L 05 C Surface defects Defects arise in



- Slides: 12

L 05 C: Surface defects • Defects arise in all stages of production and processing. CASTING • A common phase in production of metals is casting. • A melt is put in a mold and heat is extracted through the mold wall. • Crystals nucleate on the mold wall where the temperature is lowest and grow inward. This inward growth produces a columnar structure, with the lengthwise crystallographic orientation of each grain about the same, in the preferential growth direction). • For example, a cast ingot of pure copper: Last revised on April 4, 2014 by W. R. Wilcox, Clarkson University

Simulation of casting of an Al-Si alloy with nucleation of new grains in the melt. (http: //www. tms. org/pubs/journals/jom/0201/thevoz-0201. html ) View in projection mode to see the action. Microstructure depends on many things: • the alloy composition • how close the melt is to the freezing point when poured it • how rapidly it's cooled • whether the cooling's all around or mostly on the bottom • etc. Grain Refiner - added to make smaller, more uniform, equiaxed grains.

Casting of alloys • Alloy crystals tend to grow as dendrites: https: //www. youtube. com/watch? v=S 07 f. Po 45 Bv. M • If the melt falls below its melting point while being added to the mold, small crystals may have already nucleated in the melt and be floating around. • Dendrite arms may detach and float around in the melt. • After solidification is complete, grains formed by the floating crystals have random shapes and orientation. The region occupied by these in the casting is called “equiaxed. ” • Example: Ti– 47. 2 Al– 1. 50: http: //www. sciencedirect. com/science/article/pii/S 0966979507000817

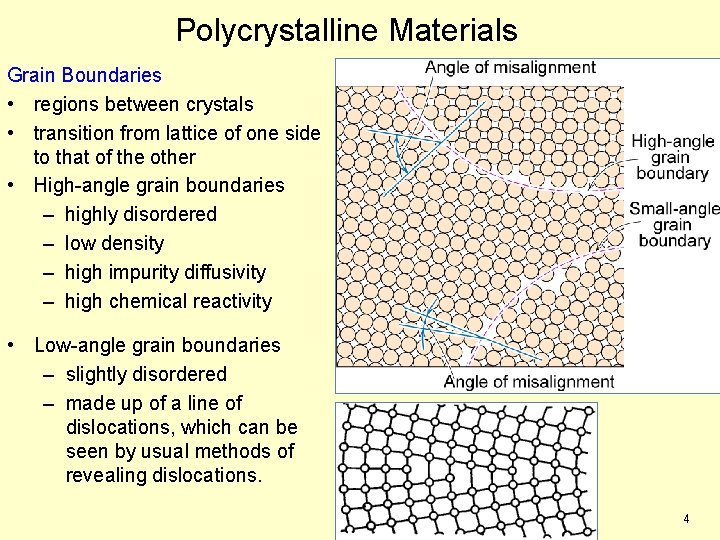
Polycrystalline Materials Grain Boundaries • regions between crystals • transition from lattice of one side to that of the other • High-angle grain boundaries – highly disordered – low density – high impurity diffusivity – high chemical reactivity • Low-angle grain boundaries – slightly disordered – made up of a line of dislocations, which can be seen by usual methods of revealing dislocations. 4

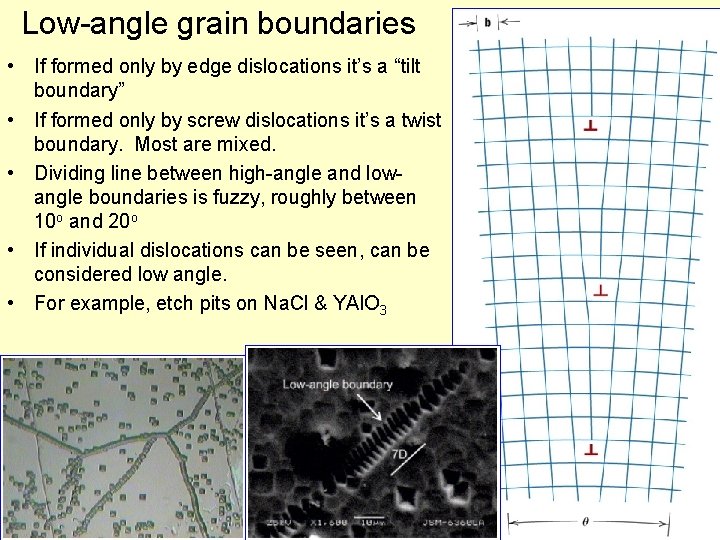
Low-angle grain boundaries • If formed only by edge dislocations it’s a “tilt boundary” • If formed only by screw dislocations it’s a twist boundary. Most are mixed. • Dividing line between high-angle and lowangle boundaries is fuzzy, roughly between 10 o and 20 o • If individual dislocations can be seen, can be considered low angle. • For example, etch pits on Na. Cl & YAl. O 3

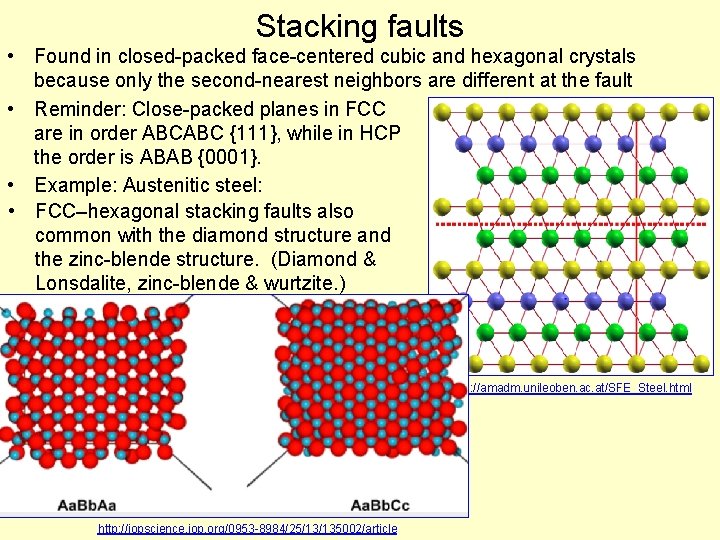
Stacking faults • Found in closed-packed face-centered cubic and hexagonal crystals because only the second-nearest neighbors are different at the fault • Reminder: Close-packed planes in FCC are in order ABCABC {111}, while in HCP the order is ABAB {0001}. • Example: Austenitic steel: • FCC–hexagonal stacking faults also common with the diamond structure and the zinc-blende structure. (Diamond & Lonsdalite, zinc-blende & wurtzite. ) http: //amadm. unileoben. ac. at/SFE_Steel. html http: //iopscience. iop. org/0953 -8984/25/13/135002/article

Twin boundaries http: //en. wikipedia. org/wiki/Crystal_twinning • Twins are two grains whose lattices are at a definite, reproducible, orientation with respect to one another. • Crystal lattices in the twins may be mirror images of one another, i. e. reflection twins. Si, for example: http: //www. tf. uni-kiel. de/matwis/amat/def_en/kap_7/backbone/r 7_1_1. html • When the two lattices share all atoms at the boundary they are called “coherent. ” Common, but not always. • Twinning can occur during plastic deformation, transformation to a different crystal structure, or crystal growth. • The mechanisms for twinning during deformation and transformation are generally well understood. • The mechanisms for twinning during crystal growth are generally unknown. • Twin boundaries are often planar, and appear as straight lines in a section. • But sometimes twin boundaries jog so that they appear curved at low magnification. • Twin boundaries are often parallel to one another. Copper, for example: http: //www. nature. com/am/journal/200904/full/am 2009128 a. html

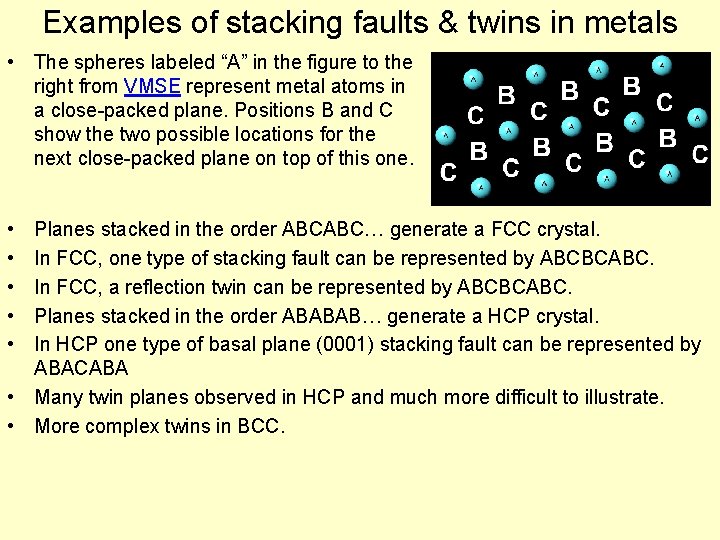
Examples of stacking faults & twins in metals • The spheres labeled “A” in the figure to the right from VMSE represent metal atoms in a close-packed plane. Positions B and C show the two possible locations for the next close-packed plane on top of this one. • • • Planes stacked in the order ABCABC… generate a FCC crystal. In FCC, one type of stacking fault can be represented by ABCBCABC. In FCC, a reflection twin can be represented by ABCBCABC. Planes stacked in the order ABABAB… generate a HCP crystal. In HCP one type of basal plane (0001) stacking fault can be represented by ABACABA • Many twin planes observed in HCP and much more difficult to illustrate. • More complex twins in BCC.

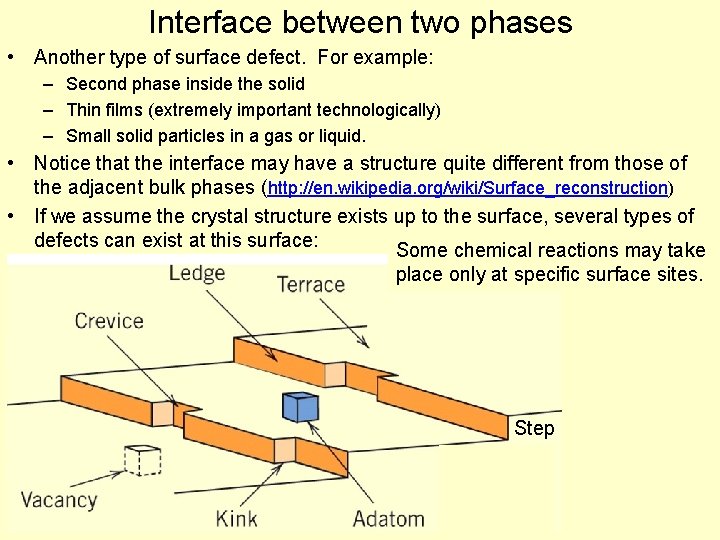
Interface between two phases • Another type of surface defect. For example: – Second phase inside the solid – Thin films (extremely important technologically) – Small solid particles in a gas or liquid. • Notice that the interface may have a structure quite different from those of the adjacent bulk phases (http: //en. wikipedia. org/wiki/Surface_reconstruction) • If we assume the crystal structure exists up to the surface, several types of defects can exist at this surface: Some chemical reactions may take place only at specific surface sites. Step

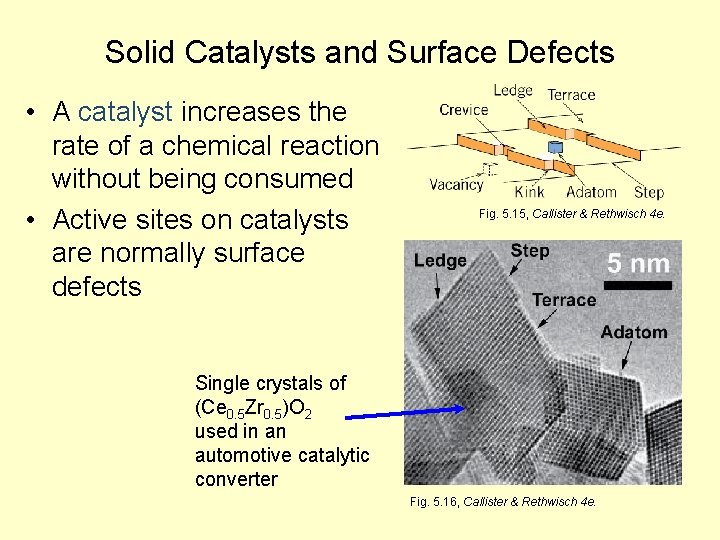
Solid Catalysts and Surface Defects • A catalyst increases the rate of a chemical reaction without being consumed • Active sites on catalysts are normally surface defects Fig. 5. 15, Callister & Rethwisch 4 e. Single crystals of (Ce 0. 5 Zr 0. 5)O 2 used in an automotive catalytic converter Fig. 5. 16, Callister & Rethwisch 4 e.

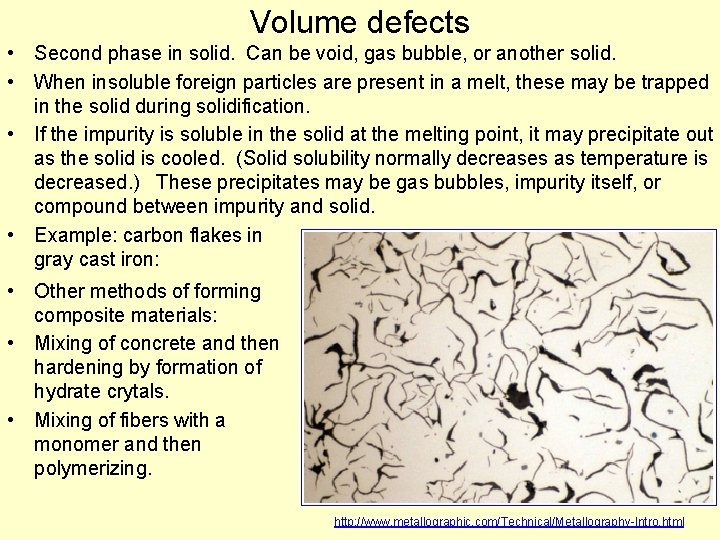
Volume defects • Second phase in solid. Can be void, gas bubble, or another solid. • When insoluble foreign particles are present in a melt, these may be trapped in the solid during solidification. • If the impurity is soluble in the solid at the melting point, it may precipitate out as the solid is cooled. (Solid solubility normally decreases as temperature is decreased. ) These precipitates may be gas bubbles, impurity itself, or compound between impurity and solid. • Example: carbon flakes in gray cast iron: • Other methods of forming composite materials: • Mixing of concrete and then hardening by formation of hydrate crytals. • Mixing of fibers with a monomer and then polymerizing. http: //www. metallographic. com/Technical/Metallography-Intro. html

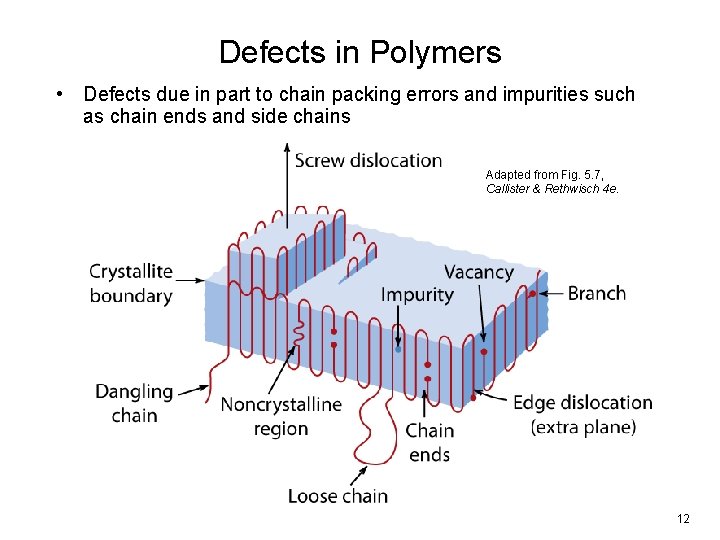
Defects in Polymers • Defects due in part to chain packing errors and impurities such as chain ends and side chains Adapted from Fig. 5. 7, Callister & Rethwisch 4 e. 12