Destruction of Organic Pollutants Through Zero Valent Metals

![Redox Processes in Environmental, Analytical, & Biological Chemistries -I] Destruction of Organic Pollutants by Redox Processes in Environmental, Analytical, & Biological Chemistries -I] Destruction of Organic Pollutants by](https://slidetodoc.com/presentation_image/2a9ed88085660b8196b36acb655e3e7e/image-2.jpg)
![I] Redox Pathways for Pollutant Destruction The Search for Alternatives for the Bulk Destruction I] Redox Pathways for Pollutant Destruction The Search for Alternatives for the Bulk Destruction](https://slidetodoc.com/presentation_image/2a9ed88085660b8196b36acb655e3e7e/image-3.jpg)






- Slides: 53

Destruction of Organic Pollutants Through Zero. Valent Metals Via Reductive and Oxidative Pathways Department of Chemistry, University of Idaho Moscow, ID 83844 -2343, Tel (208) 885 -6387 ifcheng@uidaho. edu Frank Cheng, Assistant Professor of Chemistry Graduate Students Tina Noradoun Undergraduates Tim Cantrell Matt Mc. Laughlin Kristy Henscheid 9/15/2020 Mark Engelmann Jose’ Morales Ryan Hutcheson John Doyle Ryan Neale Teri. Ann Miles Edmund Wong Kevin Breen Layne Pitcher Erik Parker 1
![Redox Processes in Environmental Analytical Biological Chemistries I Destruction of Organic Pollutants by Redox Processes in Environmental, Analytical, & Biological Chemistries -I] Destruction of Organic Pollutants by](https://slidetodoc.com/presentation_image/2a9ed88085660b8196b36acb655e3e7e/image-2.jpg)
Redox Processes in Environmental, Analytical, & Biological Chemistries -I] Destruction of Organic Pollutants by Reductive and Oxidative Pathways -RTP Reductive Dechlorination of Halocarbons -RTP Hydrogenation of Aromatics -Hydrocarbon Skeleton GC/HPLC -RTP O 2 oxidation of Organic Pollutants -II] Understanding Redox Pathways in the Mechanisms of Dietary Antioxidants -Flavonoids -Salicylate 9/15/2020 2
![I Redox Pathways for Pollutant Destruction The Search for Alternatives for the Bulk Destruction I] Redox Pathways for Pollutant Destruction The Search for Alternatives for the Bulk Destruction](https://slidetodoc.com/presentation_image/2a9ed88085660b8196b36acb655e3e7e/image-3.jpg)
I] Redox Pathways for Pollutant Destruction The Search for Alternatives for the Bulk Destruction of Organic Pollutants « Field-based Destruction of Pollutant Through O 2 Activation; RTP Mineralization. « 9/15/2020 PCB, DDT, Pentachlorophenol, phenol Organophosphorous Nerve Agents 3

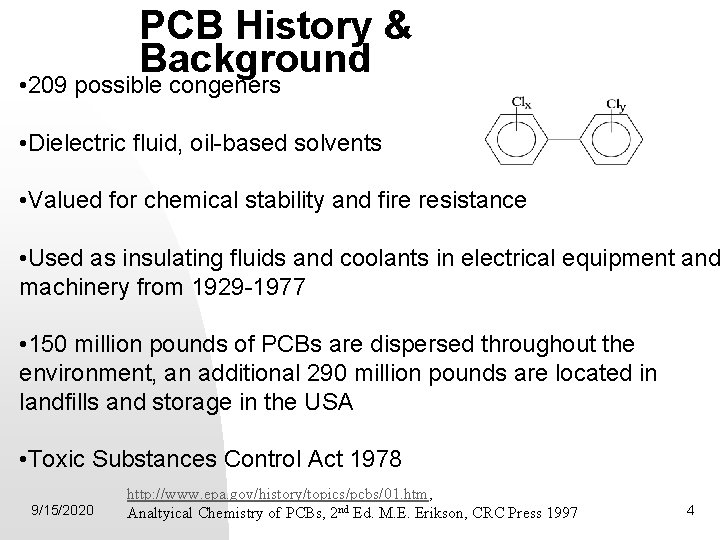
PCB History & Background • 209 possible congeners • Dielectric fluid, oil-based solvents • Valued for chemical stability and fire resistance • Used as insulating fluids and coolants in electrical equipment and machinery from 1929 -1977 • 150 million pounds of PCBs are dispersed throughout the environment, an additional 290 million pounds are located in landfills and storage in the USA • Toxic Substances Control Act 1978 9/15/2020 http: //www. epa. gov/history/topics/pcbs/01. htm, Analtyical Chemistry of PCBs, 2 nd Ed. M. E. Erikson, CRC Press 1997 4

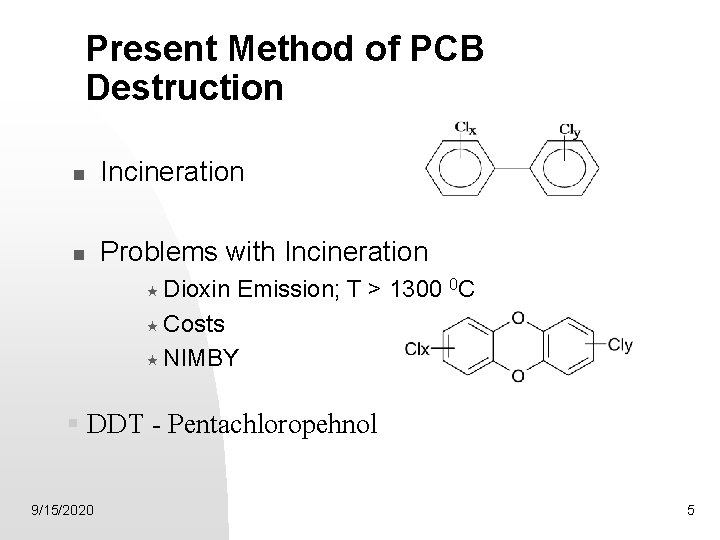
Present Method of PCB Destruction Incineration Problems with Incineration « Dioxin Emission; T > 1300 0 C « Costs « NIMBY § DDT - Pentachloropehnol 9/15/2020 5

Ideal Characteristics for Pollutant Destruction Method Minimal Costs & Environmental Impact Abundant Reagents Spent Reagents & Products Must Have Minimal Environmental Consequences if Released Mild Reaction Conditions, i. e. aqueous, room temperature and pressure, (RTP) Field Portability 9/15/2020 6

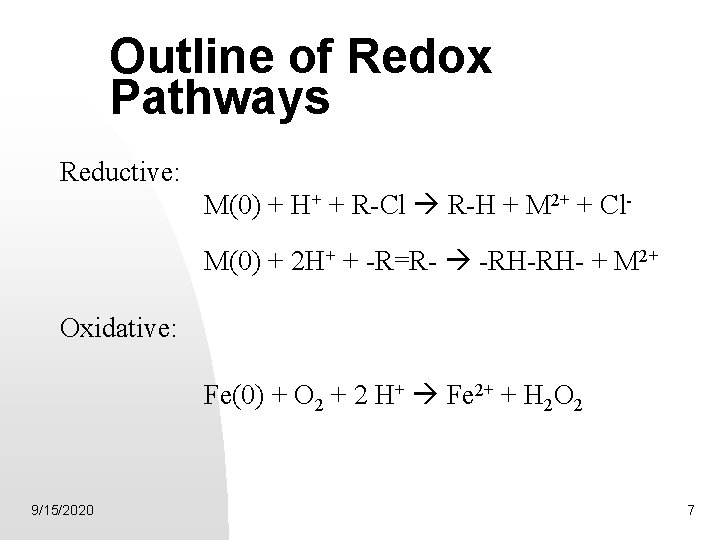
Outline of Redox Pathways Reductive: M(0) + H+ + R-Cl R-H + M 2+ + Cl. M(0) + 2 H+ + -R=R- -RH-RH- + M 2+ Oxidative: Fe(0) + O 2 + 2 H+ Fe 2+ + H 2 O 2 9/15/2020 7

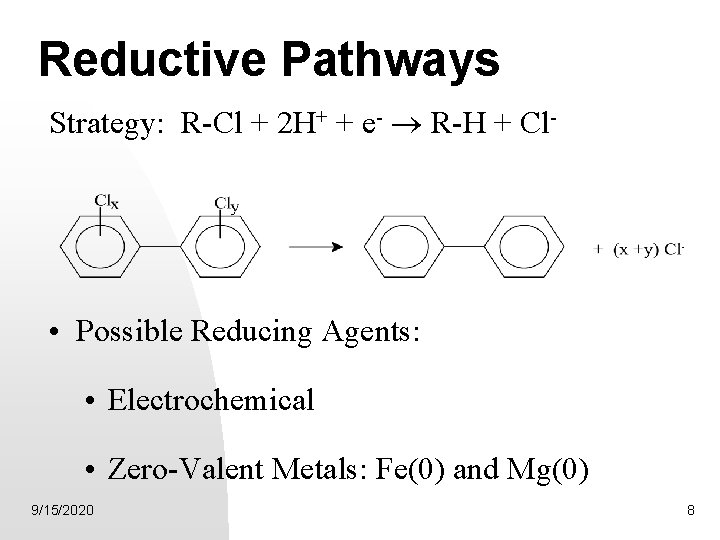
Reductive Pathways Strategy: R-Cl + 2 H+ + e- ® R-H + Cl- • Possible Reducing Agents: • Electrochemical • Zero-Valent Metals: Fe(0) and Mg(0) 9/15/2020 8

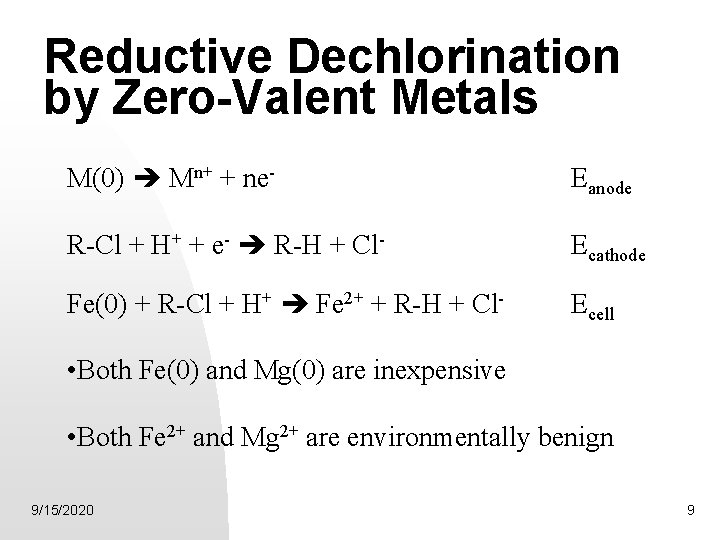
Reductive Dechlorination by Zero-Valent Metals M(0) Mn+ + ne- Eanode R-Cl + H+ + e- R-H + Cl- Ecathode Fe(0) + R-Cl + H+ Fe 2+ + R-H + Cl- Ecell • Both Fe(0) and Mg(0) are inexpensive • Both Fe 2+ and Mg 2+ are environmentally benign 9/15/2020 9

Thermodynamics of CCl 4 Dechlorination 9/15/2020 10

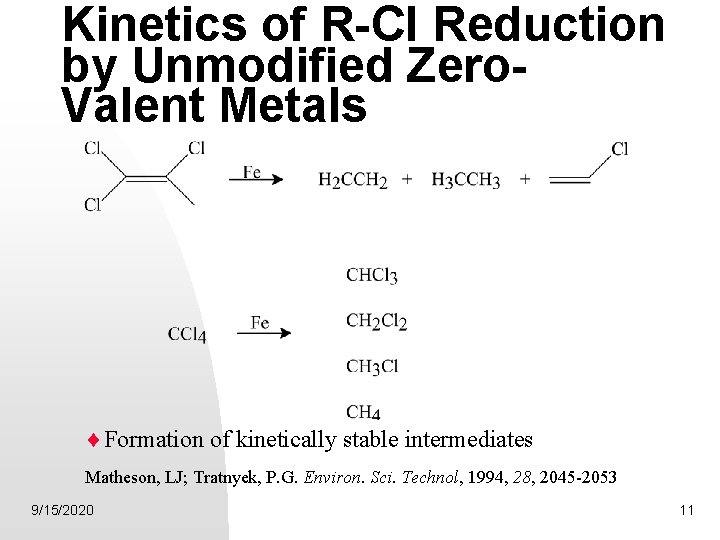
Kinetics of R-Cl Reduction by Unmodified Zero. Valent Metals Formation of kinetically stable intermediates Matheson, LJ; Tratnyek, P. G. Environ. Sci. Technol, 1994, 28, 2045 -2053 9/15/2020 11

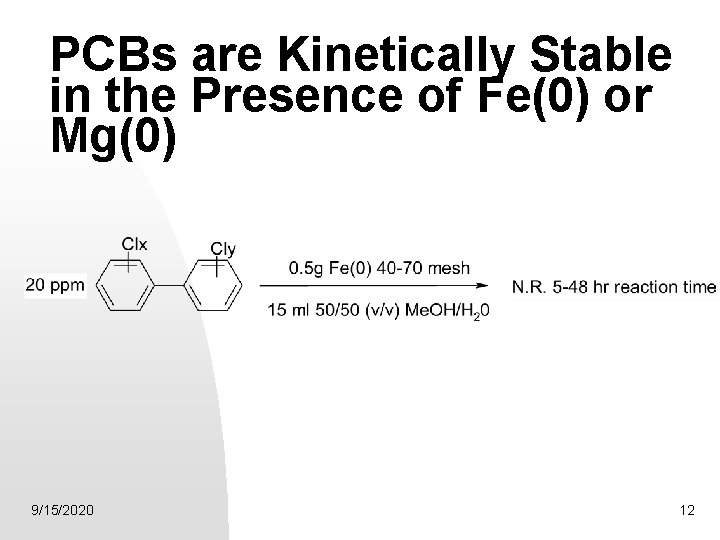
PCBs are Kinetically Stable in the Presence of Fe(0) or Mg(0) 9/15/2020 12

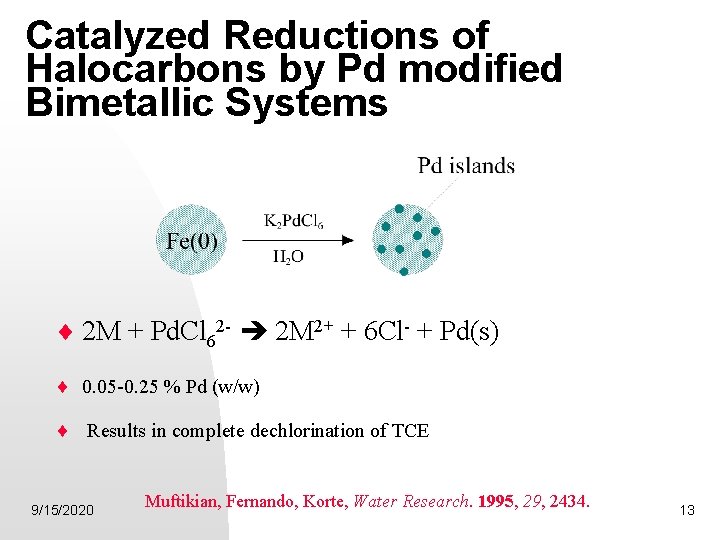
Catalyzed Reductions of Halocarbons by Pd modified Bimetallic Systems 2 M + Pd. Cl 62 - 2 M 2+ + 6 Cl- + Pd(s) 0. 05 -0. 25 % Pd (w/w) Results in complete dechlorination of TCE 9/15/2020 Muftikian, Fernando, Korte, Water Research. 1995, 29, 2434. 13

The role of Pd in hydrodehalogenation of chloro-organics by Pd/Fe Cheng, Fernando, Korte, Environ Sci &Technol, 1997, 31(4) 1074 -1078. 9/15/2020 14

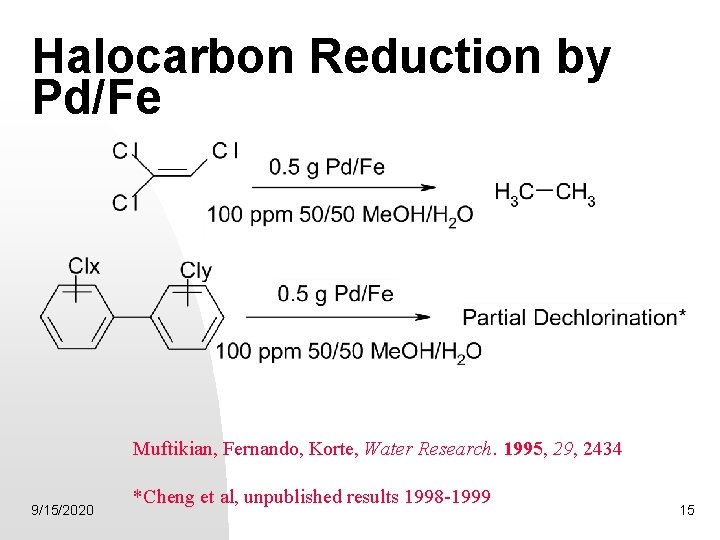
Halocarbon Reduction by Pd/Fe Muftikian, Fernando, Korte, Water Research. 1995, 29, 2434 9/15/2020 *Cheng et al, unpublished results 1998 -1999 15

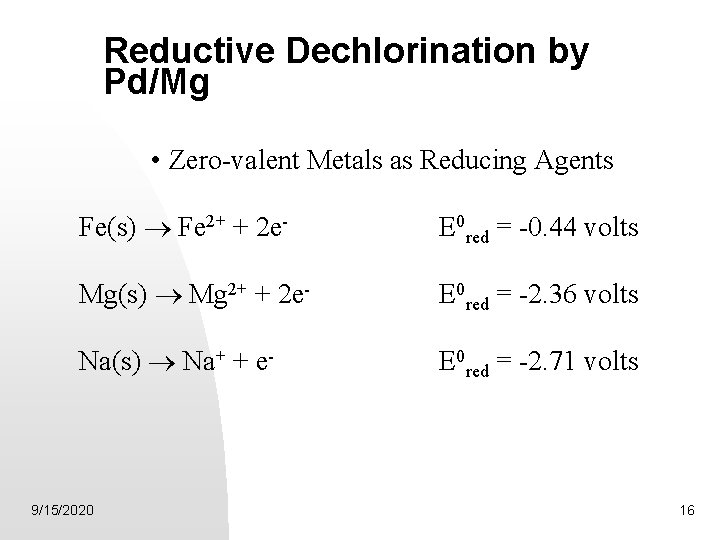
Reductive Dechlorination by Pd/Mg • Zero-valent Metals as Reducing Agents Fe(s) ® Fe 2+ + 2 e- E 0 red = -0. 44 volts Mg(s) ® Mg 2+ + 2 e- E 0 red = -2. 36 volts Na(s) ® Na+ + e- E 0 red = -2. 71 volts 9/15/2020 16

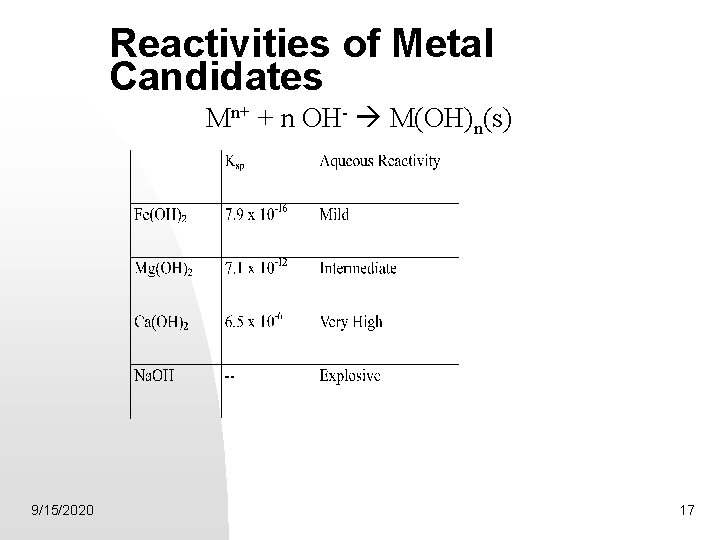
Reactivities of Metal Candidates Mn+ + n OH- M(OH)n(s) 9/15/2020 17

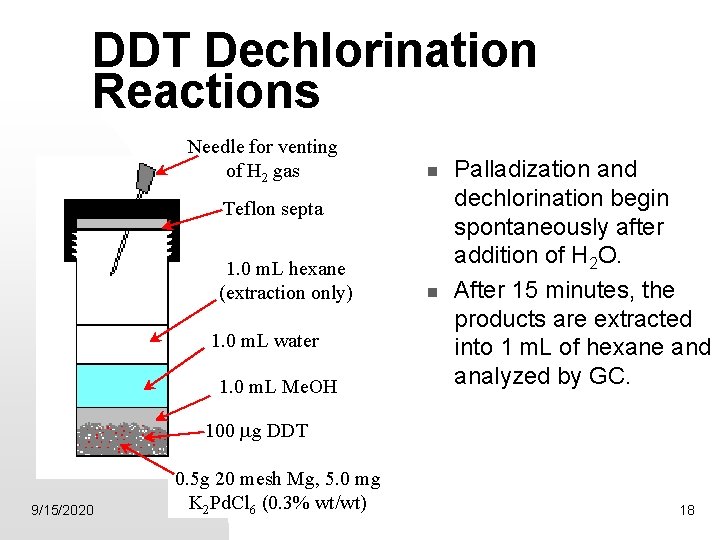
DDT Dechlorination Reactions Needle for venting of H 2 gas Teflon septa 1. 0 m. L hexane (extraction only) 1. 0 m. L water 1. 0 m. L Me. OH Palladization and dechlorination begin spontaneously after addition of H 2 O. After 15 minutes, the products are extracted into 1 m. L of hexane and analyzed by GC. 100 mg DDT 9/15/2020 0. 5 g 20 mesh Mg, 5. 0 mg K 2 Pd. Cl 6 (0. 3% wt/wt) 18

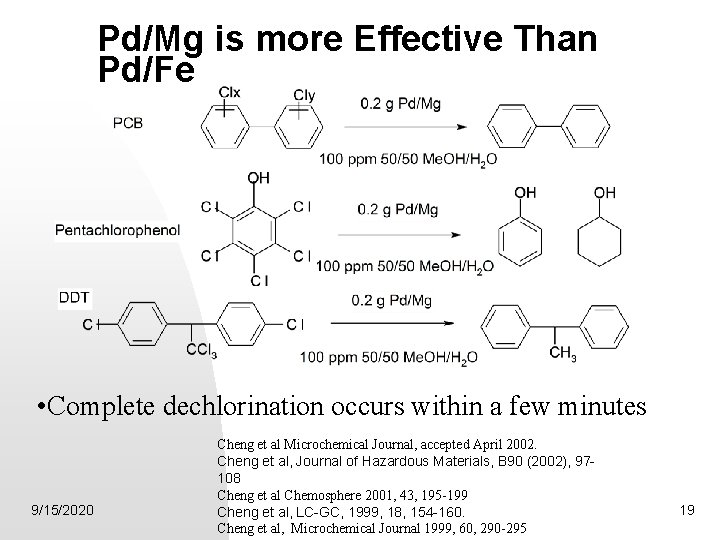
Pd/Mg is more Effective Than Pd/Fe • Complete dechlorination occurs within a few minutes 9/15/2020 Cheng et al Microchemical Journal, accepted April 2002. Cheng et al, Journal of Hazardous Materials, B 90 (2002), 97108 Cheng et al Chemosphere 2001, 43, 195 -199 Cheng et al, LC-GC, 1999, 18, 154 -160. Cheng et al, Microchemical Journal 1999, 60, 290 -295 19

Summary of Dechlorination by Pd/Mg First demonstration of the complete dechlorination of: PCP DDT PCB Reaction conditions: STP 9/15/2020 20

Hydrogenation of Phenol Common component of industrial waste streams. • As high as 1% m/m • Expensive treatment processes, i. e. high T and/or P processes, adsorption • Cyclohexanol/-one less problematic 9/15/2020 21

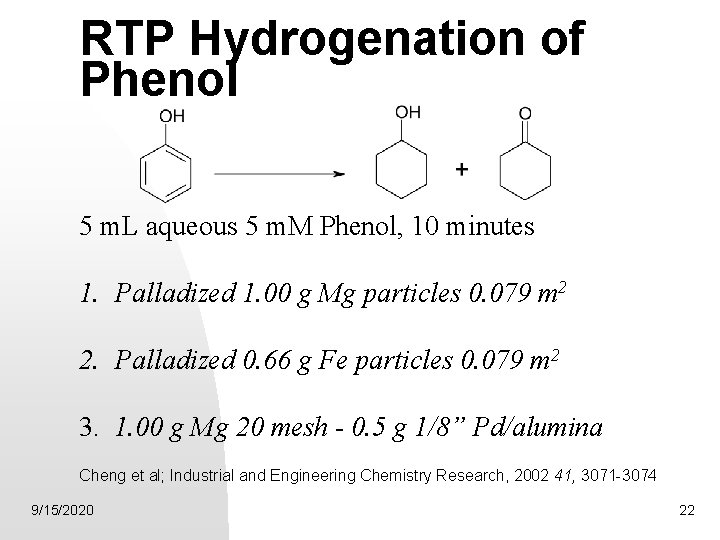
RTP Hydrogenation of Phenol 5 m. L aqueous 5 m. M Phenol, 10 minutes 1. Palladized 1. 00 g Mg particles 0. 079 m 2 2. Palladized 0. 66 g Fe particles 0. 079 m 2 3. 1. 00 g Mg 20 mesh - 0. 5 g 1/8” Pd/alumina Cheng et al; Industrial and Engineering Chemistry Research, 2002 41, 3071 -3074 9/15/2020 22

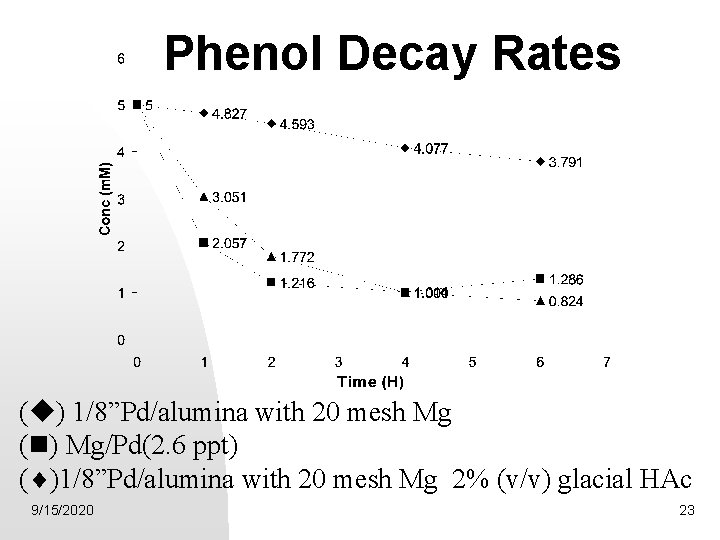
Phenol Decay Rates ( ) 1/8”Pd/alumina with 20 mesh Mg ( ) Mg/Pd(2. 6 ppt) ( )1/8”Pd/alumina with 20 mesh Mg 2% (v/v) glacial HAc 9/15/2020 23

RTP Hydrogenation Product Yields 9/15/2020 24

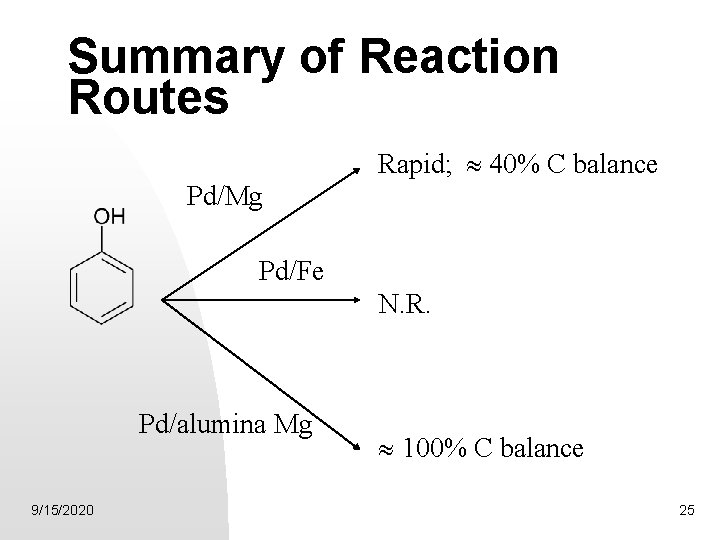
Summary of Reaction Routes Rapid; 40% C balance Pd/Mg Pd/Fe N. R. Pd/alumina Mg 9/15/2020 100% C balance 25

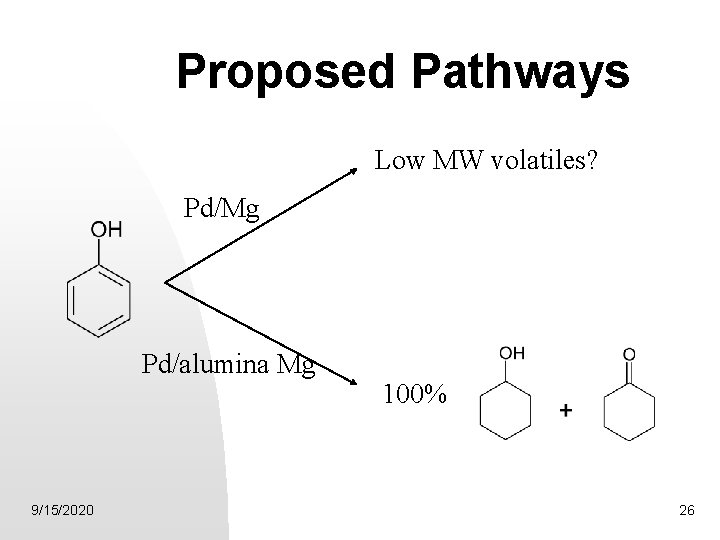
Proposed Pathways Low MW volatiles? Pd/Mg Pd/alumina Mg 9/15/2020 100% 26

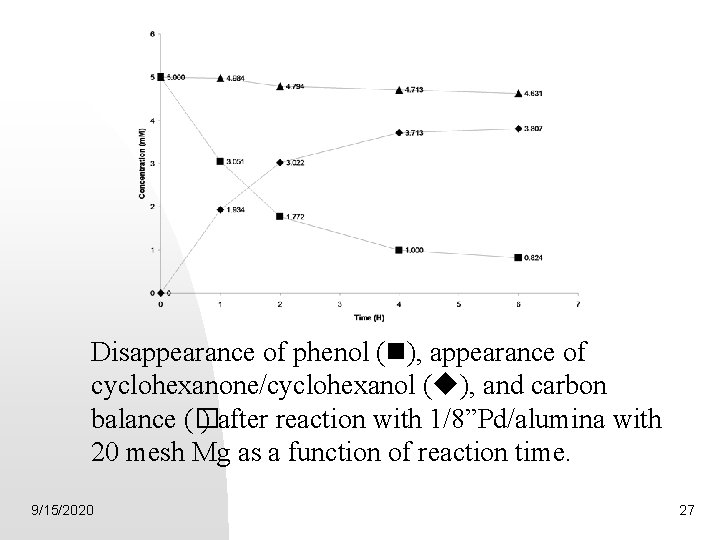
Disappearance of phenol ( ), appearance of cyclohexanone/cyclohexanol ( ), and carbon balance (� ) after reaction with 1/8”Pd/alumina with 20 mesh Mg as a function of reaction time. 9/15/2020 27

Analytical Chemistry of Halocarbon • Polychlorinated biphenyl, 209 possible conformation isomers (congeners) mixtures -problematic for GC-ECD -aging effects -environmental matrix more………. . • Complete dechlorination of PCB yields one product Biphenyl 9/15/2020 28

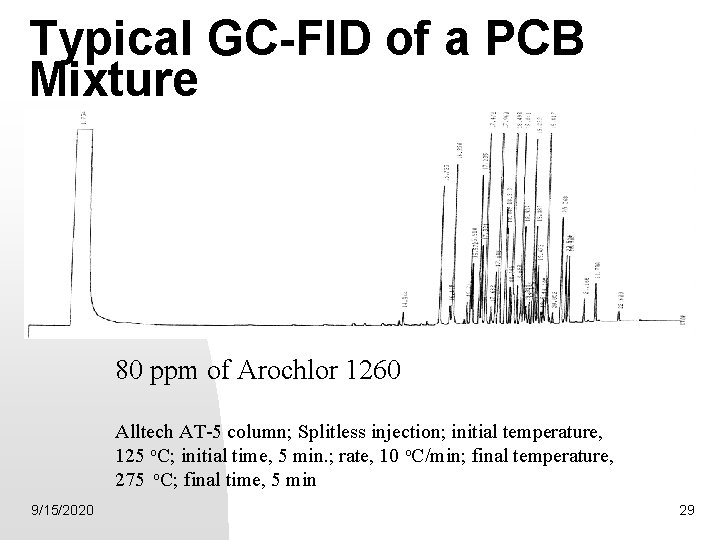
Typical GC-FID of a PCB Mixture 80 ppm of Arochlor 1260 Alltech AT-5 column; Splitless injection; initial temperature, 125 o. C; initial time, 5 min. ; rate, 10 o. C/min; final temperature, 275 o. C; final time, 5 min 9/15/2020 29

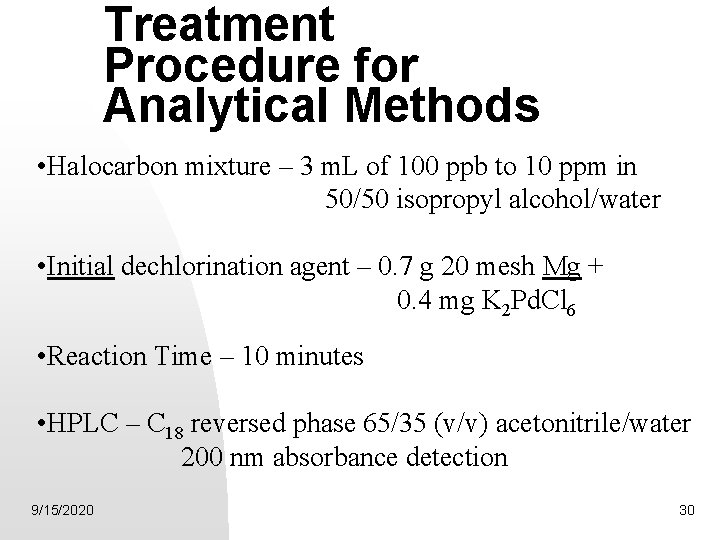
Treatment Procedure for Analytical Methods • Halocarbon mixture – 3 m. L of 100 ppb to 10 ppm in 50/50 isopropyl alcohol/water • Initial dechlorination agent – 0. 7 g 20 mesh Mg + 0. 4 mg K 2 Pd. Cl 6 • Reaction Time – 10 minutes • HPLC – C 18 reversed phase 65/35 (v/v) acetonitrile/water 200 nm absorbance detection 9/15/2020 30

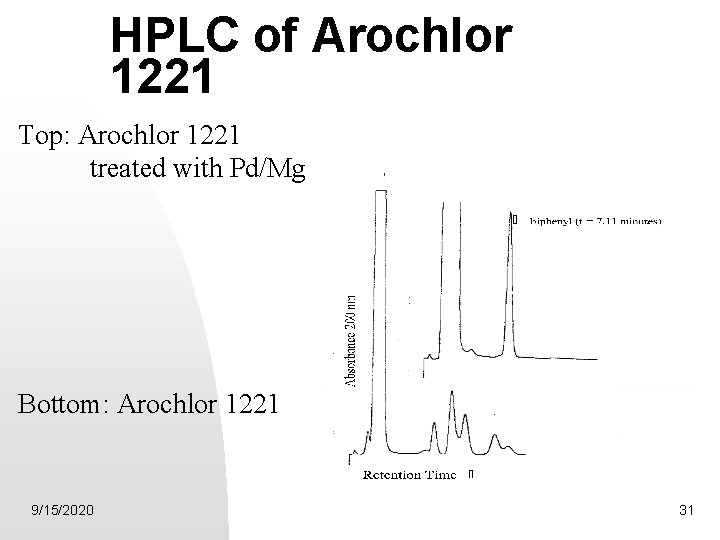
HPLC of Arochlor 1221 Top: Arochlor 1221 treated with Pd/Mg Bottom: Arochlor 1221 9/15/2020 31

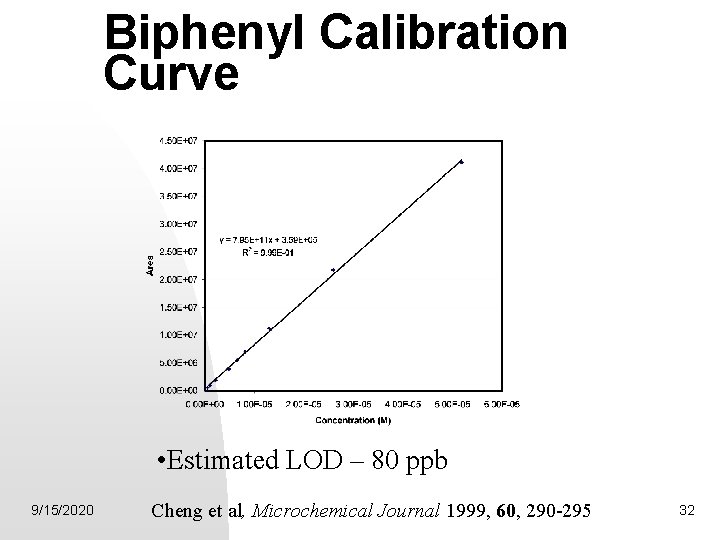
Biphenyl Calibration Curve • Estimated LOD – 80 ppb 9/15/2020 Cheng et al, Microchemical Journal 1999, 60, 290 -295 32

Biphenyl Yields From 1221 & 1260 9/15/2020 33

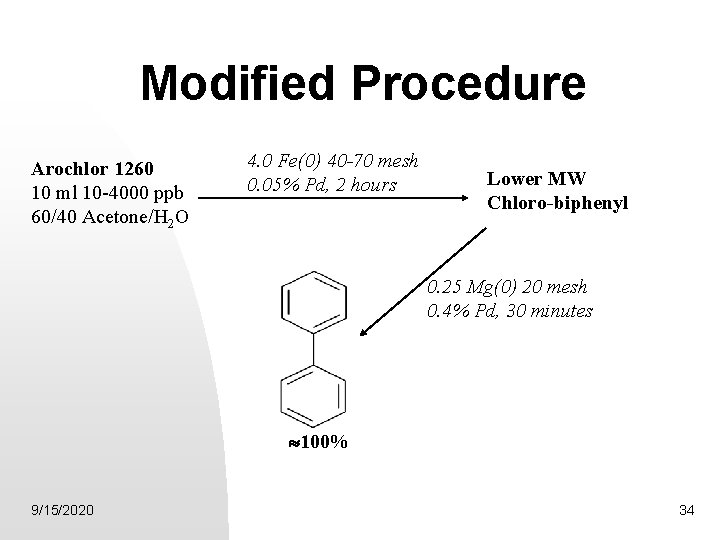
Modified Procedure Arochlor 1260 10 ml 10 -4000 ppb 60/40 Acetone/H 2 O 4. 0 Fe(0) 40 -70 mesh 0. 05% Pd, 2 hours Lower MW Chloro-biphenyl 0. 25 Mg(0) 20 mesh 0. 4% Pd, 30 minutes 100% 9/15/2020 34

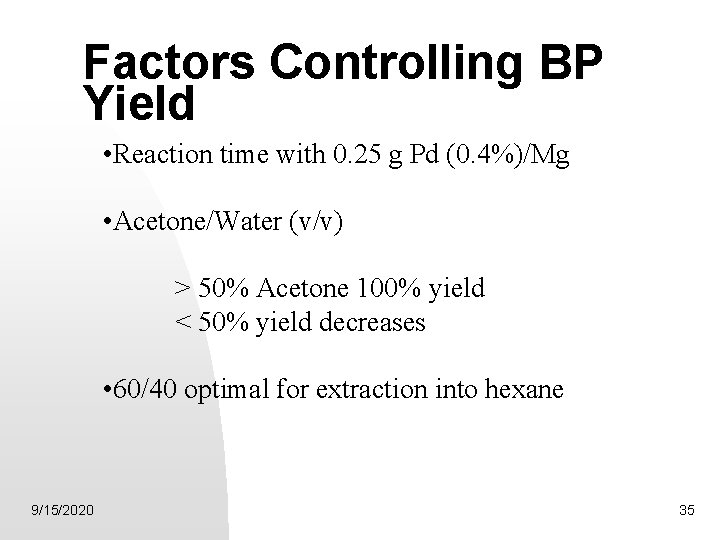
Factors Controlling BP Yield • Reaction time with 0. 25 g Pd (0. 4%)/Mg • Acetone/Water (v/v) > 50% Acetone 100% yield < 50% yield decreases • 60/40 optimal for extraction into hexane 9/15/2020 35

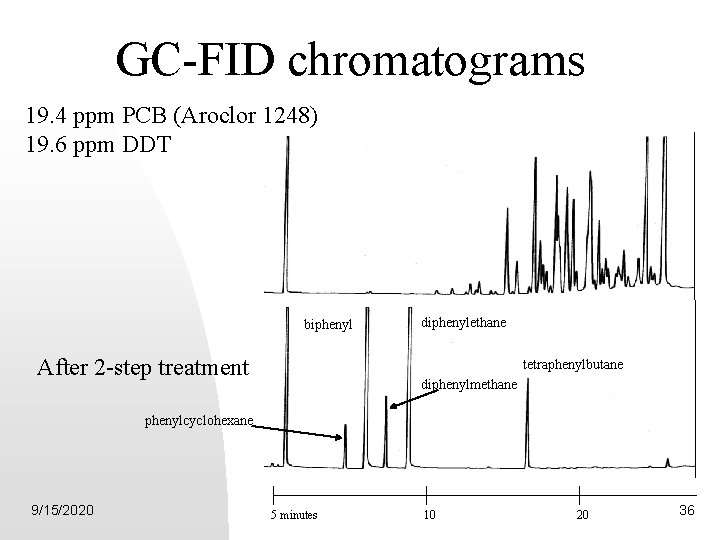
GC-FID chromatograms 19. 4 ppm PCB (Aroclor 1248) 19. 6 ppm DDT Internal Standard biphenyl After 2 -step treatment diphenylethane tetraphenylbutane diphenylmethane phenylcyclohexane 9/15/2020 5 minutes 10 20 36

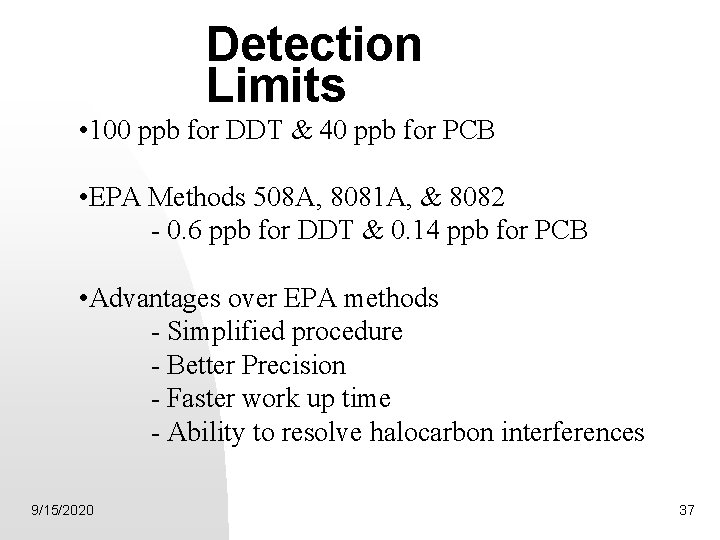
Detection Limits • 100 ppb for DDT & 40 ppb for PCB • EPA Methods 508 A, 8081 A, & 8082 - 0. 6 ppb for DDT & 0. 14 ppb for PCB • Advantages over EPA methods - Simplified procedure - Better Precision - Faster work up time - Ability to resolve halocarbon interferences 9/15/2020 37

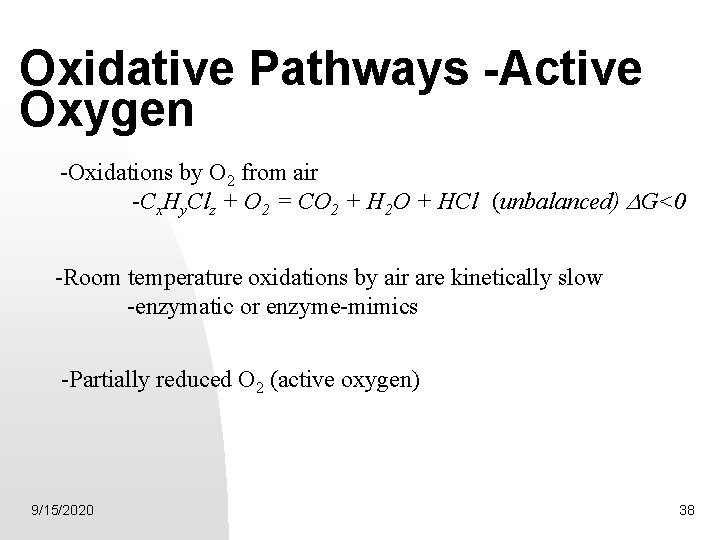
Oxidative Pathways -Active Oxygen -Oxidations by O 2 from air -Cx. Hy. Clz + O 2 = CO 2 + H 2 O + HCl (unbalanced) G<0 -Room temperature oxidations by air are kinetically slow -enzymatic or enzyme-mimics -Partially reduced O 2 (active oxygen) 9/15/2020 38

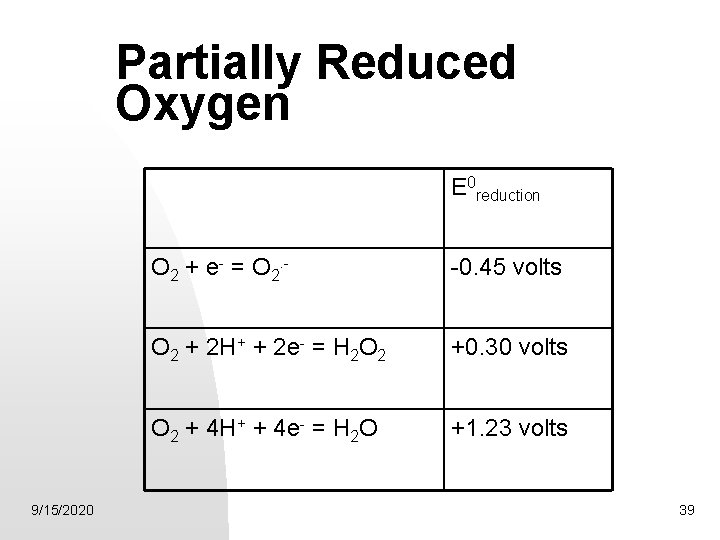
Partially Reduced Oxygen E 0 reduction 9/15/2020 O 2 + e- = O 2. - -0. 45 volts O 2 + 2 H+ + 2 e- = H 2 O 2 +0. 30 volts O 2 + 4 H+ + 4 e- = H 2 O +1. 23 volts 39

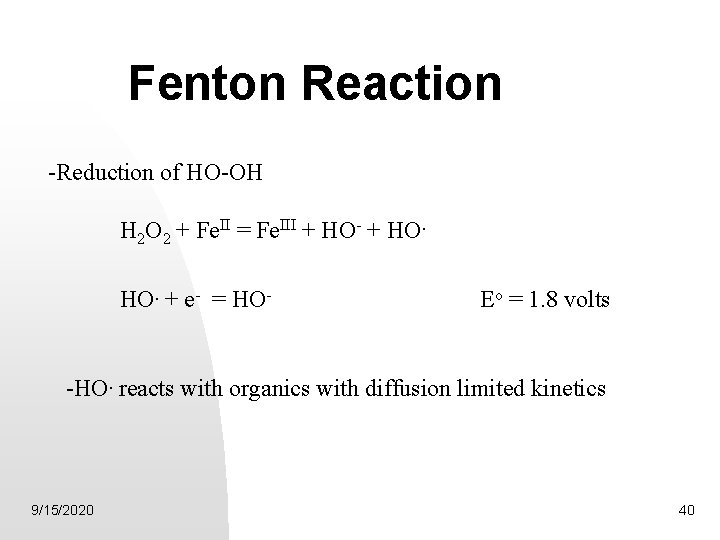
Fenton Reaction -Reduction of HO-OH H 2 O 2 + Fe. II = Fe. III + HO- + HO. + e- = HO- Eo = 1. 8 volts -HO. reacts with organics with diffusion limited kinetics 9/15/2020 40

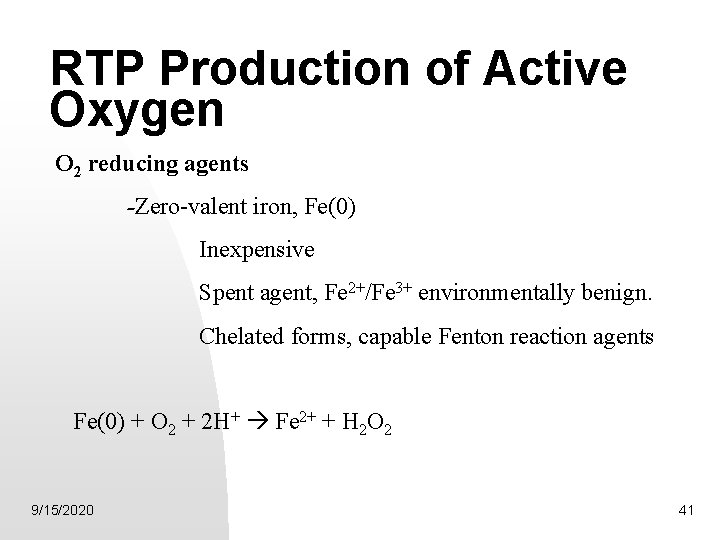
RTP Production of Active Oxygen O 2 reducing agents -Zero-valent iron, Fe(0) Inexpensive Spent agent, Fe 2+/Fe 3+ environmentally benign. Chelated forms, capable Fenton reaction agents Fe(0) + O 2 + 2 H+ Fe 2+ + H 2 O 2 9/15/2020 41

“Aero”-Fenton Reaction -Fe 2+ chelation agent - EDTA Fe. IIETDA + HOOH Fe. IIIEDTA + HO- + HO. • Inexpensive • Efficient Binding Agent for Fe 2+/3+ • Fe. IIEDTA highly effective Fenton Reaction reagent 9/15/2020 42

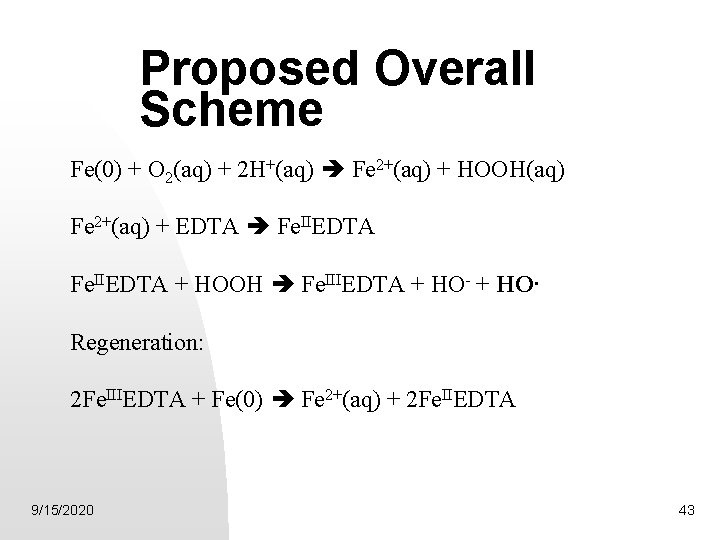
Proposed Overall Scheme Fe(0) + O 2(aq) + 2 H+(aq) Fe 2+(aq) + HOOH(aq) Fe 2+(aq) + EDTA Fe. IIEDTA + HOOH Fe. IIIEDTA + HO- + HO· Regeneration: 2 Fe. IIIEDTA + Fe(0) Fe 2+(aq) + 2 Fe. IIEDTA 9/15/2020 43

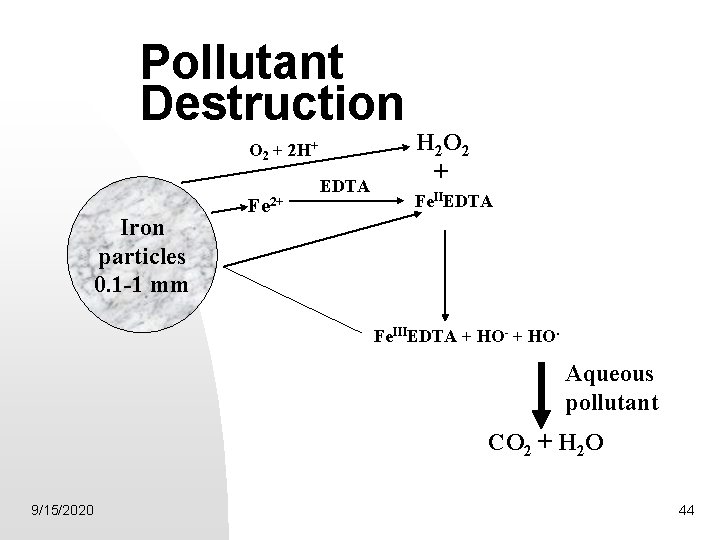
Pollutant Destruction O 2 + 2 H+ Iron particles 0. 1 -1 mm Fe 2+ EDTA H 2 O 2 + Fe. IIEDTA Fe. IIIEDTA + HO- + HO. Aqueous pollutant CO 2 + H 2 O 9/15/2020 44

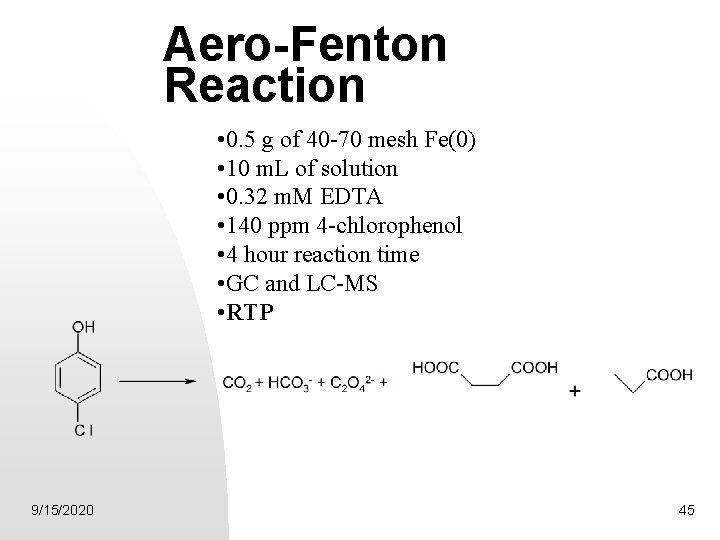
Aero-Fenton Reaction • 0. 5 g of 40 -70 mesh Fe(0) • 10 m. L of solution • 0. 32 m. M EDTA • 140 ppm 4 -chlorophenol • 4 hour reaction time • GC and LC-MS • RTP 9/15/2020 45

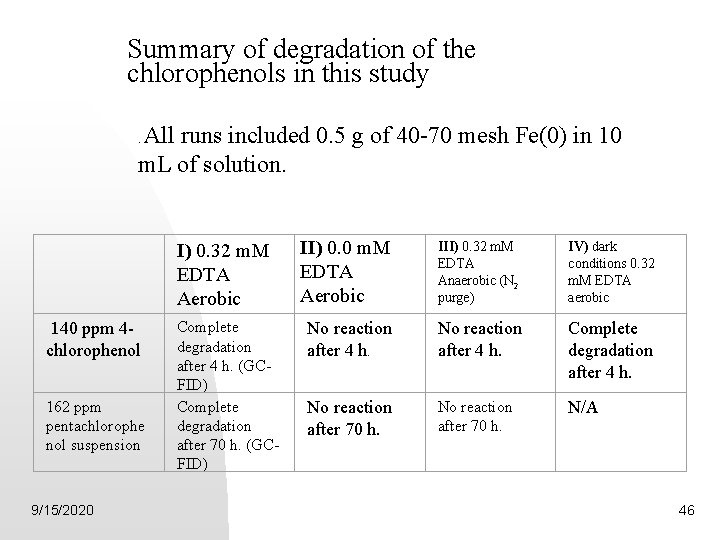
Summary of degradation of the chlorophenols in this study All runs included 0. 5 g of 40 -70 mesh Fe(0) in 10 m. L of solution. . I) 0. 32 m. M EDTA Aerobic II) 0. 0 m. M EDTA Aerobic III) 0. 32 m. M EDTA Anaerobic (N 2 purge) IV) dark conditions 0. 32 m. M EDTA aerobic 140 ppm 4 chlorophenol Complete degradation after 4 h. (GCFID) Complete degradation after 70 h. (GCFID) No reaction after 4 h. Complete degradation after 4 h. No reaction after 70 h. N/A 162 ppm pentachlorophe nol suspension 9/15/2020 46

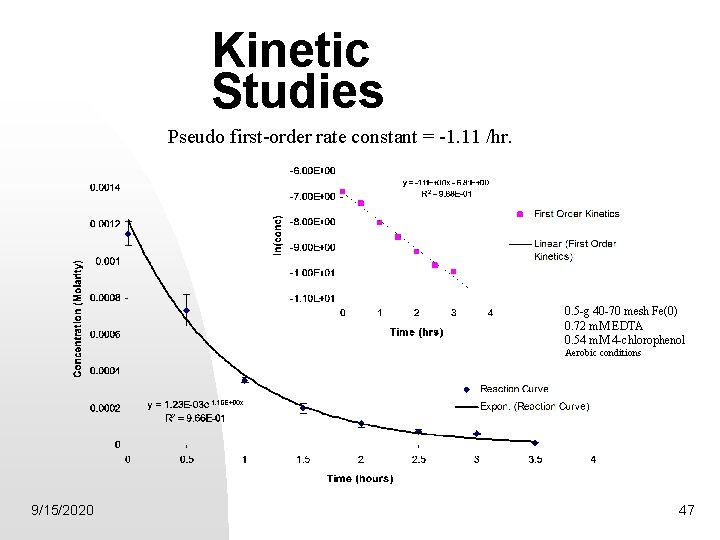
Kinetic Studies Pseudo first-order rate constant = -1. 11 /hr. 0. 5 -g 40 -70 mesh Fe(0) 0. 72 m. M EDTA 0. 54 m. M 4 -chlorophenol Aerobic conditions 9/15/2020 47

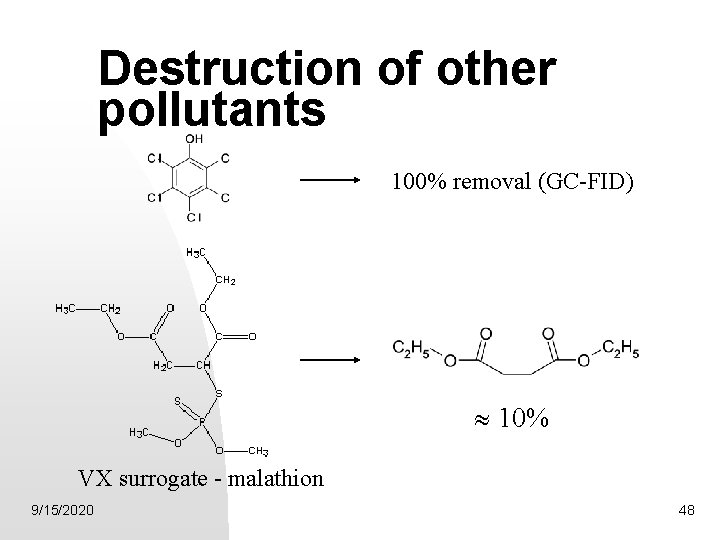
Destruction of other pollutants 100% removal (GC-FID) 10% VX surrogate - malathion 9/15/2020 48

Technical Summary Ability to degrade organic pollutants • Under room temperature, atmospheric pressure conditions • Inexpensive Reagents – Iron particles, water, air & EDTA • Unspecialized reactors • Process is easily transportable, iron particles & EDTA • Strong possibility of scale-up 9/15/2020 49

Scientific Summary • First example of abiotic RTP activation of O 2 able to oxidize destructively organics • Control experiments indicate process is dependent on Fe(0), EDTA, air, and water 9/15/2020 50

Future Investigations • Mechanisms – Understanding the process • Kinetics – Speeding up the process • Search for an oxidatively stable iron chelate • Survey of Pollutants – oxidizable and nonoxidizable functional groups • Application & Scale-up 9/15/2020 51

Acknowledgements 9/15/2020 52

Acknowledgements • Thank you for Attention • University of Idaho Research Foundation • Mark Engelmann – Ph. D. candidate – Dechlorination • Jose’ Morales – M. S. 2001 – Hydrogenation • Tina Noradoun – Ph. D. candidate – Aero-Fenton 9/15/2020 53
 Planting more trees is called
Planting more trees is called Primary pollutants and secondary pollutants
Primary pollutants and secondary pollutants Examples of primary pollutants and secondary pollutants
Examples of primary pollutants and secondary pollutants Natural science grade 7 term 2 matter and materials
Natural science grade 7 term 2 matter and materials Ferrous metals vs non ferrous metals
Ferrous metals vs non ferrous metals Metals vs nonmetals
Metals vs nonmetals Metalloids vs metals
Metalloids vs metals Matter and materials (grade 7 worksheets)
Matter and materials (grade 7 worksheets) Periodic table metals and nonmetals
Periodic table metals and nonmetals Zero defect zero effect
Zero defect zero effect Unità di governo cnc
Unità di governo cnc Inorganic gaseous pollutants of air
Inorganic gaseous pollutants of air Ozone layer depletion
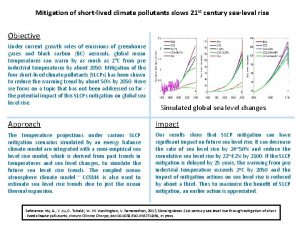
Ozone layer depletion Short lived climate pollutants
Short lived climate pollutants Inorganic gaseous pollutants of air
Inorganic gaseous pollutants of air Secondary pollutants examples
Secondary pollutants examples Before and after global warming
Before and after global warming Secondary air pollutants
Secondary air pollutants Indoor air pollutants
Indoor air pollutants Primary and secondary pollutants difference
Primary and secondary pollutants difference Primary and secondary pollutants
Primary and secondary pollutants Major air pollutants
Major air pollutants Primary vs secondary pollutants
Primary vs secondary pollutants Why do thermal inversion layers trap pollutants
Why do thermal inversion layers trap pollutants What are the secondary air pollutants
What are the secondary air pollutants Primary vs secondary pollution
Primary vs secondary pollution Air pollutants
Air pollutants Stock pollutants
Stock pollutants Pollutants
Pollutants Habitat destruction
Habitat destruction Joint destruction
Joint destruction Joint destruction
Joint destruction Iron mountain data destruction
Iron mountain data destruction Lets get a lock for this thing cartoon
Lets get a lock for this thing cartoon Deasy destruction
Deasy destruction A wise economist asks a question
A wise economist asks a question Usps secure destruction
Usps secure destruction When was it made
When was it made Destruction
Destruction Destruction
Destruction Locusts in things fall apart
Locusts in things fall apart Hypokemi
Hypokemi Destruction document honfleur
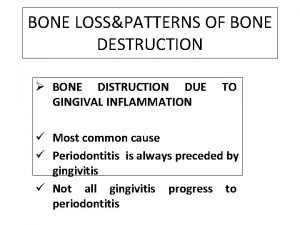
Destruction document honfleur Patterns of bone destruction
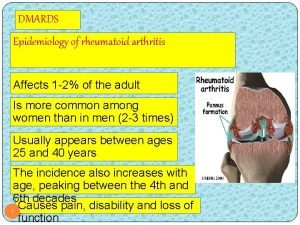
Patterns of bone destruction Benjamin spock apush
Benjamin spock apush Destruction operator
Destruction operator Subcutaneous nodules
Subcutaneous nodules Lord byron the destruction of sennacherib analysis
Lord byron the destruction of sennacherib analysis Reconstruction of automobile destruction
Reconstruction of automobile destruction Destruction of mankind
Destruction of mankind Vertical bone loss
Vertical bone loss Central buttressing bone formation
Central buttressing bone formation Mutually assured destruction cuban missile crisis
Mutually assured destruction cuban missile crisis Destruction of mankind
Destruction of mankind