DFT study of point and more complex defects


- Slides: 34

DFT study of point and more complex defects in bcc Fe and fcc Ni Fe- bcc Ni - fcc Dr. Dmytro KANDASKALOV D. Connétable, C. Mijoule, D. Monceau Institut Jean Lamour, UMR CNRS 7198, Université de Lorraine, 54011 Nancy, France 1

Defects in materials Real materials often have different types of defects These defects are: Ø present in thermal equilibrium Ø generated during material processing Ø generated during its functional life Defects Point: Øvacancies Øimpurities and Complex: Ødislocations Øgrain boundaries Øcavities … These defects can influence the different properties of the material such as: Ø plasticity Ø diffusion Ø electric conductivity The theoretical simulations are a complementary tool for the experimental study 2

Research Topics Ni (fcc) Ø Study the Sulfur in the solid solution and on the surfaces: solubility, segregation and adsorption Fe (bcc) Ø Formation of small cavities V 1 -V 15 : stability in bulk and surfaces, diffusion DFT PAW « Projected Augmented Wave » pseudopotential Ni: 10 valence electrons: 4 s 2 3 d 8 Fe: 8 valence electrons: 4 s 2 3 d 6 Non local exchange-correlation functional – GGA-PW 91 Periodic approach is used 3

Part I Study of sulfur in the γ-Ni (fcc) 1. Motivations 2. Sulfur in the bulk: γ Ni - fcc • Insertion vs substitution • Sulfur complexes Sm in Vn vacancies • Diffusion and migration 3. Sulfur on the Ni(100) and Ni(111) surfaces: • adsorption • segregation 4. Conclusion 4

Motivations S has a harmful role on the properties of the transition metals (i. e. Ni). S segregates at grain boundaries and adsorbs at the surface Ø Reduction of the catalytic activity of the materials* Ø Embrittlement induced by segregation Ø Embrittlement of multilayer interface Ni-S is the experimental model for the fundamental understanding of the segregation** Ø It is necessary to obtain the precise kinetic and thermodynamic data of S in the Ni such as: • Solubility • Migration • Segregation/adsorption energies on the interfaces * R. Brill, S. Tauster, Ber. Bunsen. -Ges. Phys. Chem. 67 (1963) 390 ** N. Barbouth and J. Oudar, Mémoires et Etudes Sc Revue de Métallurgie 12 (1989) 777 5

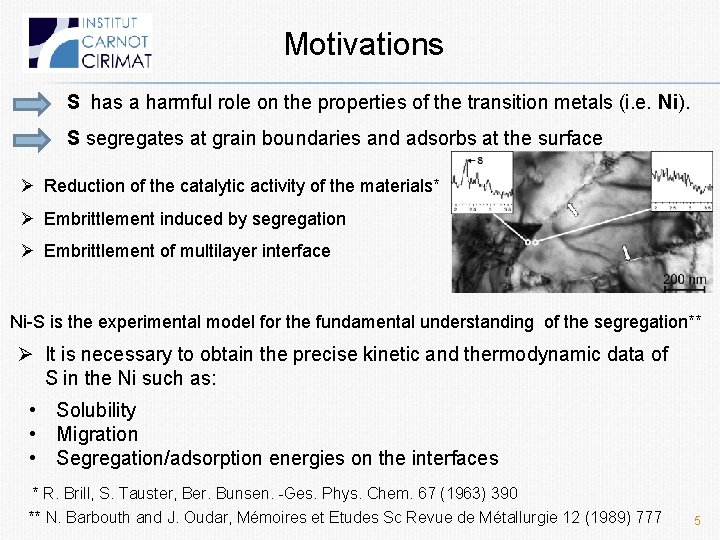
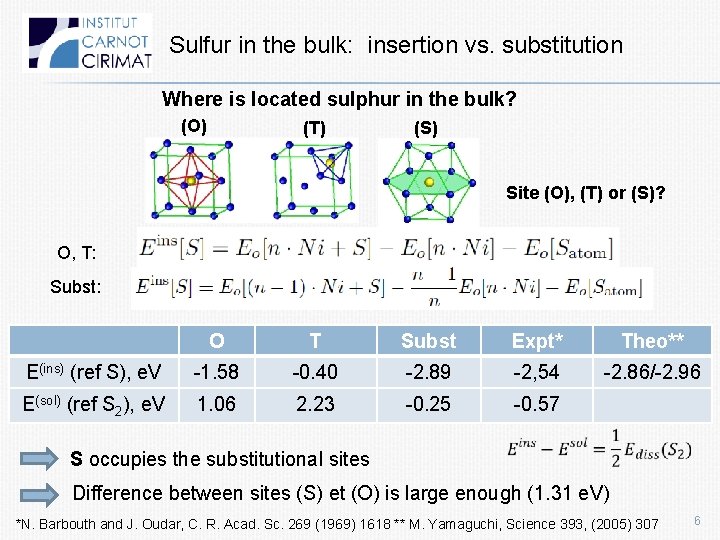
Sulfur in the bulk: insertion vs. substitution Where is located sulphur in the bulk? (O) (T) (S) Site (O), (T) or (S)? O, T: Subst: O T Subst Expt* Theo** E(ins) (ref S), e. V -1. 58 -0. 40 -2. 89 -2, 54 -2. 86/-2. 96 E(sol) (ref S 2), e. V 1. 06 2. 23 -0. 25 -0. 57 S occupies the substitutional sites Difference between sites (S) et (O) is large enough (1. 31 e. V) *N. Barbouth and J. Oudar, C. R. Acad. Sc. 269 (1969) 1618 ** M. Yamaguchi, Science 393, (2005) 307 6

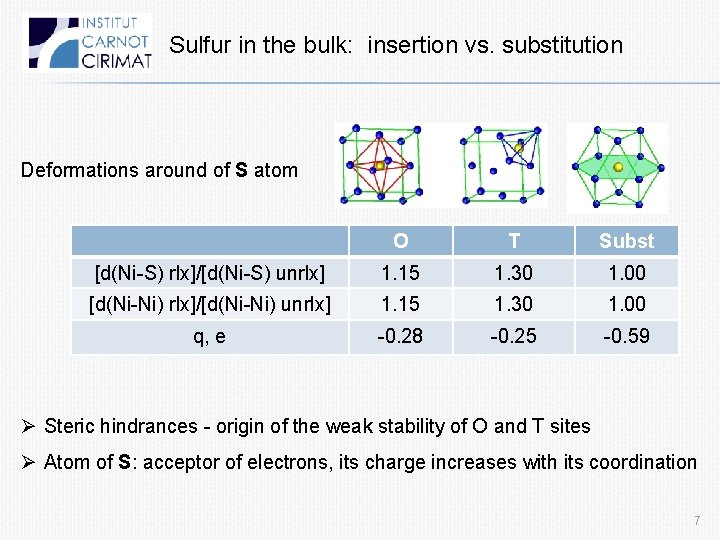
Sulfur in the bulk: insertion vs. substitution Deformations around of S atom O T Subst [d(Ni-S) rlx]/[d(Ni-S) unrlx] 1. 15 1. 30 1. 00 [d(Ni-Ni) rlx]/[d(Ni-Ni) unrlx] 1. 15 1. 30 1. 00 q, e -0. 28 -0. 25 -0. 59 Ø Steric hindrances - origin of the weak stability of O and T sites Ø Atom of S: acceptor of electrons, its charge increases with its coordination 7

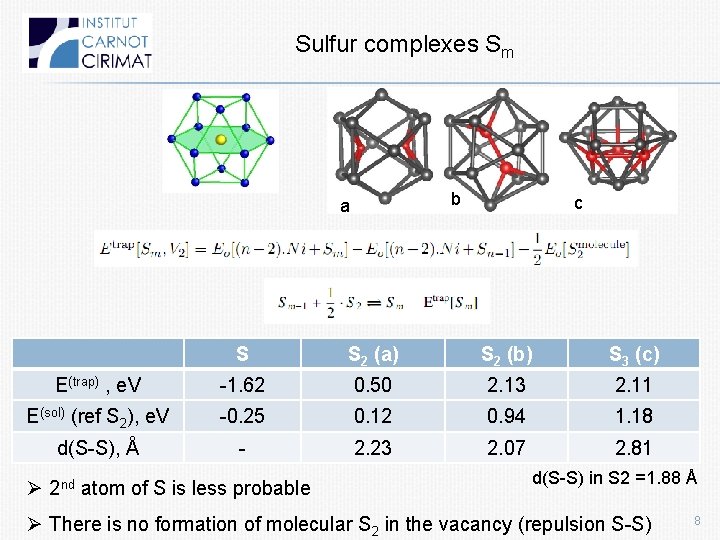
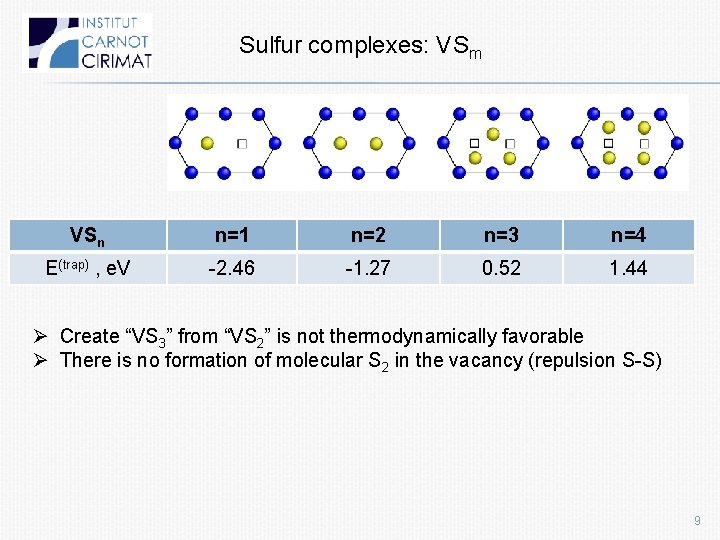
Sulfur complexes Sm b a c S S 2 (a) S 2 (b) S 3 (c) E(trap) , e. V -1. 62 0. 50 2. 13 2. 11 E(sol) (ref S 2), e. V -0. 25 0. 12 0. 94 1. 18 d(S-S), Å - 2. 23 2. 07 2. 81 Ø 2 nd atom of S is less probable d(S-S) in S 2 =1. 88 Å Ø There is no formation of molecular S 2 in the vacancy (repulsion S-S) 8

Sulfur complexes: VSm VSn n=1 n=2 n=3 n=4 E(trap) , e. V -2. 46 -1. 27 0. 52 1. 44 Ø Create “VS 3” from “VS 2” is not thermodynamically favorable Ø There is no formation of molecular S 2 in the vacancy (repulsion S-S) 9

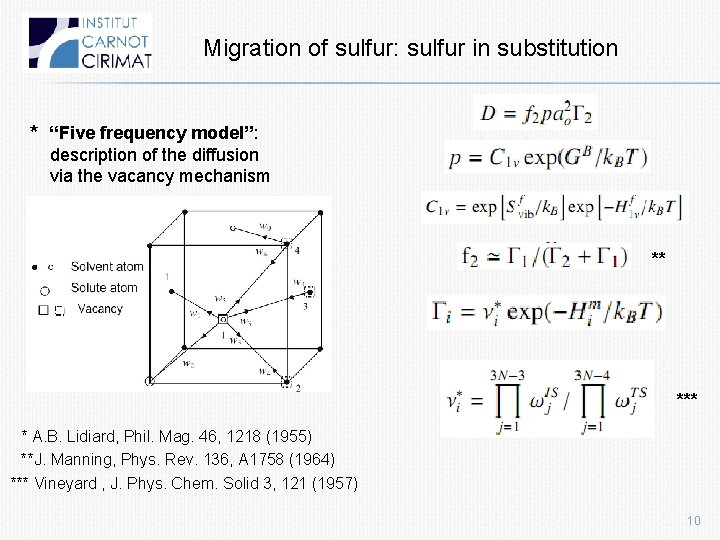
Migration of sulfur: sulfur in substitution * “Five frequency model”: description of the diffusion via the vacancy mechanism ** * A. B. Lidiard, Phil. Mag. 46, 1218 (1955) **J. Manning, Phys. Rev. 136, A 1758 (1964) *** Vineyard , J. Phys. Chem. Solid 3, 121 (1957) 10

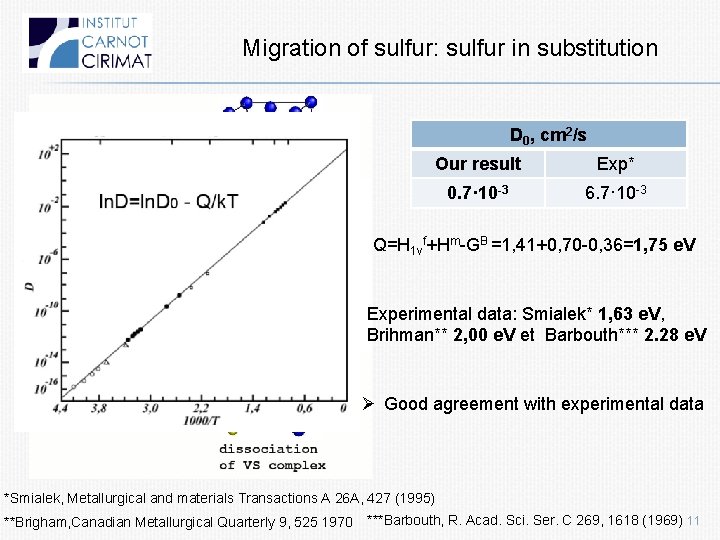
Migration of sulfur: sulfur in substitution D 0, cm 2/s Our result Exp* 0. 7· 10 -3 6. 7· 10 -3 Q=H 1 vf+Hm-GB =1, 41+0, 70 -0, 36=1, 75 e. V Experimental data: Smialek* 1, 63 e. V, Brihman** 2, 00 e. V et Barbouth*** 2. 28 e. V Ø Good agreement with experimental data *Smialek, Metallurgical and materials Transactions A 26 A, 427 (1995) **Brigham, Canadian Metallurgical Quarterly 9, 525 1970 ***Barbouth, R. Acad. Sci. Ser. C 269, 1618 (1969) 11

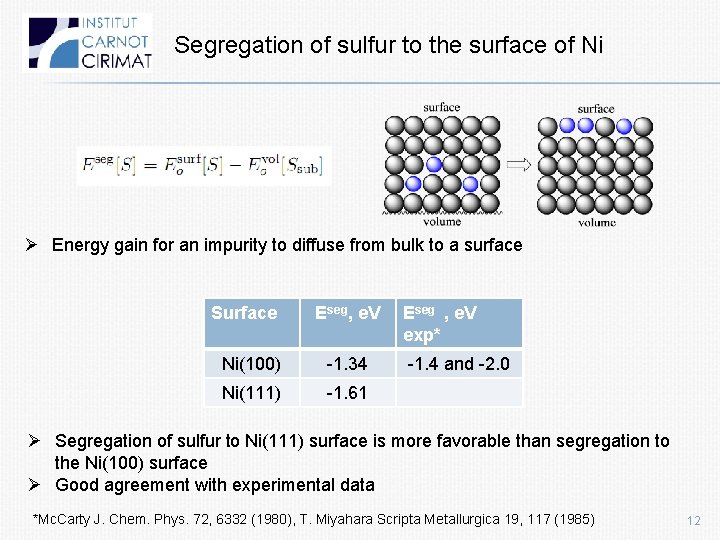
Segregation of sulfur to the surface of Ni Ø Energy gain for an impurity to diffuse from bulk to a surface Surface Eseg, e. V Ni(100) -1. 34 Ni(111) -1. 61 Eseg , e. V exp* -1. 4 and -2. 0 Ø Segregation of sulfur to Ni(111) surface is more favorable than segregation to the Ni(100) surface Ø Good agreement with experimental data *Mc. Carty J. Chem. Phys. 72, 6332 (1980), T. Miyahara Scripta Metallurgica 19, 117 (1985) 12

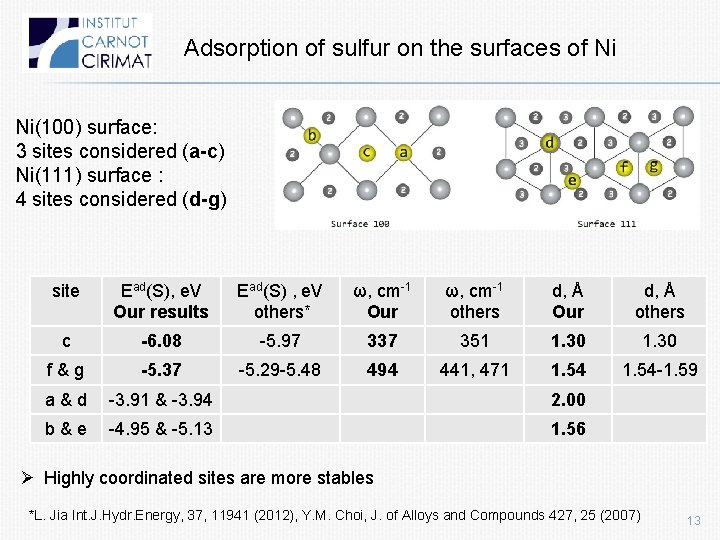
Adsorption of sulfur on the surfaces of Ni Ni(100) surface: 3 sites considered (a-c) Ni(111) surface : 4 sites considered (d-g) site Ead(S), e. V Our results Ead(S) , e. V others* ω, cm-1 Our ω, cm-1 others d, Å Our d, Å others c -6. 08 -5. 97 337 351 1. 30 f&g -5. 37 -5. 29 -5. 48 494 441, 471 1. 54 -1. 59 a&d -3. 91 & -3. 94 2. 00 b&e -4. 95 & -5. 13 1. 56 Ø Highly coordinated sites are more stables *L. Jia Int. J. Hydr. Energy, 37, 11941 (2012), Y. M. Choi, J. of Alloys and Compounds 427, 25 (2007) 13

Part I Conclusions Ø The sulfur atom takes the substitutional site in the solid solution Ø In the case of substitution, it’s difficult to form small complexes Vn. Sm Ø Vacancy mechanism of S diffusion : agreement with experimental results Ø Adsorption of S: highly coordinated sites are more stables Ø Adsorption of S: segregation of sulfur to the Ni (111) surface is more favorable 14

Part II Study of multivacancies in Fe Fe- bcc 1. Motivations 2. Monovacancy: bulk and surfaces 3. Divacancies and trivacancies 4. Middle size multivacancies Vn n=4 -15 5. Conclusion 15

Motivations Ø Iron is important in the differents domains (for example aeronautics, nuclear) Irradiation => defects, cavities Oxidation at high temperature=> injection of vacancies, formation of cavities Ø Vacancies are involved in the diffusion in the solid solution Ø Multivacancies lead to a modification of the microstructure of the material and its properties: decrease of the life-time of the material. 16

Monovacancy: formation energy Concentration of monovacancies : Formation energy of V 1 : Calculation of formation entalphy by DFT Ø Formation energy obtained: Ø The exp data: 1. 5 -2. 0 e. V, other data DFT: 1. 95 -2. 20 e. V 17

Monovacancy: diffusion Diffusion law: To calculate D two parameters should be known: Ø D 0 – coefficient of diffusion Ø Em 1 v – migration energy Schematic representation of multivacancy migration: 18

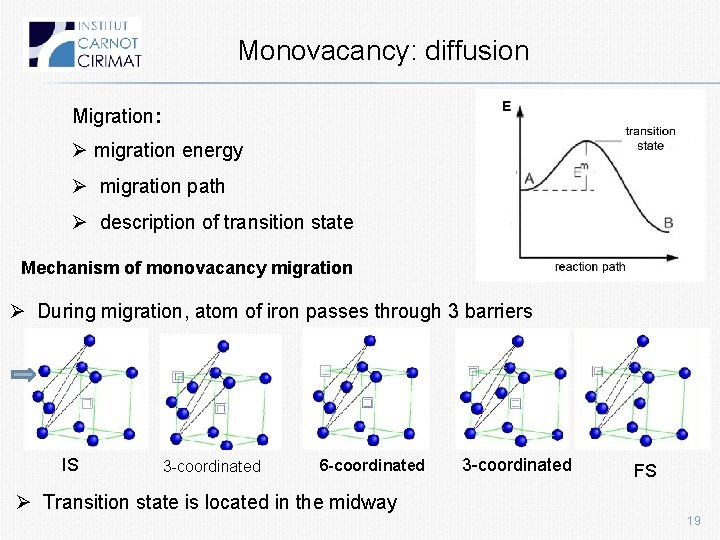
Monovacancy: diffusion Migration: Ø migration energy Ø migration path Ø description of transition state Mechanism of monovacancy migration Ø During migration, atom of iron passes through 3 barriers IS 3 -coordinated 6 -coordinated 3 -coordinated FS Ø Transition state is located in the midway 19

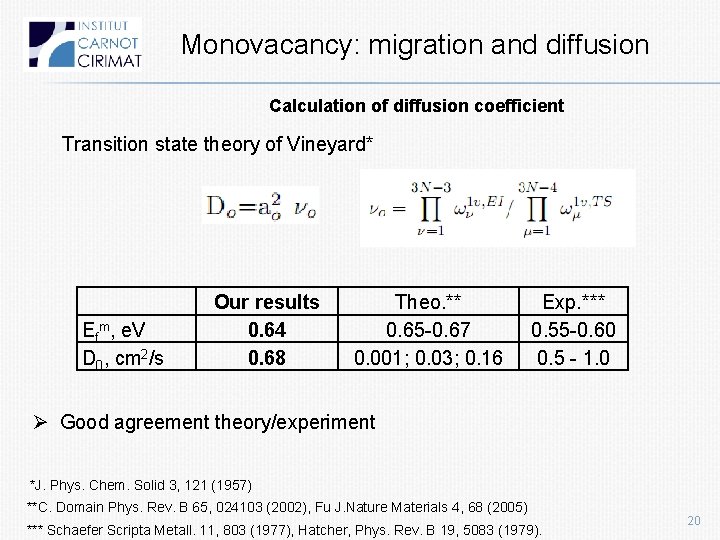
Monovacancy: migration and diffusion Calculation of diffusion coefficient Transition state theory of Vineyard* Efm, e. V D 0, cm 2/s Our results 0. 64 0. 68 Theo. ** 0. 65 -0. 67 0. 001; 0. 03; 0. 16 Exp. *** 0. 55 -0. 60 0. 5 - 1. 0 Ø Good agreement theory/experiment *J. Phys. Chem. Solid 3, 121 (1957) **C. Domain Phys. Rev. B 65, 024103 (2002), Fu J. Nature Materials 4, 68 (2005) *** Schaefer Scripta Metall. 11, 803 (1977), Hatcher, Phys. Rev. B 19, 5083 (1979). 20

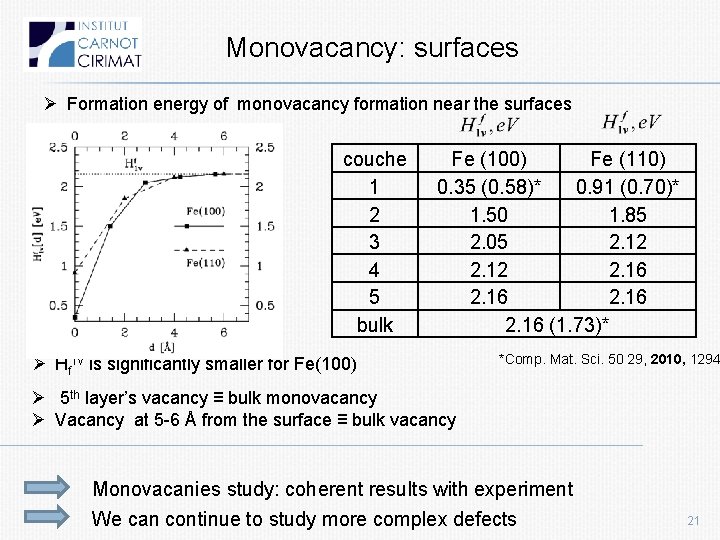
Monovacancy: surfaces Ø Formation energy of monovacancy formation near the surfaces couche 1 2 3 4 5 bulk Fe (100) Fe (110) 0. 35 (0. 58)* 0. 91 (0. 70)* 1. 50 1. 85 2. 05 2. 12 2. 16 (1. 73)* Ø Hf 1 v is significantly smaller for Fe(100) *Comp. Mat. Sci. 50 29, 2010, 1294 Ø 5 th layer’s vacancy ≡ bulk monovacancy Ø Vacancy at 5 -6 Å from the surface ≡ bulk vacancy Monovacanies study: coherent results with experiment We can continue to study more complex defects 21

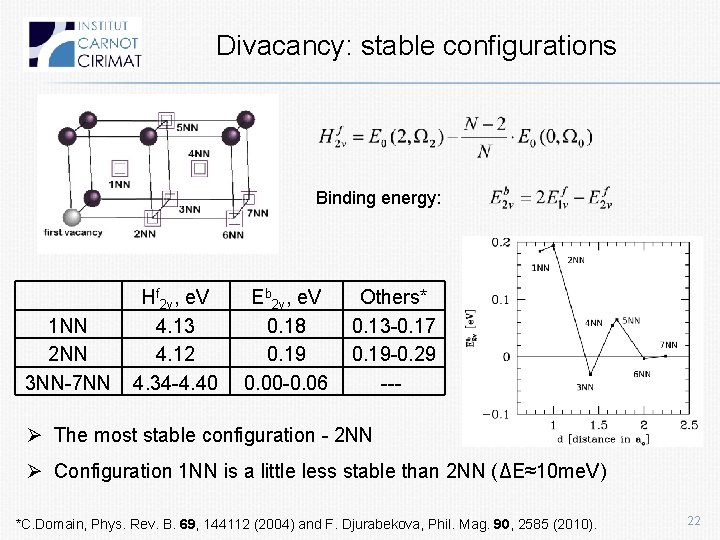
Divacancy: stable configurations Binding energy: 1 NN 2 NN 3 NN-7 NN Hf 2 v, e. V 4. 13 4. 12 4. 34 -4. 40 Eb 2 v, e. V 0. 18 0. 19 0. 00 -0. 06 Others* 0. 13 -0. 17 0. 19 -0. 29 --- Ø The most stable configuration - 2 NN Ø Configuration 1 NN is a little less stable than 2 NN (ΔE≈10 me. V) *C. Domain, Phys. Rev. B. 69, 144112 (2004) and F. Djurabekova, Phil. Mag. 90, 2585 (2010). 22

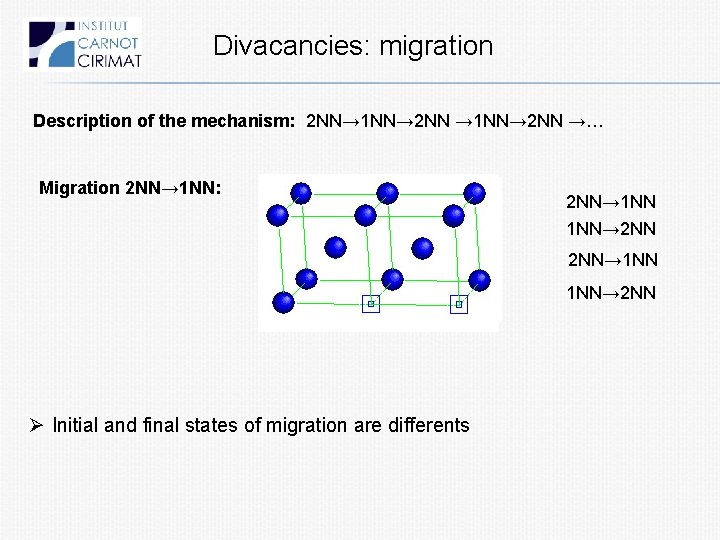
Divacancies: migration Description of the mechanism: 2 NN→ 1 NN→ 2 NN →… Migration 2 NN→ 1 NN: 2 NN→ 1 NN 1 NN→ 2 NN Ø Initial and final states of migration are differents

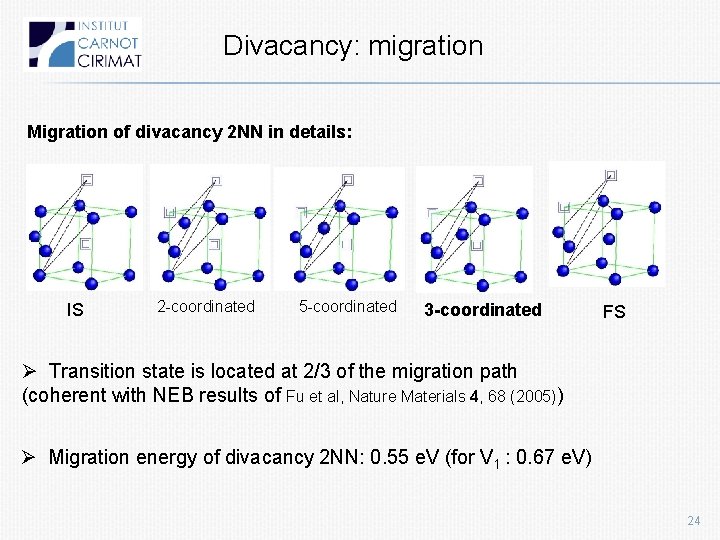
Divacancy: migration Migration of divacancy 2 NN in details: IS 2 -coordinated 5 -coordinated 3 -coordinated FS Ø Transition state is located at 2/3 of the migration path (coherent with NEB results of Fu et al, Nature Materials 4, 68 (2005)) Ø Migration energy of divacancy 2 NN: 0. 55 e. V (for V 1 : 0. 67 e. V) 24

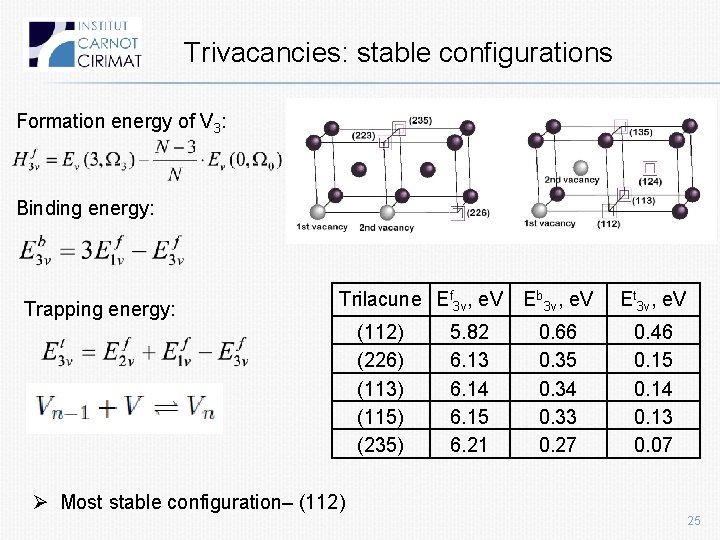
Trivacancies: stable configurations Formation energy of V 3: Binding energy: Trapping energy: Trilacune Ef 3 v, e. V (112) (226) (113) (115) (235) 5. 82 6. 13 6. 14 6. 15 6. 21 Eb 3 v, e. V Et 3 v, e. V 0. 66 0. 35 0. 34 0. 33 0. 27 0. 46 0. 15 0. 14 0. 13 0. 07 Ø Most stable configuration– (112) 25

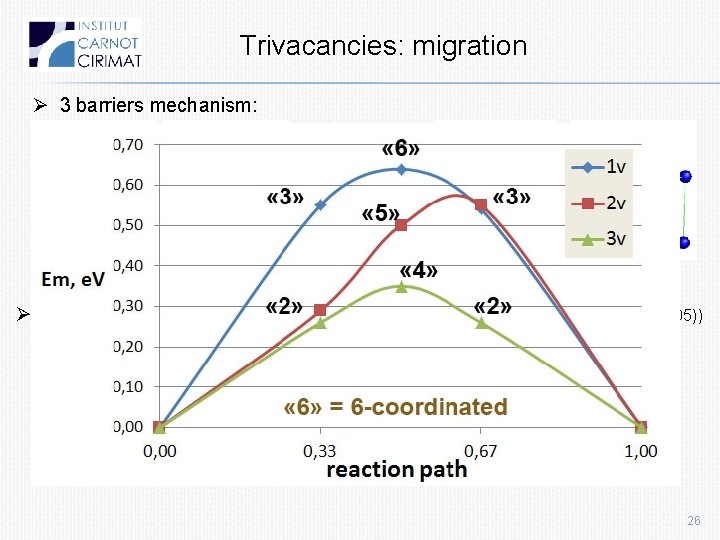
Trivacancies: migration Ø 3 barriers mechanism: IS 2 -coordinated 4 -coordinated 2 -coordinated FS Ø Em 0. 36 e. V (coherent with NEB results of Fu: 0. 35 e. V Nature Materials 4, 68 (2005)) 26

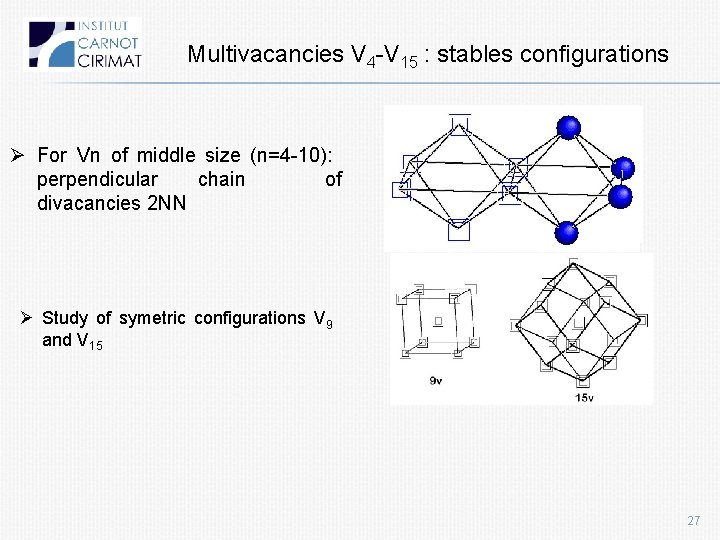
Multivacancies V 4 -V 15 : stables configurations Ø For Vn of middle size (n=4 -10): perpendicular chain of divacancies 2 NN Ø Study of symetric configurations V 9 and V 15 27

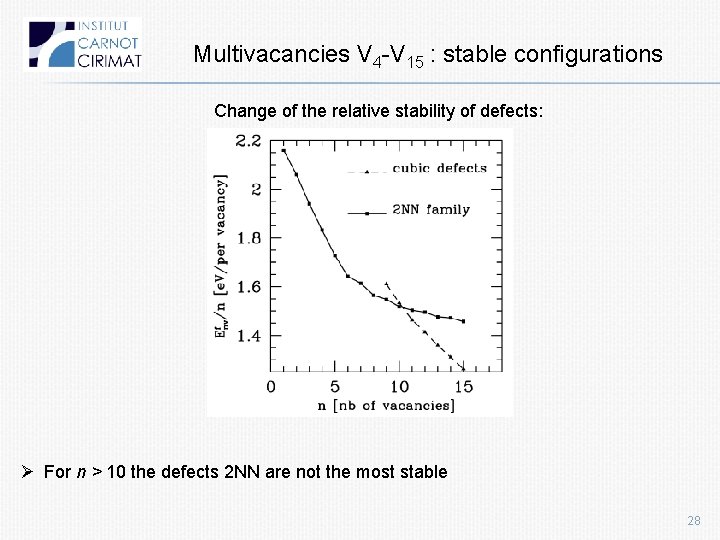
Multivacancies V 4 -V 15 : stable configurations Change of the relative stability of defects: Ø For n > 10 the defects 2 NN are not the most stable 28

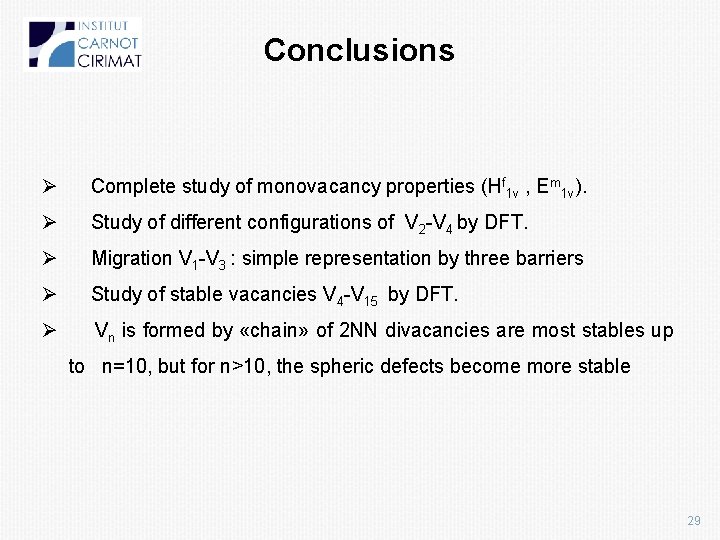
Conclusions Ø Complete study of monovacancy properties (Hf 1 v , Em 1 v). Ø Study of different configurations of V 2 -V 4 by DFT. Ø Migration V 1 -V 3 : simple representation by three barriers Ø Study of stable vacancies V 4 -V 15 by DFT. Ø Vn is formed by «chain» of 2 NN divacancies are most stables up to n=10, but for n>10, the spheric defects become more stable 29

Thank for your attention! 30

Remerciements Centre de calcul CALMIP Ecole Doctorale Science De la Matière (ED 482) Personnes concernées dans le travail de ma thèse aux niveaux scientifique et administratif 31

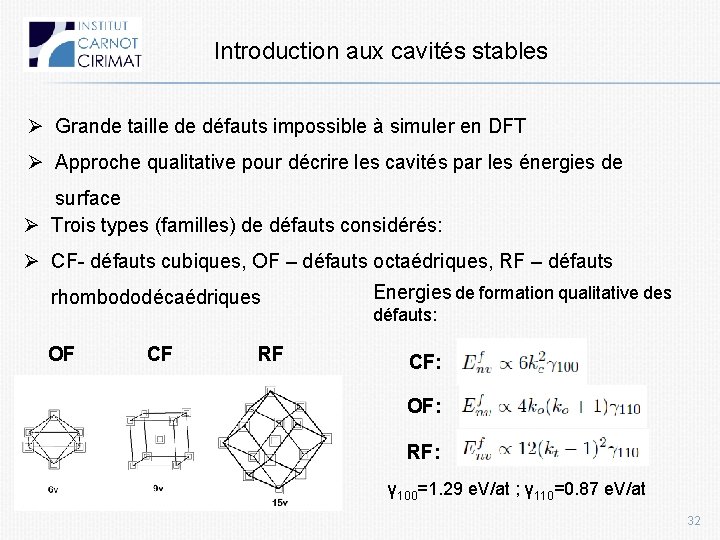
Introduction aux cavités stables Ø Grande taille de défauts impossible à simuler en DFT Ø Approche qualitative pour décrire les cavités par les énergies de surface Ø Trois types (familles) de défauts considérés: Ø CF- défauts cubiques, OF – défauts octaédriques, RF – défauts rhombododécaédriques OF CF RF Energies de formation qualitative des défauts: CF: OF: RF: γ 100=1. 29 e. V/at ; γ 110=0. 87 e. V/at 32

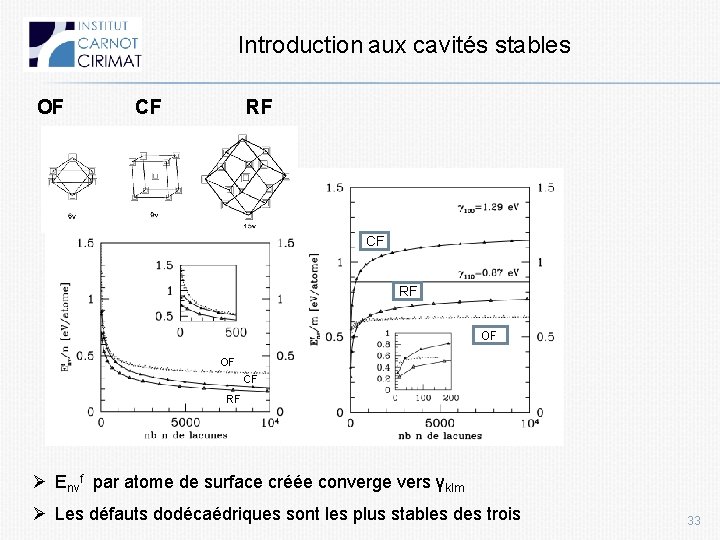
Introduction aux cavités stables OF CF RF OF OF CF RF Ø Envf par atome de surface créée converge vers γklm Ø Les défauts dodécaédriques sont les plus stables des trois 33

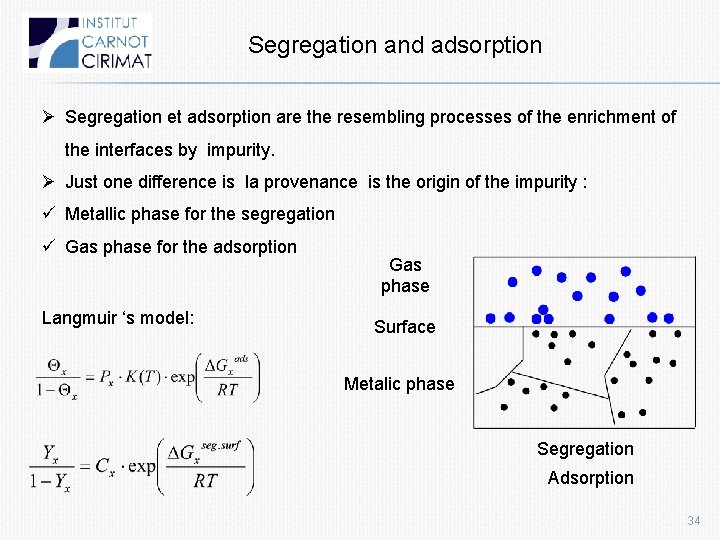
Segregation and adsorption Ø Segregation et adsorption are the resembling processes of the enrichment of the interfaces by impurity. Ø Just one difference is la provenance is the origin of the impurity : ü Metallic phase for the segregation ü Gas phase for the adsorption Langmuir ‘s model: Gas phase Surface Metalic phase Segregation Adsorption 34