History of the Atom Democritus u Greek Philosopher









- Slides: 17

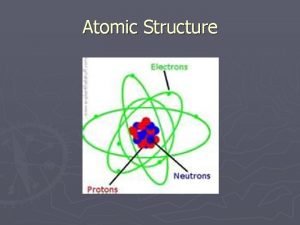
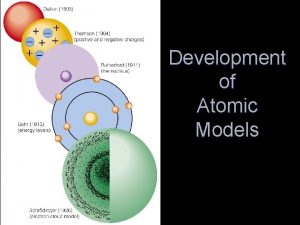
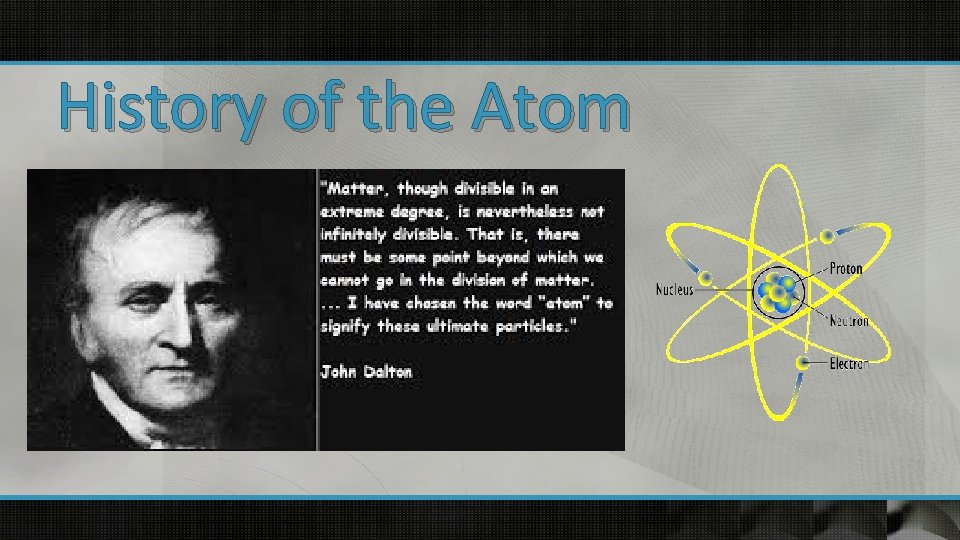
History of the Atom

Democritus u Greek Philosopher (460 -370 B. C. ) îAmong the first to suggest the existence of atoms. îBelieved that atoms were indivisible & indestructible îAgreed with later scientific theory

Democritus u His theory did not explain chemical behavior. u Lacked experimental support. u His approach was not based on the scientific method.

John Dalton u English chemist & School Teacher (1766 -1844) u 2000 years after Democritus u Used experimental methods to transform Democritus’ ideas into scientific theory.

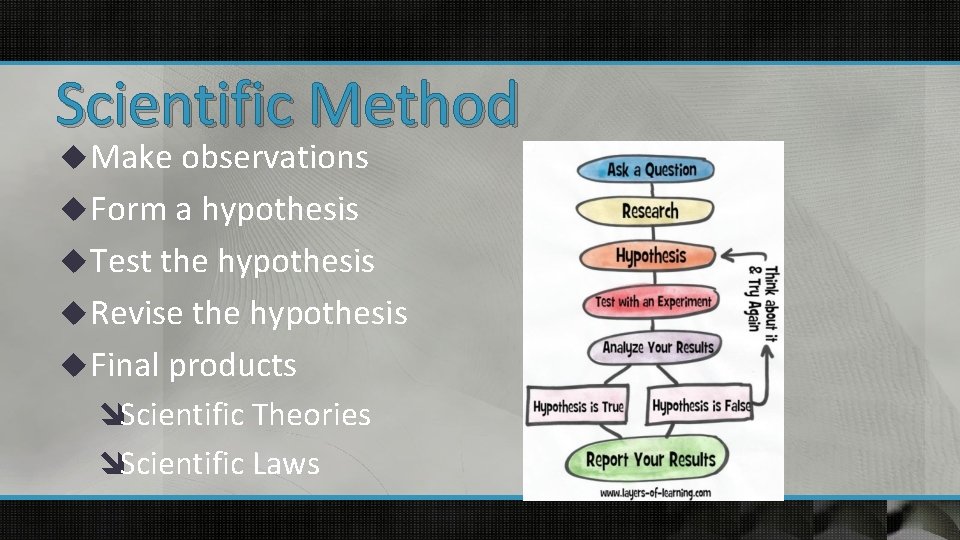
Scientific Method u Make observations u Form a hypothesis u Test the hypothesis u Revise the hypothesis u Final products îScientific Theories îScientific Laws

John Dalton’s Atomic Theory u All elements are composed of tiny indivisible particles called atoms u Atoms of the same element are identical. u The atoms of any one element are different than those from any other element.

John Dalton’s Atomic Theory u Atoms of different elements can chemically combine in simple whole number ratios to form compounds. u Chemical reactions occur when atoms are separated, joined or rearranged. Atoms themselves are never changed into atoms of another element as a result of a chemical reaction

J. J. Thomson u English Physicist (1856 -1940). u Discovered the electron u An electron is a negatively charged subatomic particle.

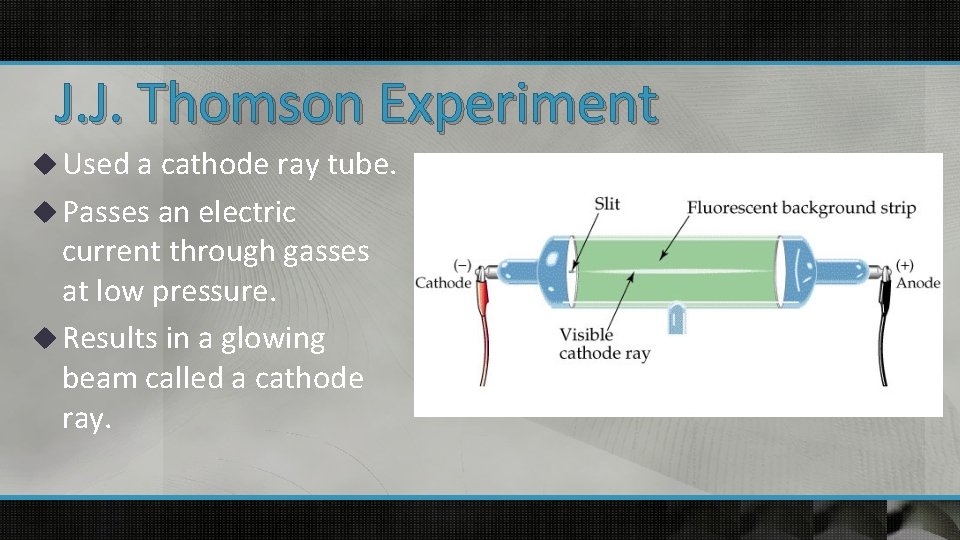
J. J. Thomson Experiment u Used a cathode ray tube. u Passes an electric current through gasses at low pressure. u Results in a glowing beam called a cathode ray.

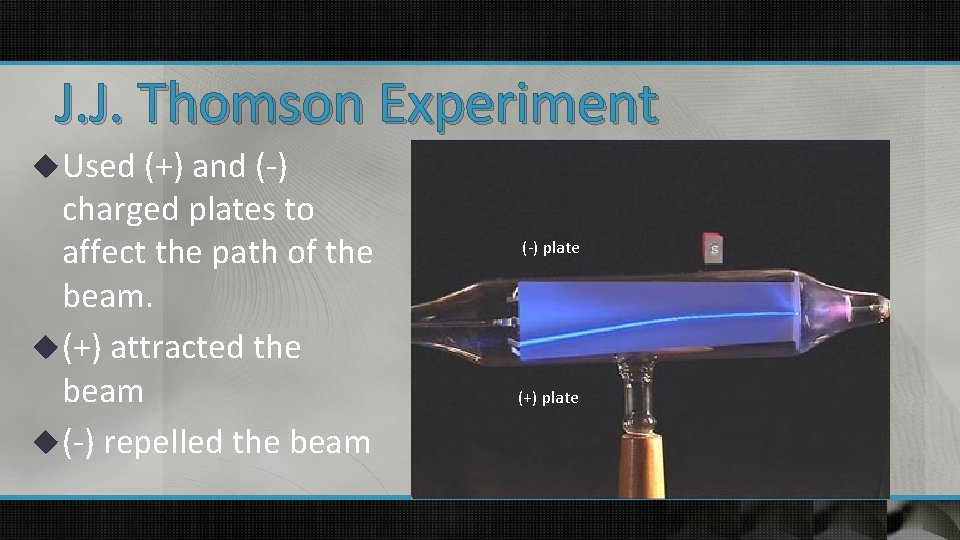
J. J. Thomson Experiment u Used (+) and (-) charged plates to affect the path of the beam. u (+) attracted the beam u (-) repelled the beam (-) plate (+) plate

J. J. Thomson Experiment u Repeated the experiment with different gasses with the same results. u Hypothesized that a cathode ray is a stream of tiny negatively charged particles which he called electrons. u Concluded that electrons must be a part of all atoms.

Robert Millikan u U. S. Physicist (1868 -1953). u Calculated the charge and mass of an electron. u 1 unit of negative charge u 1/1840 the mass of a hydrogen atom.

Protons u Since electrons are negatively charged and atoms are positively charged, atoms must contain a positive charge as well. u Electric charge must be carried on a unit of matter. u Electric charges always occur in whole number multiples of a single basic unit. u Therefore atoms must contain a particle that is positively charged.

Eugen Goldstein u German Physicist (1850 -1930). u Confirmed positively charged particles when he observed rays travelling in the opposite direction of the cathode rays in the cathode tube. u From the (+) cathode to the () anode.

Eugen Goldstein u Concluded that the ray was composed of positively charged particles. u Named them protons. u A proton has a mass of about 1840 times that of an electron.

Eugen Goldstein u Concluded that the ray was composed of positively charged particles. u Named them protons. u A proton has a mass of about 1840 times that of an electron.

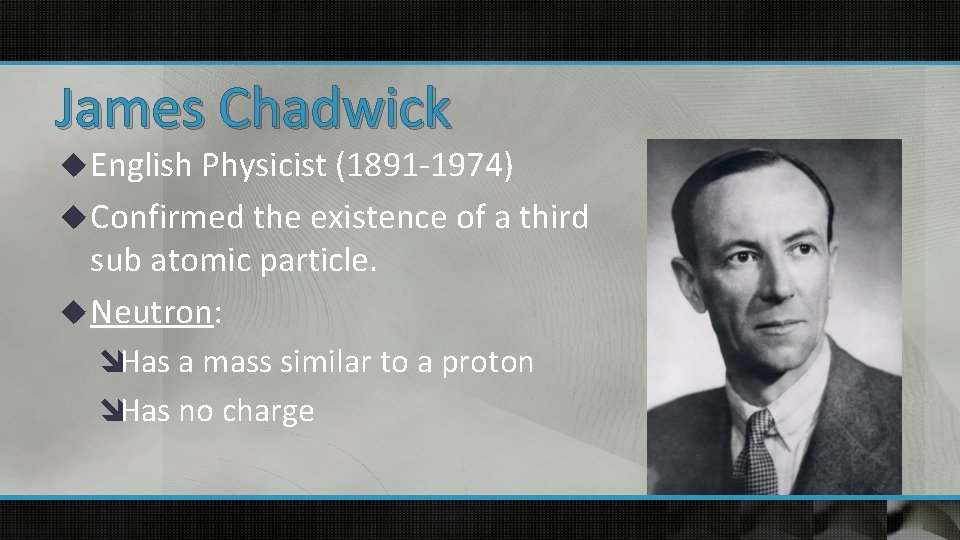
James Chadwick u English Physicist (1891 -1974) u Confirmed the existence of a third sub atomic particle. u Neutron: îHas a mass similar to a proton îHas no charge
 Greek philosopher democritus
Greek philosopher democritus Democritus atom modeli
Democritus atom modeli Which greek philosopher defined the art of persuasion
Which greek philosopher defined the art of persuasion Aristotle is the student of
Aristotle is the student of Was aristotle a philosopher
Was aristotle a philosopher Ethos, pathos and logos
Ethos, pathos and logos Where does tragedy come from
Where does tragedy come from Democritus atomic theory
Democritus atomic theory Teori atom democritus
Teori atom democritus Kelemahan teori atom dalton
Kelemahan teori atom dalton Democritus atomic theory model
Democritus atomic theory model Nuclear symbol notation
Nuclear symbol notation Democritus atom modeli
Democritus atom modeli Atom table of contents
Atom table of contents Democritus atomic theory
Democritus atomic theory Teori atom democritus
Teori atom democritus Democritus atom modeli
Democritus atom modeli Teori atom democritus
Teori atom democritus