Democritus Theory Greek Philosopher in the 4 th






















- Slides: 37


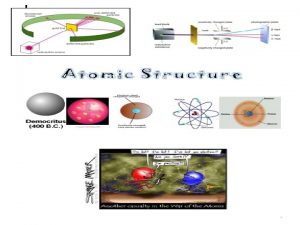
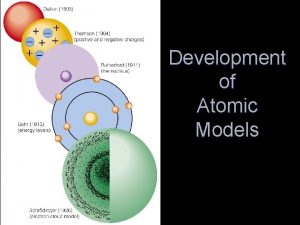
Democritus Theory Greek Philosopher in the 4 th century B. C - believed that all matter consisted of extremely small particles - suggested these particles are made of invisible units called atoms - term atom is derived from a Greek word meaning “unable to divide” - believed there were different types of atoms, liquids: round, smooth solids: rough, prickly - unable to provide evidence that an atom existed, therefore many people were very skeptical

Dalton’s Atomic Theory John Dalton - interested in predicting the weather SO. . studied the behavior of gases in the air, concluded that a gas consists of individual particles Evidence - masses of elements as they combined to form compounds always produced the same ratio no matter what the size of the sample ex. carbon dioxide - 1 carbon 2 oxygen: 1: 2 ratio

Dalton’s Atomic Theory - used a Greek concept of the atom and the 3 laws to give the atomic theory a scientific basis

Dalton’s Atomic Theory Cont. 5 Principles 1. All matter is made of indivisible and indestructible atoms 2. All atoms of a given element are identical in their physical and chemical properties 3. Atoms of different elements differ in their physical and chemical properties 4. Atoms of different elements combine in simple whole-numbers ratios to form compounds

Dalton’s Atomic Theory Cont. 5. Chemical reactions consist of the combination, separation, or rearrangement of atoms - theory explained most of the chemical data of the day and was readily accepted - evidence since has shown the first two principles are not valid; overlooked that most atoms will combined with other of their own kind - NOT discarded only modified

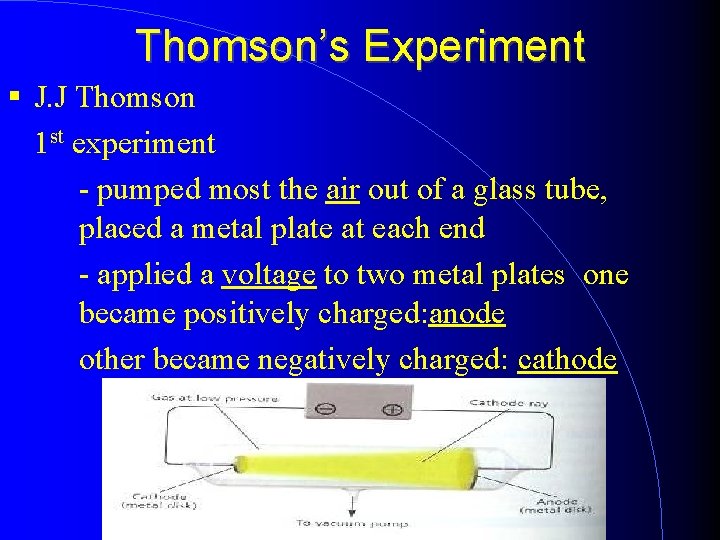
Thomson’s Experiment J. J Thomson 1 st experiment - pumped most the air out of a glass tube, placed a metal plate at each end - applied a voltage to two metal plates one became positively charged: anode other became negatively charged: cathode

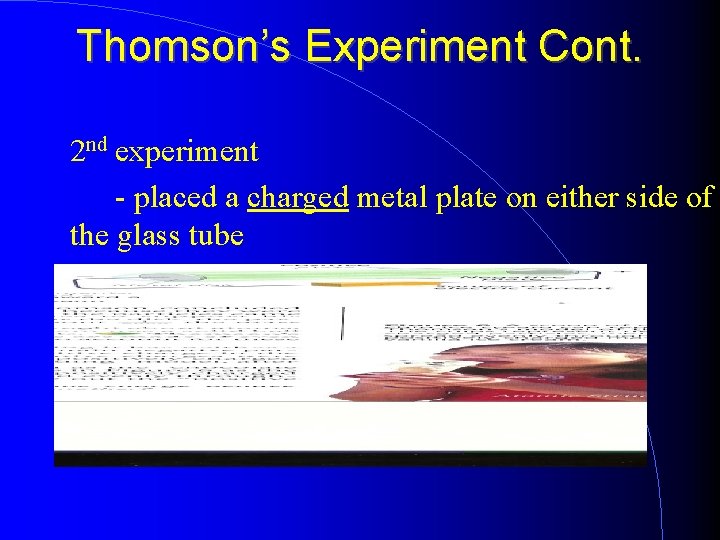
Thomson’s Experiment Cont. 2 nd experiment - placed a charged metal plate on either side of the glass tube

Thomson’s Experiment Cont. Observations 1 st experiment - glowing ray emerged between the cathode and anode 2 nd experiment - charged plates caused the beam to deflect/bend - repelled from the negative - attracted to the positive Conclusion - beam of light (stream of charged particles) - negative

Thomson’s Experiment Cont. Uses - TV screens - computer monitors - radar displays *Later became known as cathode rays due to their origin now known as an electron beam - 1 st to provide evidence that atoms are made of smaller particles - revised Dalton’s model

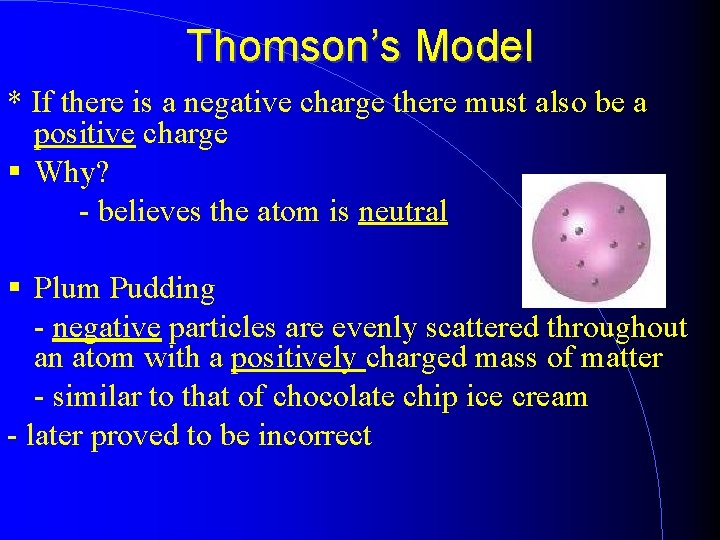
Thomson’s Model * If there is a negative charge there must also be a positive charge Why? - believes the atom is neutral Plum Pudding - negative particles are evenly scattered throughout an atom with a positively charged mass of matter - similar to that of chocolate chip ice cream - later proved to be incorrect

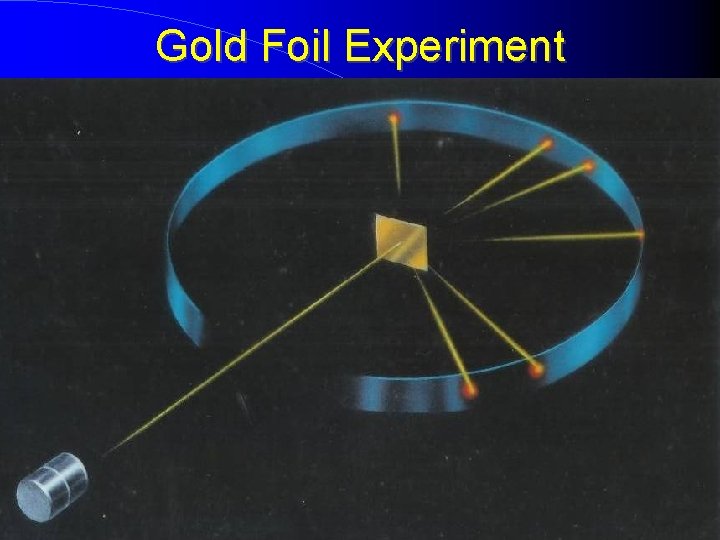
Rutherford’s Theory Ernest Rutherford - a former student of Thomson came up with a more accurate picture of the atom in 1909 -oversaw the now famous gold foil experiment Gold Foil Experiment Hypothesis - alpha particles are thousands of times more massive, hence they would not be impeded as it passed through the “atomic pudding” - beam of positively charged particles, alpha particles from a radioactive source was directed through a sheet of very thin gold foil

Gold Foil Experiment

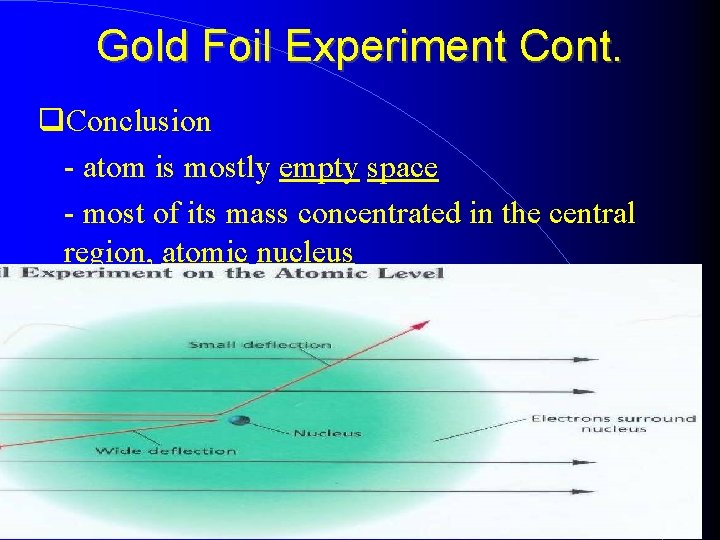
Gold Foil Experiment Cont. Observations - nearly all passed through undeflected and produced spots of light - some were widely deflected, and a few bounced straight back What massive object did they hit? atomic nucleus, an extremely dense positively charged center of the atom

Gold Foil Experiment Cont. Why did the others pass through then?

Gold Foil Experiment Cont. Conclusion - atom is mostly empty space - most of its mass concentrated in the central region, atomic nucleus

Gold Foil Experiment Cont. - the nucleus and surrounding electrons occupy only a tiny fraction of the atomic volume - diameter of an atom is generally about 10, 000 times greater than the diameter of its nucleus * If the nucleus were the size of the period at the end of this sentence, the outer edges of the atom would be located some 3. 3 meters away *

Gold Foil Experiment Cont. Rutherford’s Model - all of the atoms positive charge is concentrated in the nucleus, which only takes up a very small amount of the atom Can we then say we are mainly empty space?

Objectives Identify three subatomic particles Understand how subatomic particle was discovered Compare the properties of the subatomic particles Distinguish between atomic number and mass number Calculate the number of protons, electrons and neutrons in an atom

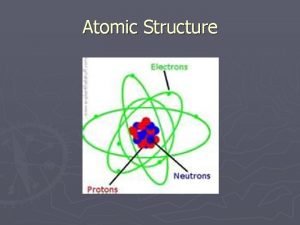
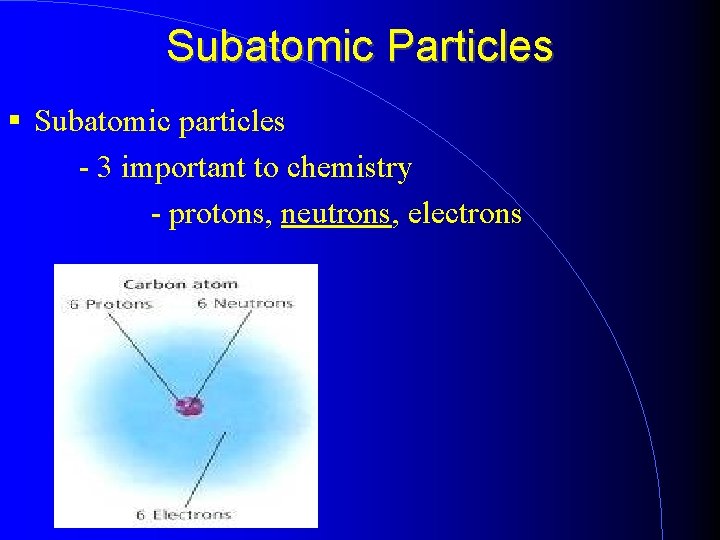
Subatomic Particles Subatomic particles - 3 important to chemistry - protons, neutrons, electrons

Protons Definition - a positively charge subatomic particle that is found in the nucleus of an atom About Protons - proton is nearly 2000 times more massive than the electron, but equal in charge and opposite in sign to the electron - number of protons in the nucleus is electrically balanced by an equal number of electrons ex. oxygen atom: contains 8 electrons and protons: neutral atom, no net charge

Electron Definition - a negatively charged subatomic particle that is found in the space outside the nucleus - name comes from the Greek word for amber - Amber: material discovered by early Greeks that was found to exhibit the effects of electrical charging ex. Ben Franklin: Key/Kite - lead others to experiment with electric currents through gases in sealed tubes

Neutrons Definition - is a neutral subatomic particle that is found in the nucleus of the atom - mass almost exactly equal to that of the proton

Comparing Subatomic Particles

Atomic Number Definition - number of protons in the atom ex. Oxygen 8 p + 8 n = 16 - elements are classified by this number - continues up to 119 - unique to a given element - all atoms are electrically neutral, meaning the number of electrons must equal the number of protons - this arrangement of elements by their atomic numbers makes up the periodic table - Usually located at the upper left hand corner

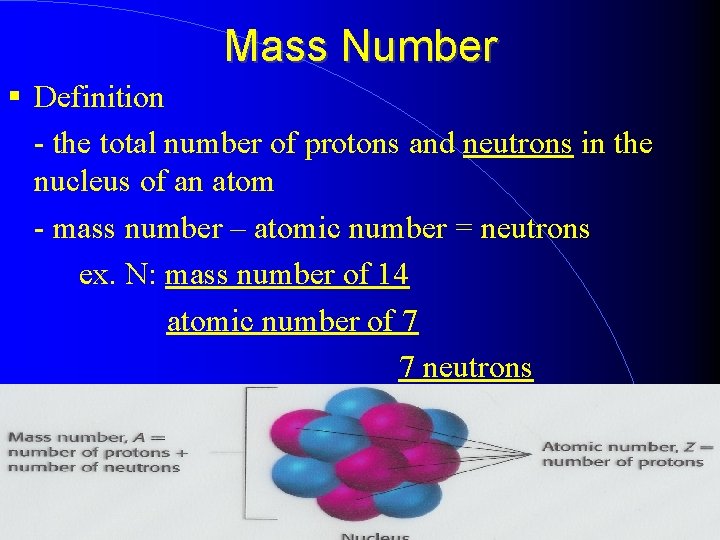
Mass Number Definition - the total number of protons and neutrons in the nucleus of an atom - mass number – atomic number = neutrons ex. N: mass number of 14 atomic number of 7 7 neutrons

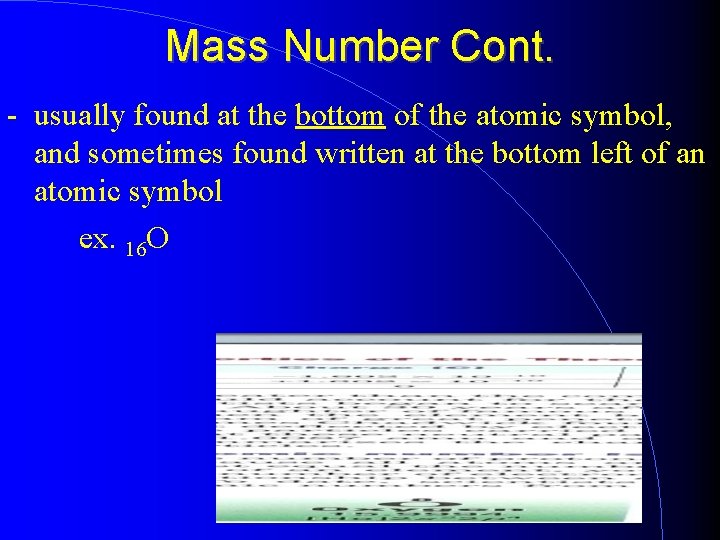
Mass Number Cont. - usually found at the bottom of the atomic symbol, and sometimes found written at the bottom left of an atomic symbol ex. 16 O

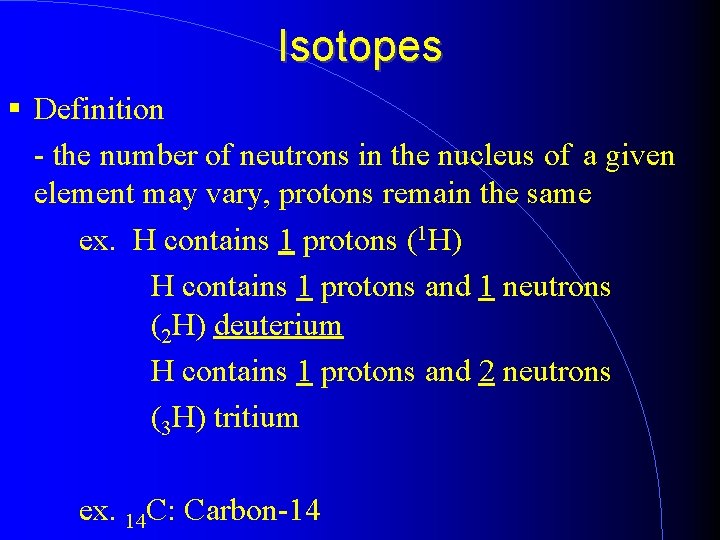
Mass Number - although a given type of atom will usually contain a certain number of neutrons in the nucleus, a small percentage will not ex. most hydrogen atoms contain no neutrons - a small percentage contain one neutron and a smaller percentage two neutrons What do we call atoms with a different number of neutrons? - isotopes

Isotopes Definition - the number of neutrons in the nucleus of a given element may vary, protons remain the same ex. H contains 1 protons (1 H) H contains 1 protons and 1 neutrons (2 H) deuterium H contains 1 protons and 2 neutrons (3 H) tritium ex. 14 C: Carbon-14

Atomic Mass Definition - mass of an atom in atomic mass units (amu) - atoms have very little mass - equal to 1/12 th of the mass of carbon - often an average mass - weighted mass AMU or the Dalton (Da) - equal to 1. 6605402 x 10 -27 kg

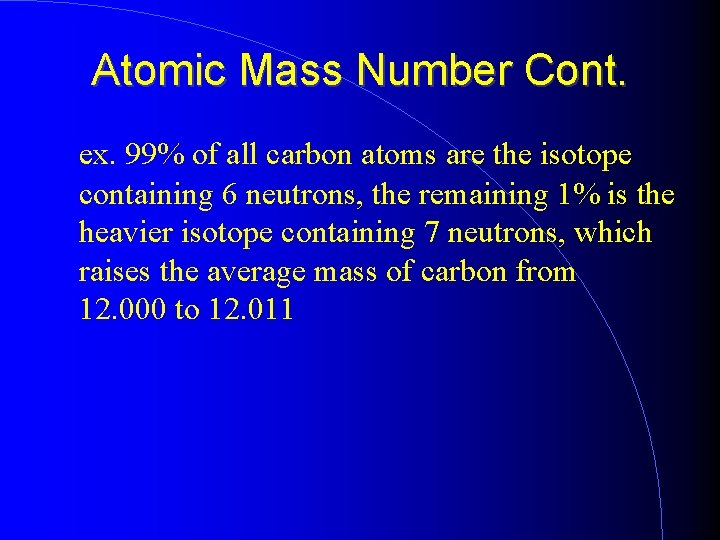
Atomic Mass Number Cont. ex. 99% of all carbon atoms are the isotope containing 6 neutrons, the remaining 1% is the heavier isotope containing 7 neutrons, which raises the average mass of carbon from 12. 000 to 12. 011

Objectives Describe Bohr’s model of the atom and the evidence for energy levels Explain how the electron cloud model represents the behavior and locations of electrons in atoms

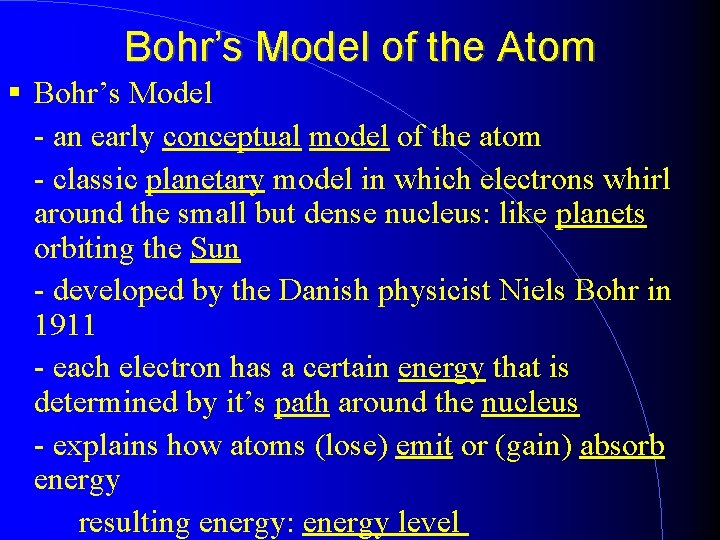
Bohr’s Model of the Atom Bohr’s Model - an early conceptual model of the atom - classic planetary model in which electrons whirl around the small but dense nucleus: like planets orbiting the Sun - developed by the Danish physicist Niels Bohr in 1911 - each electron has a certain energy that is determined by it’s path around the nucleus - explains how atoms (lose) emit or (gain) absorb energy resulting energy: energy level

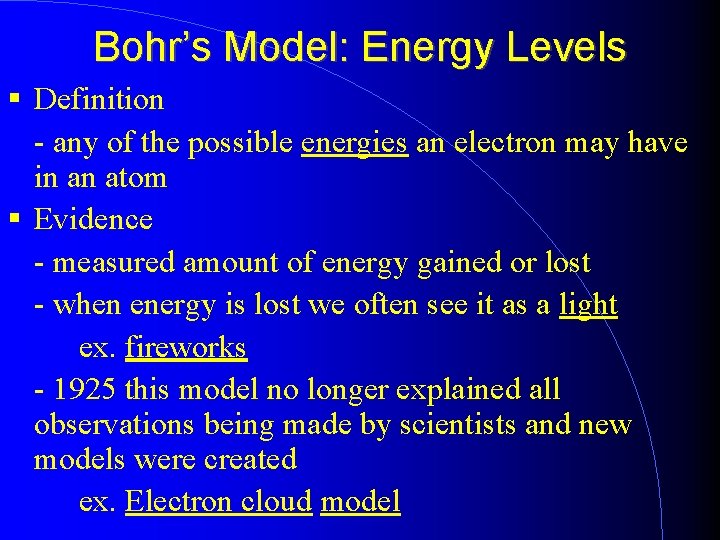
Bohr’s Model: Energy Levels Definition - any of the possible energies an electron may have in an atom Evidence - measured amount of energy gained or lost - when energy is lost we often see it as a light ex. fireworks - 1925 this model no longer explained all observations being made by scientists and new models were created ex. Electron cloud model

Electron Cloud Model - visual model of the most likely locations for electrons in an atom Orbitals - a region in an atom where there is a high probability of finding an electron ex. propeller on a helicopter (you know its there you see a blur, can’t pinpoint exact location) - 4 orbitals s – 2 houses 2 ep – 3 houses 6 ed – 5 houses 10 e-

Orbitals - electrons occupy the lowest energy levels first - electrons in the outermost energy levels of an atoms are called valence electron

Electron Configurations Definition - arrangement of electrons in the orbitals of an atom (similar to seating assignments on an airplane) - when all electrons at their lowest energies this is called ground state
 Democritus greek model
Democritus greek model Which greek philosopher defined the art of persuasion
Which greek philosopher defined the art of persuasion Aristotle was the student of
Aristotle was the student of Was aristotle a philosopher
Was aristotle a philosopher What greek philosopher named the appeals ethos pathos logos
What greek philosopher named the appeals ethos pathos logos Where does tragedy come from
Where does tragedy come from Democritus greek model
Democritus greek model 460 bc atomic structure
460 bc atomic structure Democritus atomic model
Democritus atomic model Bc atomic mass
Bc atomic mass Democritus atomic theory model
Democritus atomic theory model Atomic models timeline
Atomic models timeline The philosopher king plato
The philosopher king plato American philosopher
American philosopher Harry potter spisateljica
Harry potter spisateljica Gma grube
Gma grube Social contract philosopher
Social contract philosopher French philosopher mathematician
French philosopher mathematician Italian physicist mathematician astronomer and philosopher
Italian physicist mathematician astronomer and philosopher Philosopher translator secretary architecture
Philosopher translator secretary architecture The philosopher's way 5th edition
The philosopher's way 5th edition Philosopher of cordoba
Philosopher of cordoba Arabic indic numerals
Arabic indic numerals David armstrong philosopher
David armstrong philosopher Weeping philosopher
Weeping philosopher Philosopher giving a lecture on the orrery
Philosopher giving a lecture on the orrery Jagger philosopher
Jagger philosopher Philosopher puns
Philosopher puns Simile
Simile The school of athens
The school of athens Enlightened philosopher pathfinder
Enlightened philosopher pathfinder What is the greek miracle in greek mythology
What is the greek miracle in greek mythology History of the atom democritus
History of the atom democritus Democritus atom model
Democritus atom model Democritus contribution
Democritus contribution Aristotle democritus
Aristotle democritus Perkembangan model atom
Perkembangan model atom Nuclear symbol notation
Nuclear symbol notation