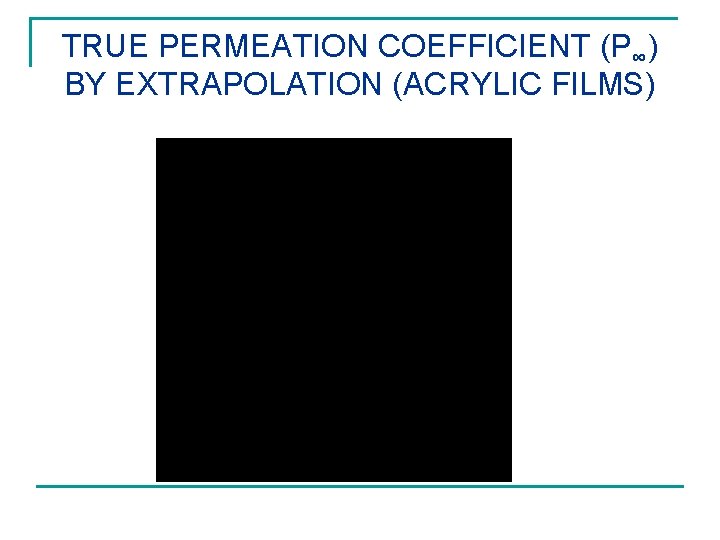
TRUE PERMEATION COEFFICIENT (P∞) BY EXTRAPOLATION (ACRYLIC FILMS)

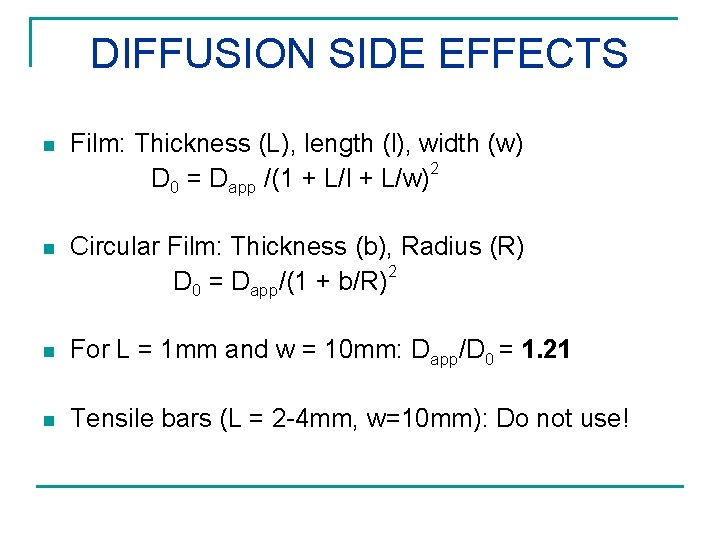
DIFFUSION SIDE EFFECTS n Film: Thickness (L), length (l), width (w) D 0 = Dapp /(1 + L/l + L/w)2 n Circular Film: Thickness (b), Radius (R) D 0 = Dapp/(1 + b/R)2 n For L = 1 mm and w = 10 mm: Dapp/D 0 = 1. 21 n Tensile bars (L = 2 -4 mm, w=10 mm): Do not use!

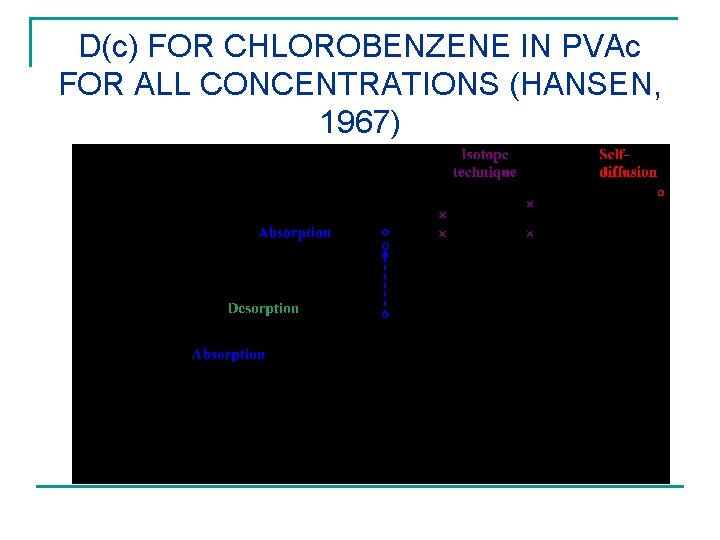
UNIQUE DATA USED IN FOLLOWING n The system chlorobenzene in poly(vinyl acetate) has been studied extensively with all relevant data reported in my thesis and subsequent journal articles. See the next slides. Absorption data from one equilibrium to another, desorption data from different equilibria to vacuum, and film drying (years) all present a unified and coherent picture of solvent diffusion in polymers, if one accounts for concentration dependence and significant surface effects when present.

D(c) FOR CHLOROBENZENE IN PVAc FOR ALL CONCENTRATIONS (HANSEN, 1967)

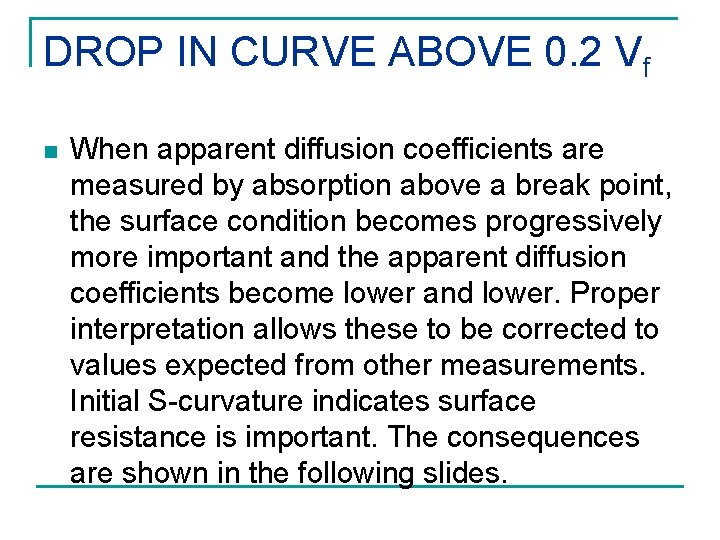
DROP IN CURVE ABOVE 0. 2 Vf n When apparent diffusion coefficients are measured by absorption above a break point, the surface condition becomes progressively more important and the apparent diffusion coefficients become lower and lower. Proper interpretation allows these to be corrected to values expected from other measurements. Initial S-curvature indicates surface resistance is important. The consequences are shown in the following slides.

DESORPTION AND ABSORPTION GIVE SAME D(c) WITH CORRECTION (HANSEN 1967, 2004)

ABSORPTION WITH CORRECTIONS (Fa) REQUIRED FOR D(c) AND FB FOR Rs

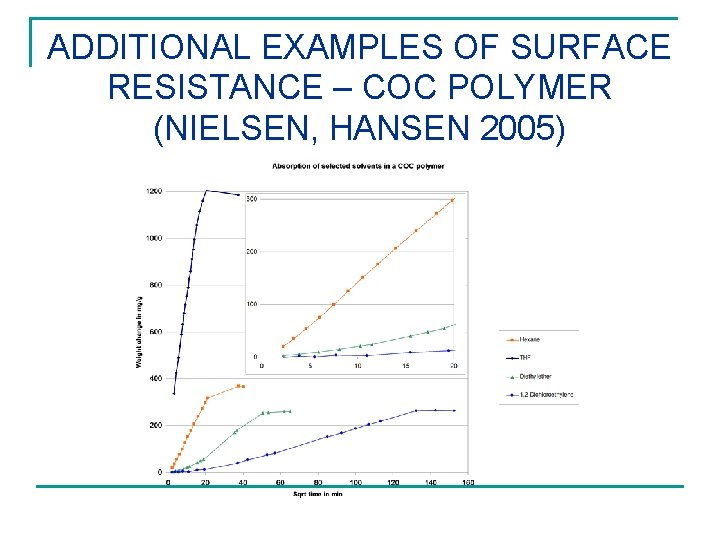
ADDITIONAL EXAMPLES OF SURFACE RESISTANCE – COC POLYMER (NIELSEN, HANSEN 2005)

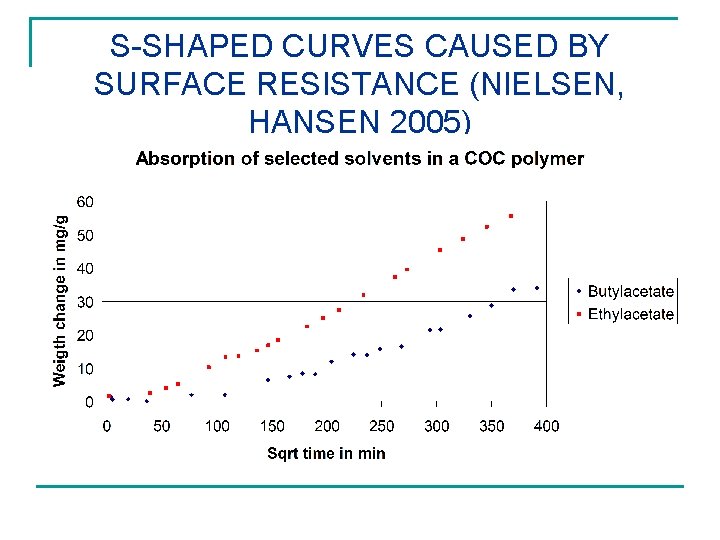
S-SHAPED CURVES CAUSED BY SURFACE RESISTANCE (NIELSEN, HANSEN 2005)

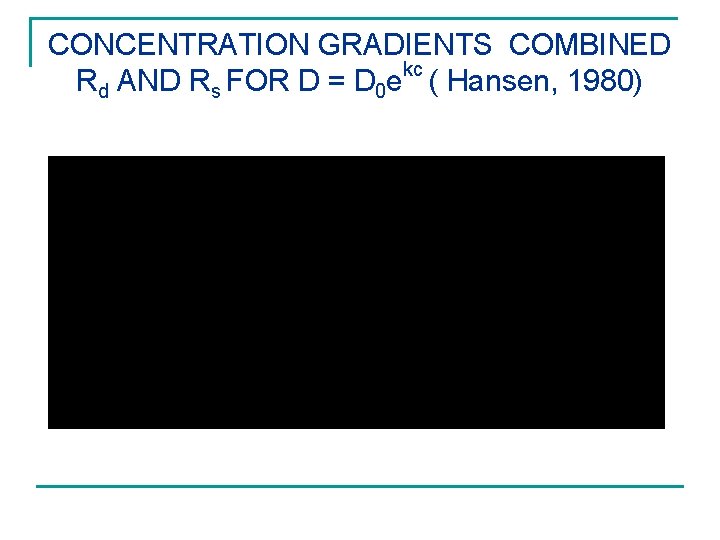
ABSORPTION – CASE II AND SUPER CASE II CAUSED BY COMBINED ( Hansen, 1980) kc Rd and Rs for D = D 0 e

CONCENTRATION GRADIENTS COMBINED kc Rd AND Rs FOR D = D 0 e ( Hansen, 1980)

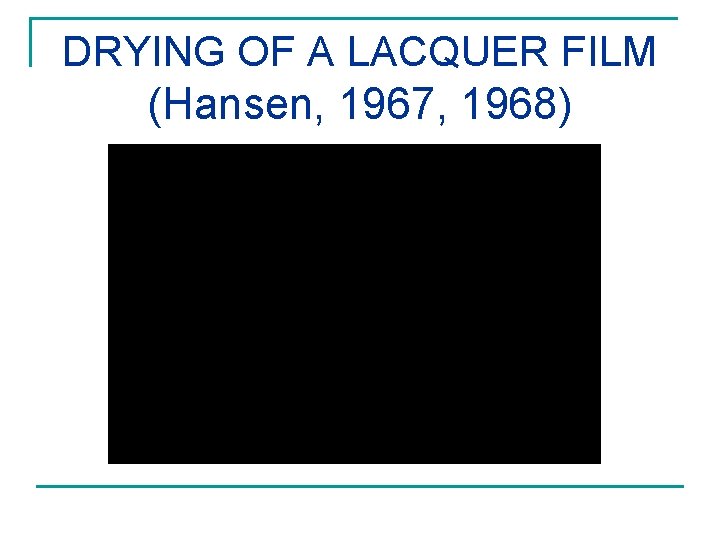
DRYING OF A LACQUER FILM (Hansen, 1967, 1968)

RELATIVE SOLVENT RETENTION (HANSEN, 1967) MOLECULAR SIZE AND SHAPE

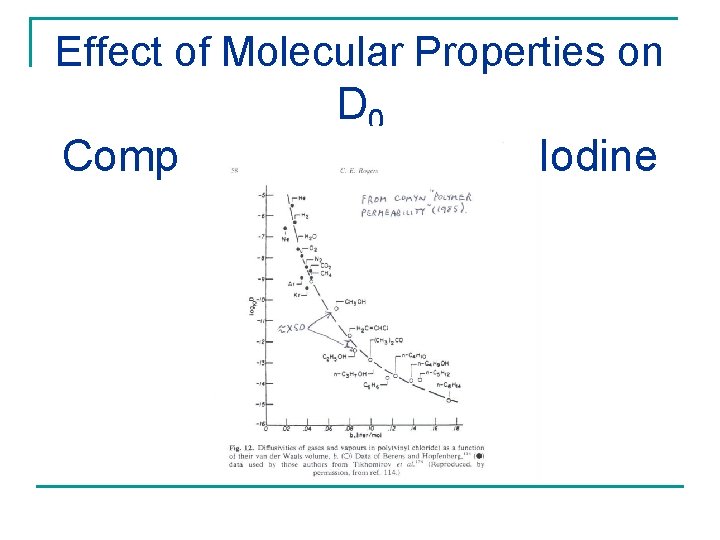
Effect of Molecular Properties on D 0 Compare Methanol with Iodine

GENERAL ARTICLE APPEARS EXPLAINING “ANOMALIES” USING DIFFUSION EQUATION n Much of the above has been presented in Chapter 16 of Second Edition of Hansen Solubility Parameters: A User’s Handbook, CRC Press, 2007. The following article: Hansen CM. The significance of the surface condition in solutions to the diffusion equation: explaining "anomalous" sigmoidal, Case II, and Super Case II absorption behavior. Eur Polym J 2010; 46; 651662 contains the next slides.

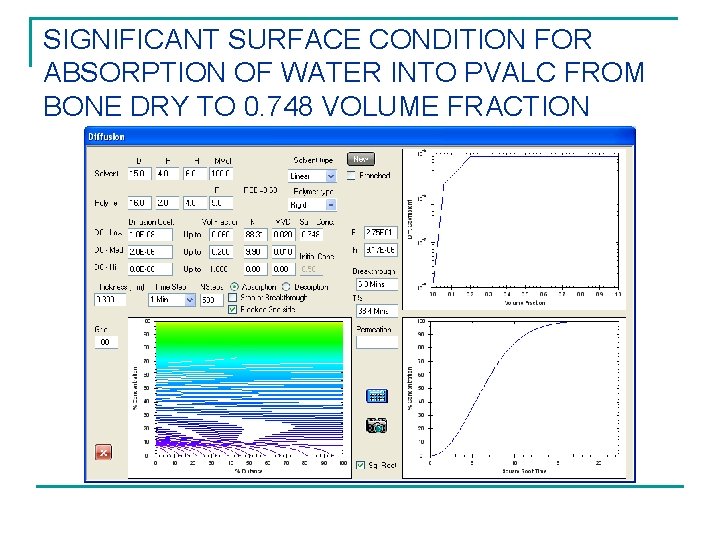
SIGNIFICANT SURFACE CONDITION FOR ABSORPTION OF WATER INTO PVALC FROM BONE DRY TO 0. 748 VOLUME FRACTION

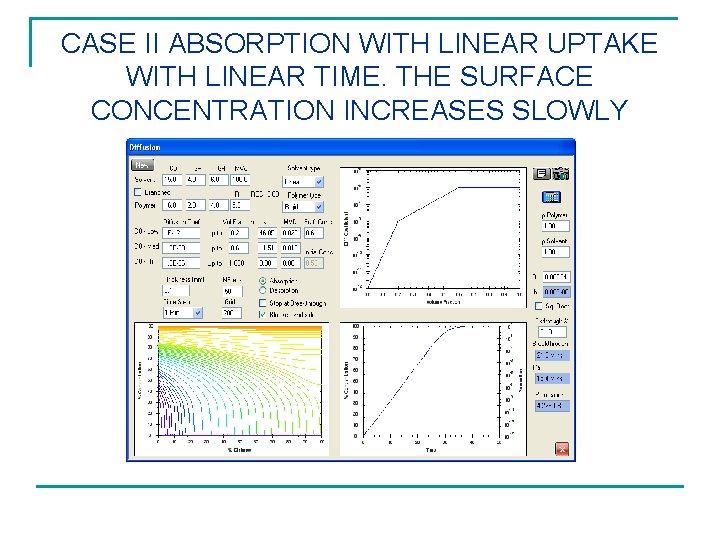
CASE II ABSORPTION WITH LINEAR UPTAKE WITH LINEAR TIME. THE SURFACE CONCENTRATION INCREASES SLOWLY

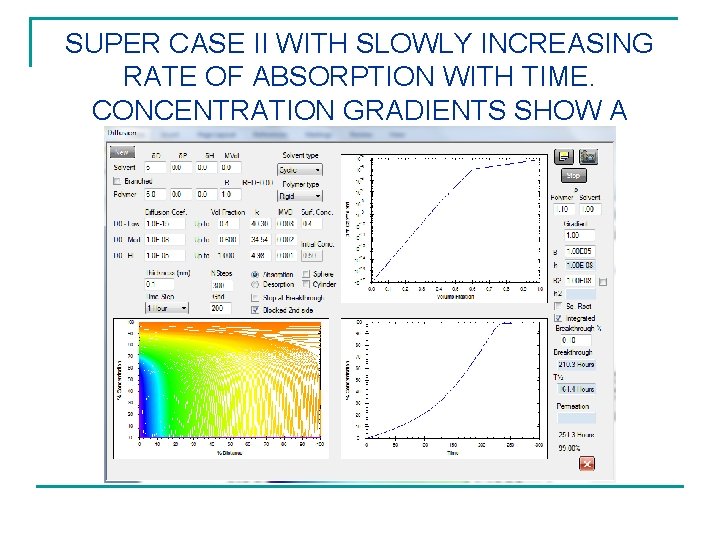
SUPER CASE II WITH SLOWLY INCREASING RATE OF ABSORPTION WITH TIME. CONCENTRATION GRADIENTS SHOW A FRONT.

HANSEN IS “EXTRANEOUS”: PETROPOULOS et. al n n Petropoulos JH Sanopoulou M Papadokostaki KG. Physically insightful modeling of non-Fickian kinetic energy regimes encountered in fundamental studies of isothermal sorption of swelling agents in polymeric media. Eur Polym J 2011; 47: 20532062. Hansen extraneous, challenges included

Hansen cannot explain these data! Next two slides do explain these data

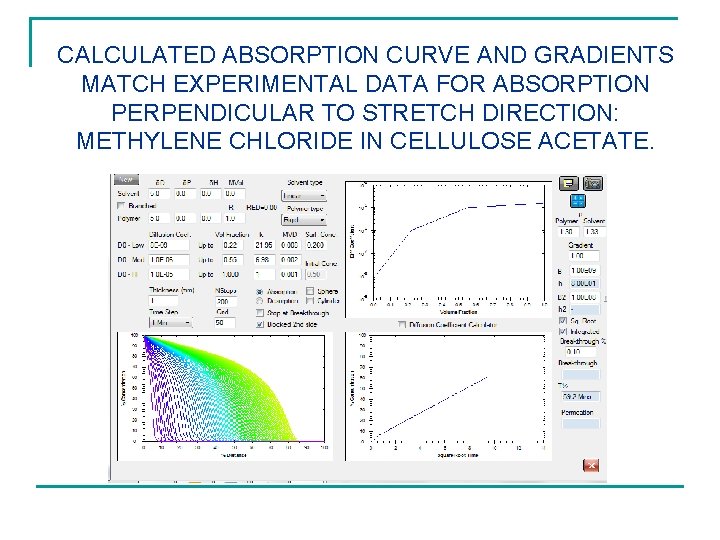
CALCULATED ABSORPTION CURVE AND GRADIENTS MATCH EXPERIMENTAL DATA FOR ABSORPTION PERPENDICULAR TO STRETCH DIRECTION: METHYLENE CHLORIDE IN CELLULOSE ACETATE.

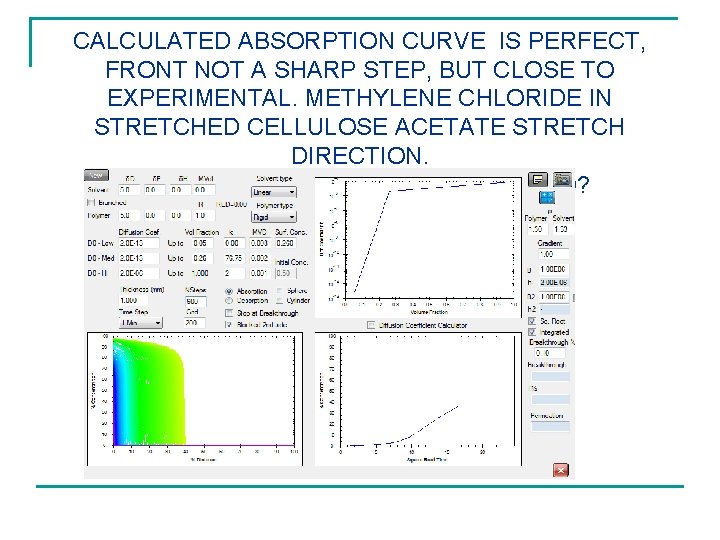
CALCULATED ABSORPTION CURVE IS PERFECT, FRONT NOT A SHARP STEP, BUT CLOSE TO EXPERIMENTAL. METHYLENE CHLORIDE IN STRETCHED CELLULOSE ACETATE STRETCH DIRECTION. ARE INITIAL CONDITIONS MAINTAINED?

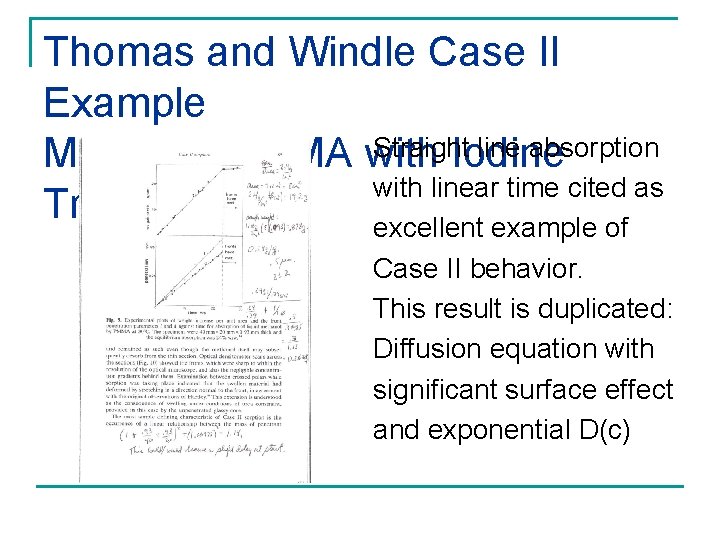
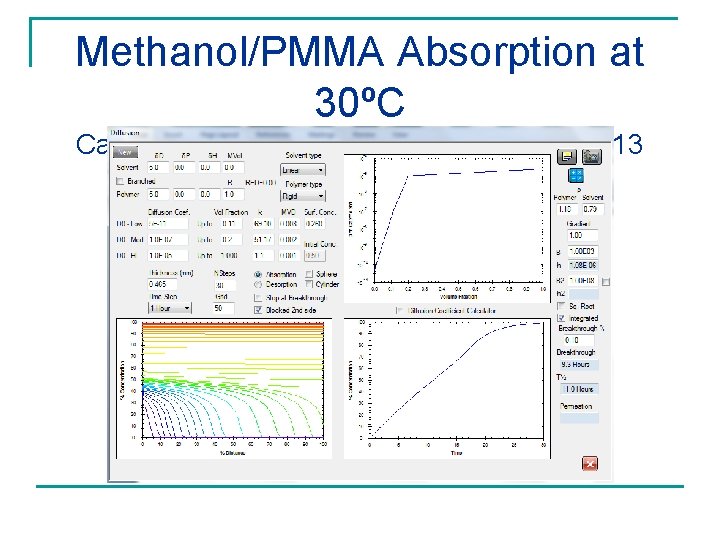
Thomas and Windle Case II Example Straight line absorption Methanol/PMMA with Iodine with linear time cited as Tracer excellent example of Case II behavior. This result is duplicated: Diffusion equation with significant surface effect and exponential D(c)

Thomas and Windle Case II Example Windle, “Case II Sorption” in Comyn, Polymer Permeability Iodine tracer lags methanol (1985) in PMMA at 30°C showing apparent step-like gradient. Methanol does not have this “advancing sharp front”. Iodine tracer far too slow as shown in the next slide. Methanol gradients become flat at longer time.

Methanol/PMMA Absorption at 30ºC Calculated Concentration Gradients Flat at 13 hours

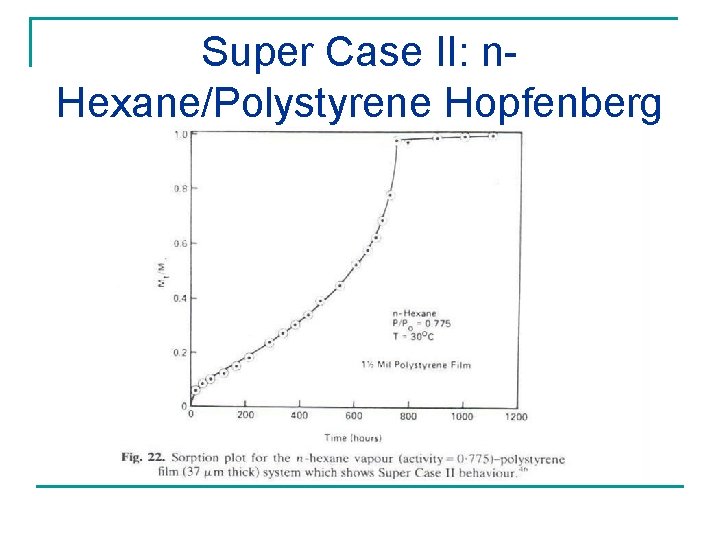
Super Case II: n. Hexane/Polystyrene Hopfenberg and Coworkers

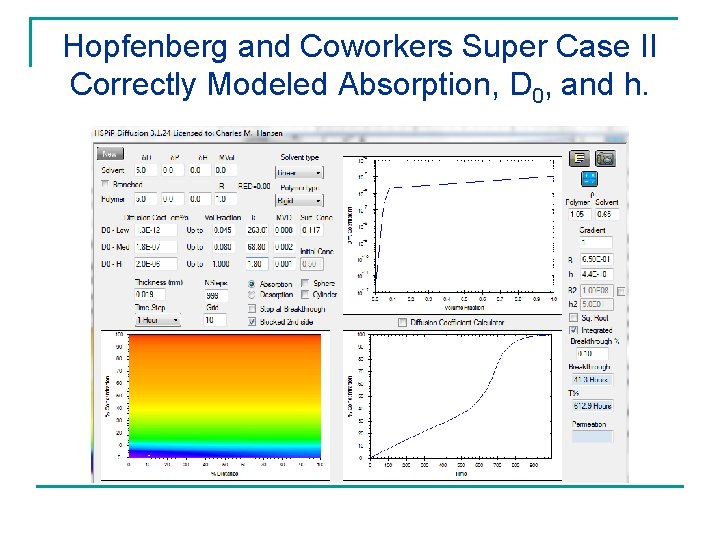
Hopfenberg and Coworkers Super Case II Correctly Modeled Absorption, D 0, and h.

CONCLUSION: STRESS RELAXATION NEED NOT BE INVOKED. Stress relaxation phenomena need not be invoked to explain the cases examined including Thomas and Windle Case II, Super Case II, and Sigmoidal examples or the studies of Petropoulos and coworkers. The diffusion equation seems to fully describe all of these studies when the a significant surface condition is included and exponential diffusion coefficients are used.

DIFFUSION IN POLYMERS SUMMARY n n n n Laws of Diffusion Generalized Solutions to these Laws Concentration Dependent Coefficients Surface Condition involved with ”Anomalies” Combine These - No Anomalies Predict Missing Data from Limited Results Estimate Behavior at Different Conditions Improved understanding

Thank you for your attention! For further contact please visit: www. hansen-solubility. com