Dalla non inferiorita alla superiorita i NAO sono














- Slides: 43

Dalla “non inferiorita ” alla “superiorita ”: i NAO sono tutti uguali? Ph. D, MD Sergio Agosti Dirigente medico SOC Cardiologia Ospedale Novi Ligure

Agenda • Perché un DOAC • Differenze metodologiche trials DOACs • Differenze farmacocinetiche e farmacodinamiche • Aderenza e compliance • Malattia coronarica • In tutte le situazioni

Agenda • Perché un DOAC • Differenze metodologiche trials DOACs • Differenze farmacocinetiche e farmacodinamiche • Aderenza e compliance • Malattia coronarica • In tutte le situazioni

Lancet, published online December 4, 2013

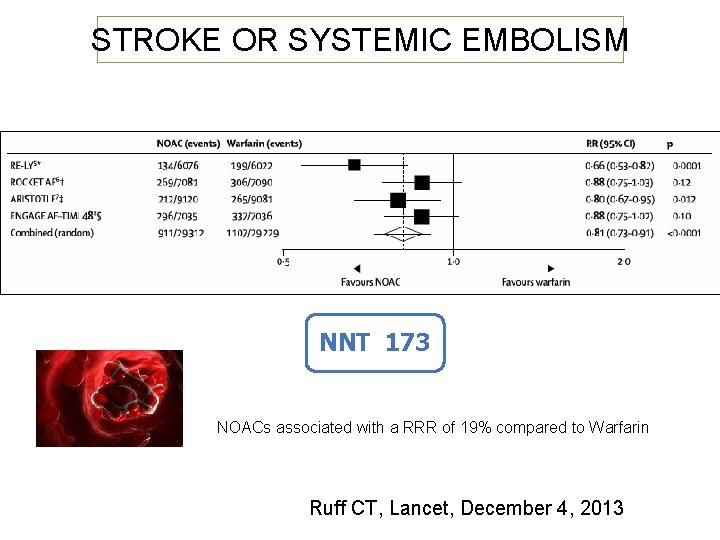
STROKE OR SYSTEMIC EMBOLISM NNT 173 NOACs associated with a RRR of 19% compared to Warfarin Ruff CT, Lancet, December 4, 2013

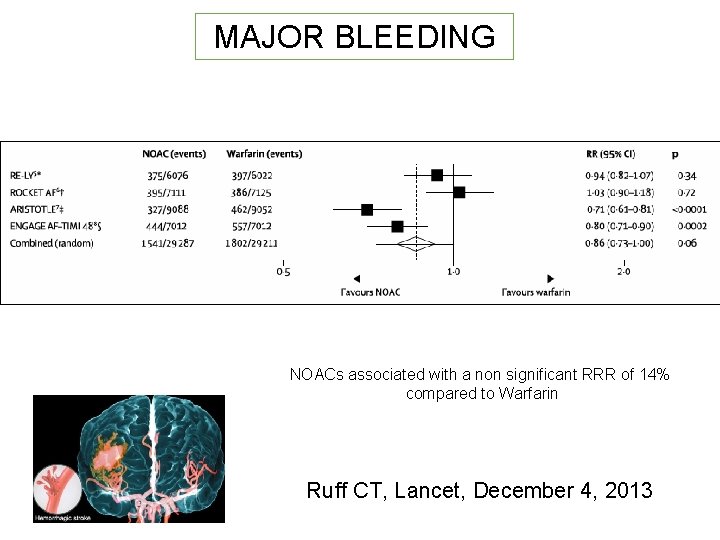
MAJOR BLEEDING NOACs associated with a non significant RRR of 14% compared to Warfarin Ruff CT, Lancet, December 4, 2013

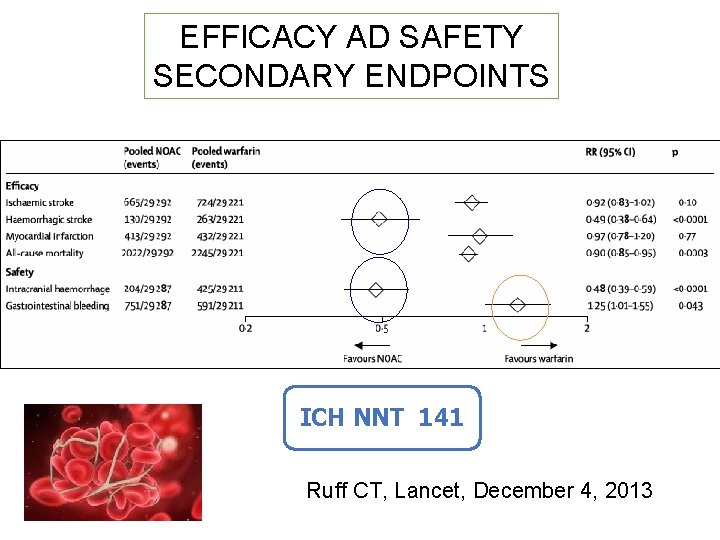
EFFICACY AD SAFETY SECONDARY ENDPOINTS ICH NNT 141 Ruff CT, Lancet, December 4, 2013

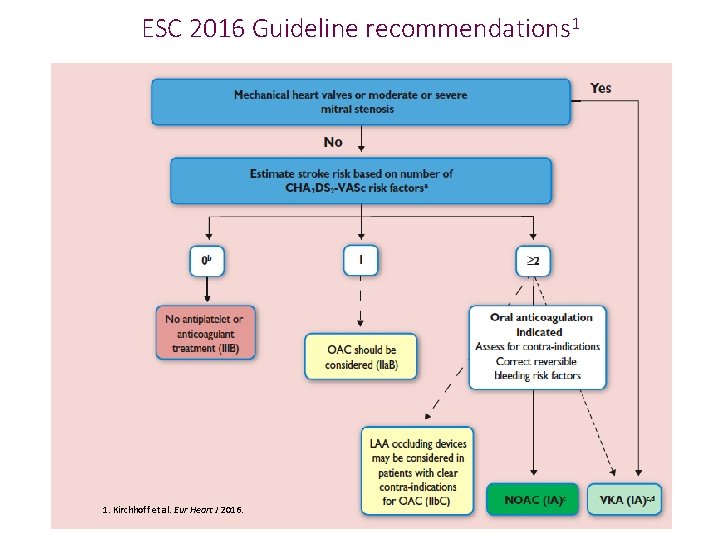
ESC 2016 Guideline recommendations 1 1. Kirchhoff et al. Eur Heart J 2016.

Agenda • Perché un DOAC • Differenze metodologiche trials DOACs • Differenze farmacocinetiche e farmacodinamiche • Aderenza e compliance • Malattia coronarica • In tutte le situazioni

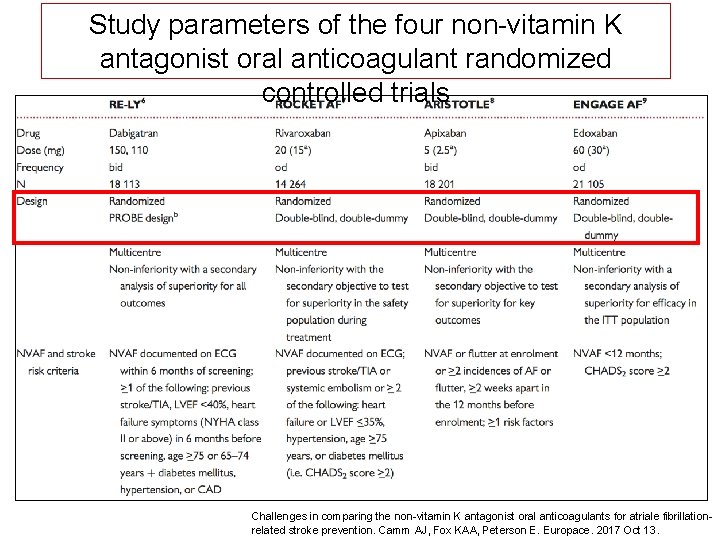
Study parameters of the four non-vitamin K antagonist oral anticoagulant randomized controlled trials Challenges in comparing the non-vitamin K antagonist oral anticoagulants for atriale fibrillationrelated stroke prevention. Camm AJ, Fox KAA, Peterson E. Europace. 2017 Oct 13.

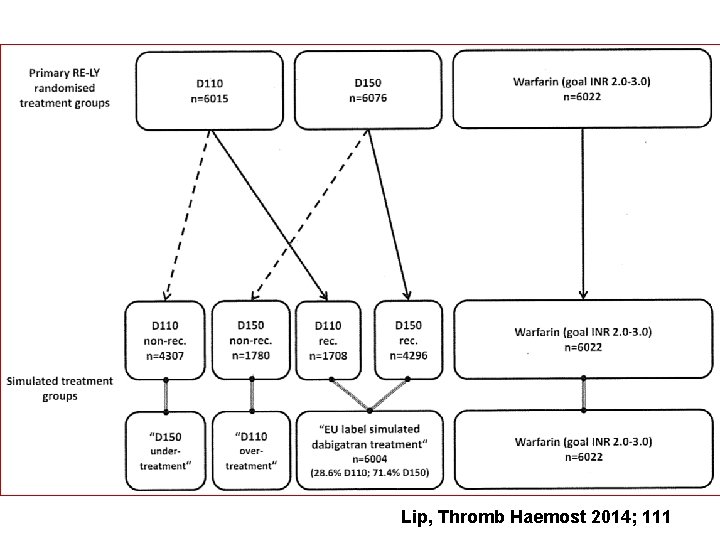
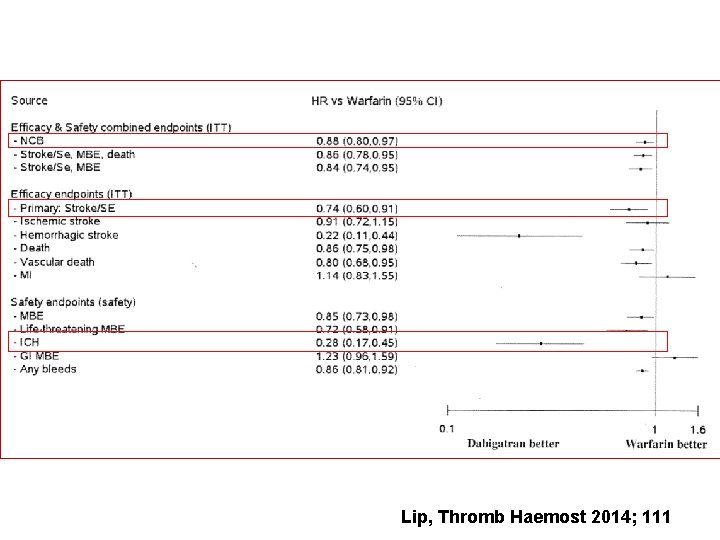
Lip, Thromb Haemost 2014; 111

Lip, Thromb Haemost 2014; 111

Lip, Thromb Haemost 2014; 111

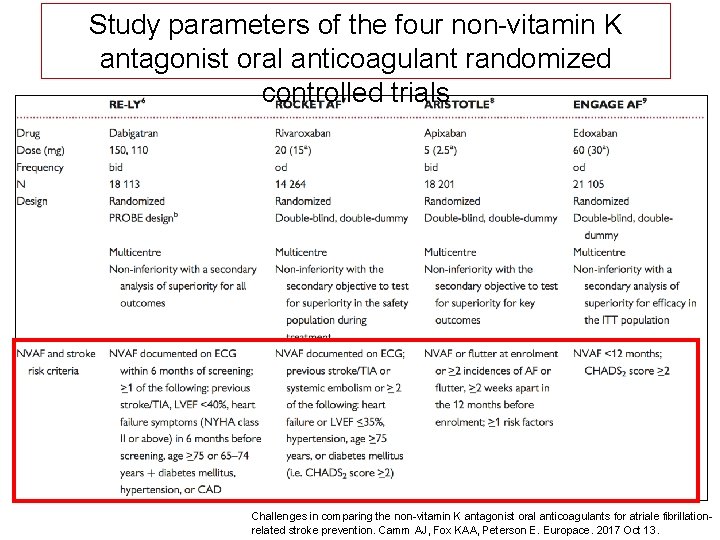
Study parameters of the four non-vitamin K antagonist oral anticoagulant randomized controlled trials Challenges in comparing the non-vitamin K antagonist oral anticoagulants for atriale fibrillationrelated stroke prevention. Camm AJ, Fox KAA, Peterson E. Europace. 2017 Oct 13.

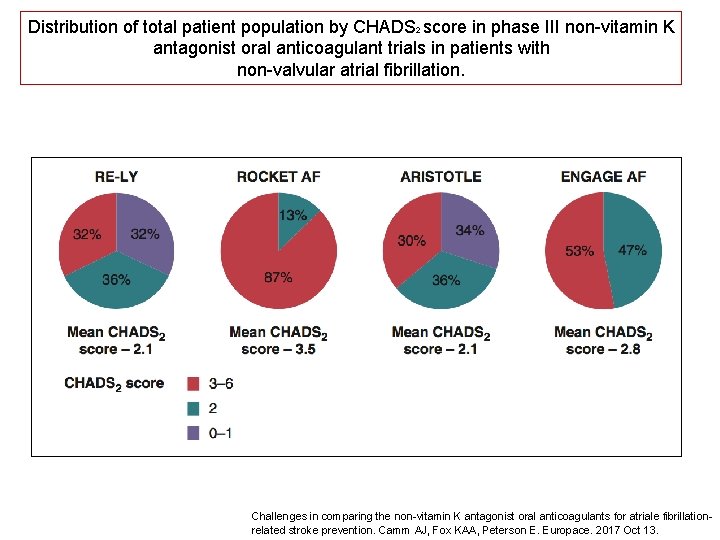
Distribution of total patient population by CHADS 2 score in phase III non-vitamin K antagonist oral anticoagulant trials in patients with non-valvular atrial fibrillation. Challenges in comparing the non-vitamin K antagonist oral anticoagulants for atriale fibrillationrelated stroke prevention. Camm AJ, Fox KAA, Peterson E. Europace. 2017 Oct 13.

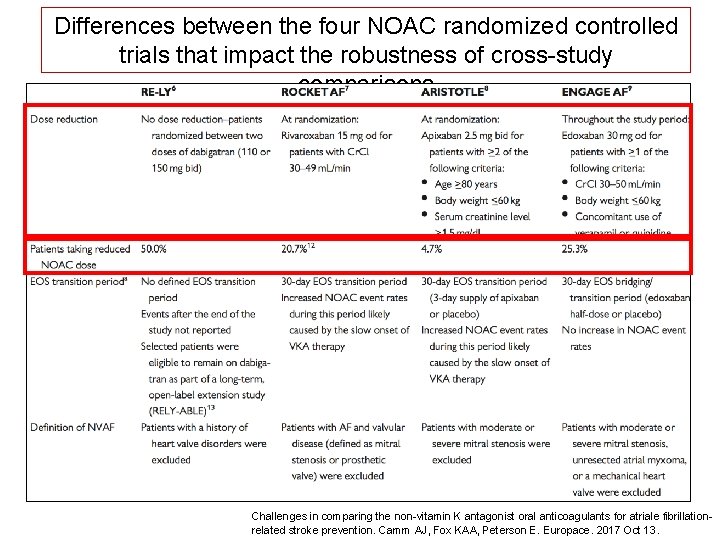
Differences between the four NOAC randomized controlled trials that impact the robustness of cross-study comparisons Challenges in comparing the non-vitamin K antagonist oral anticoagulants for atriale fibrillationrelated stroke prevention. Camm AJ, Fox KAA, Peterson E. Europace. 2017 Oct 13.

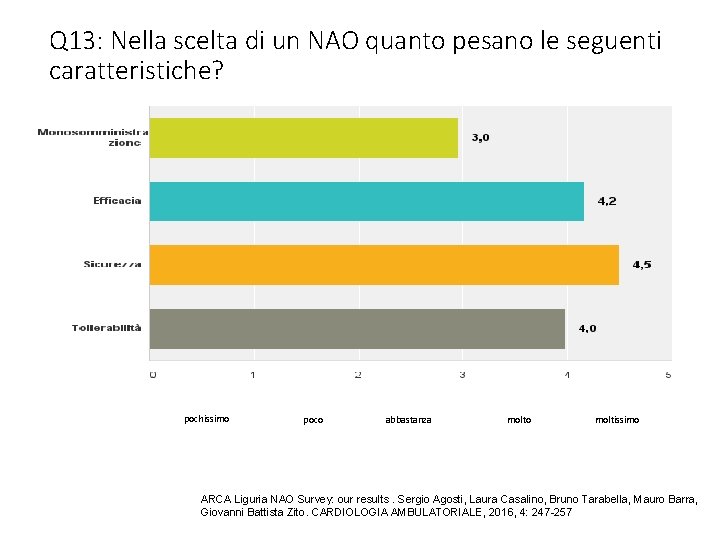
Q 13: Nella scelta di un NAO quanto pesano le seguenti caratteristiche? pochissimo poco abbastanza molto moltissimo ARCA Liguria NAO Survey: our results. Sergio Agosti, Laura Casalino, Bruno Tarabella, Mauro Barra, Giovanni Battista Zito. CARDIOLOGIA AMBULATORIALE, 2016, 4: 247 -257

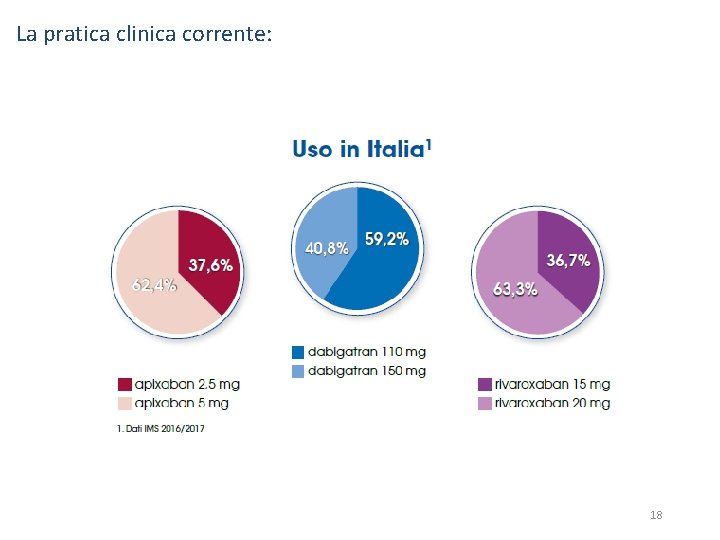
La pratica clinica corrente: 18

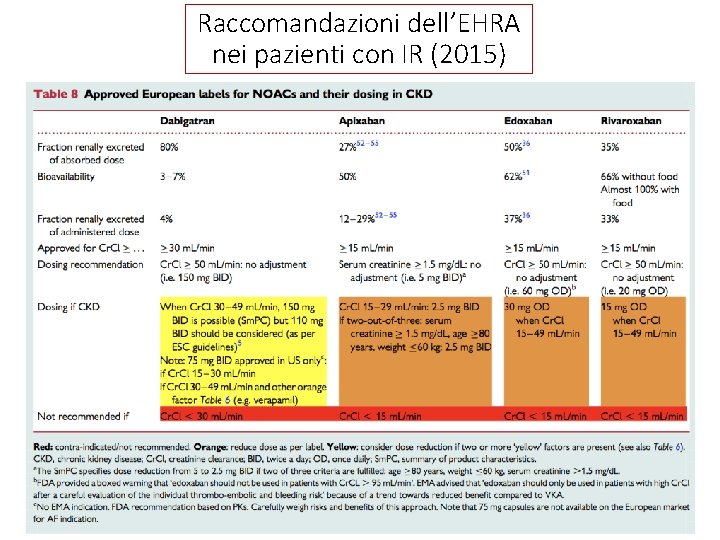
Raccomandazioni dell’EHRA nei pazienti con IR (2015)

Agenda • Perché un DOAC • Differenze metodologiche trials DOACs • Differenze farmacocinetiche e farmacodinamiche • Aderenza e compliance • Malattia coronarica • In tutte le situazioni

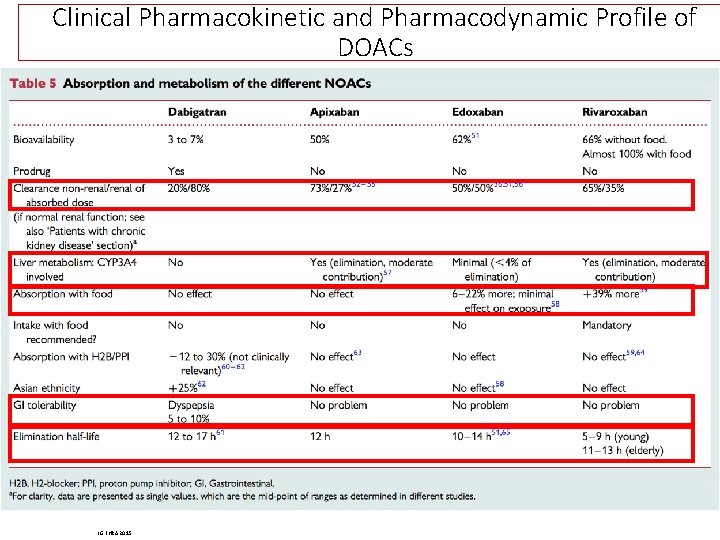
Clinical Pharmacokinetic and Pharmacodynamic Profile of DOACs LG EHRA 2015

Agenda • Perché un DOAC • Differenze metodologiche trials DOACs • Differenze farmacocinetiche e farmacodinamiche • Aderenza e compliance • Malattia coronarica • In tutte le situazioni

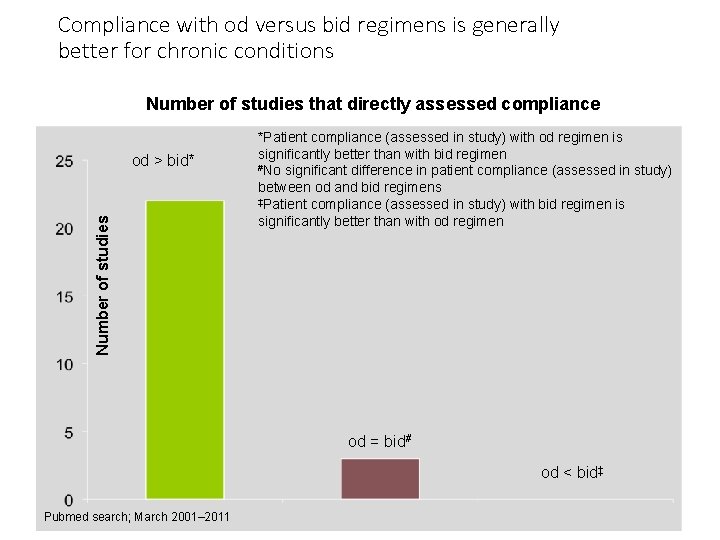
Compliance with od versus bid regimens is generally better for chronic conditions Number of studies that directly assessed compliance Number of studies od > bid* *Patient compliance (assessed in study) with od regimen is significantly better than with bid regimen #No significant difference in patient compliance (assessed in study) between od and bid regimens ‡Patient compliance (assessed in study) with bid regimen is significantly better than with od regimen od = bid# od < bid‡ Pubmed search; March 2001– 2011

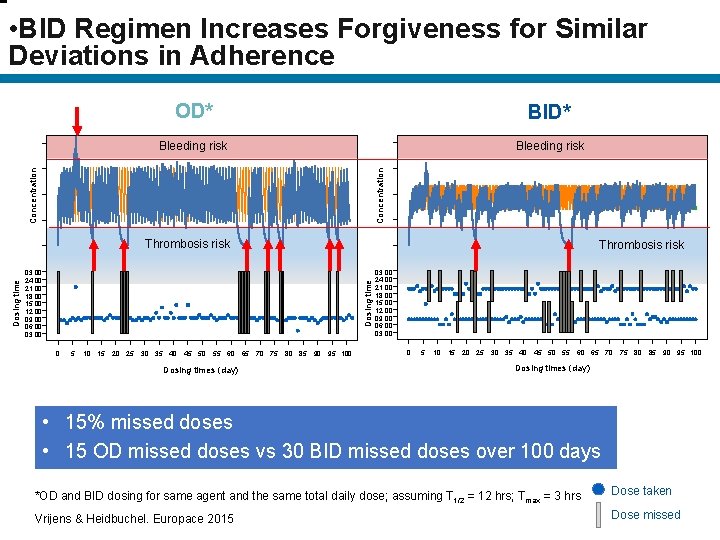
• BID Regimen Increases Forgiveness for Similar Deviations in Adherence OD* BID* Bleeding risk Concentration Thrombosis risk Dosing time 03: 00 24: 00 21: 00 18: 00 15: 00 12: 00 09: 00 06: 00 03: 00 0 5 10 15 20 25 30 35 40 45 50 55 60 65 70 75 80 85 90 95 100 Dosing times (day) Dosing time Bleeding risk Thrombosis risk 03: 00 24: 00 21: 00 18: 00 15: 00 12: 00 09: 00 06: 00 03: 00 0 5 10 15 20 25 30 35 40 45 50 55 60 65 70 75 80 85 90 95 100 Dosing times (day) • 15% missed doses • 15 OD missed doses vs 30 BID missed doses over 100 days *OD and BID dosing for same agent and the same total daily dose; assuming T 1/2 = 12 hrs; Tmax = 3 hrs Dose taken Vrijens & Heidbuchel. Europace 2015 Dose missed

Agenda • Perché un DOAC • Differenze metodologiche trials DOACs • Differenze farmacocinetiche e farmacodinamiche • Aderenza e compliance • Malattia coronarica • In tutte le situazioni

There is an unmet need in the management of patients with AF undergoing PCI 20– 30% of patients with AF and an indication for continuous OAC have coexisting CAD and therefore may require PCI An estimated 1– 2 million anticoagulated patients in Europe are candidates for PCI procedures Stenting requires follow-up treatment with antiplatelets, which puts anticoagulated patients at higher risk of bleeding CAD, coronary artery disease; PCI, percutaneous coronary intervention; Lip et al. Thromb Haemost 2010 26

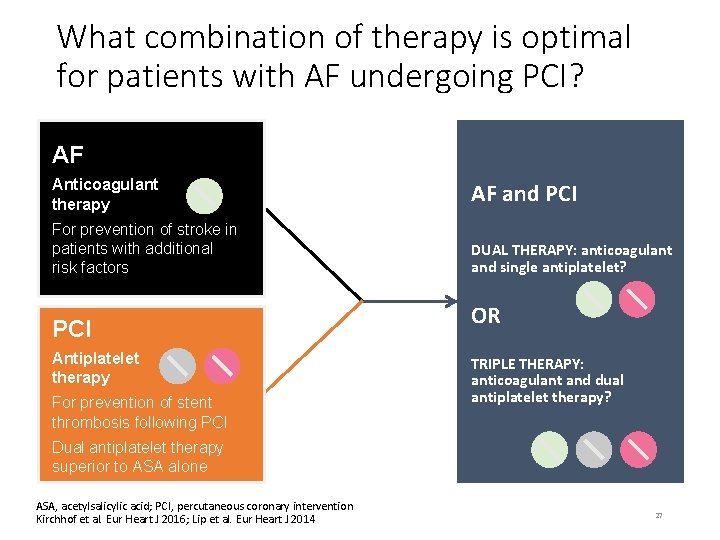
What combination of therapy is optimal for patients with AF undergoing PCI? AF Anticoagulant therapy AF and PCI For prevention of stroke in patients with additional risk factors DUAL THERAPY: anticoagulant and single antiplatelet? PCI Antiplatelet therapy For prevention of stent thrombosis following PCI OR TRIPLE THERAPY: anticoagulant and dual antiplatelet therapy? Dual antiplatelet therapy superior to ASA alone ASA, acetylsalicylic acid; PCI, percutaneous coronary intervention Kirchhof et al. Eur Heart J 2016; Lip et al. Eur Heart J 2014 27

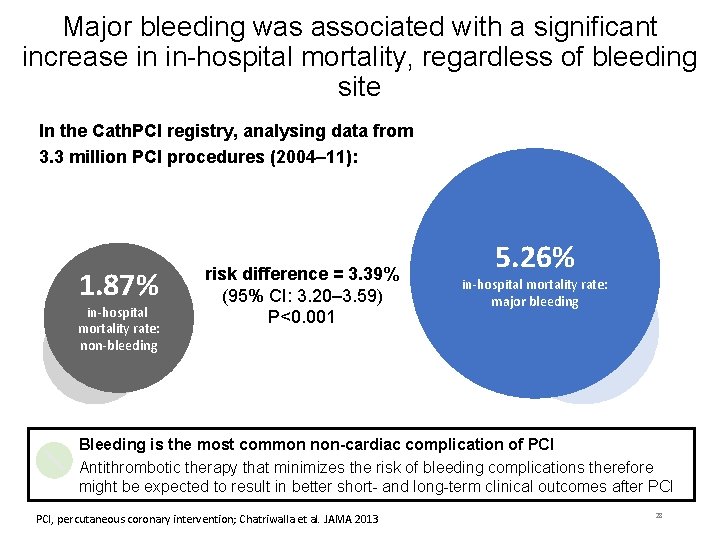
Major bleeding was associated with a significant increase in in-hospital mortality, regardless of bleeding site In the Cath. PCI registry, analysing data from 3. 3 million PCI procedures (2004– 11): 1. 87% in-hospital mortality rate: non-bleeding risk difference = 3. 39% (95% CI: 3. 20– 3. 59) P<0. 001 5. 26% in-hospital mortality rate: major bleeding Bleeding is the most common non-cardiac complication of PCI Antithrombotic therapy that minimizes the risk of bleeding complications therefore might be expected to result in better short- and long-term clinical outcomes after PCI, percutaneous coronary intervention; Chatriwalla et al. JAMA 2013 28

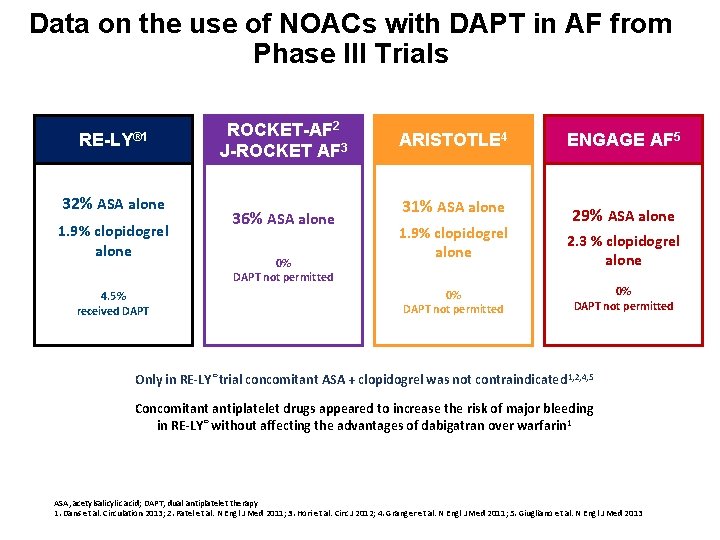
Data on the use of NOACs with DAPT in AF from Phase III Trials RE-LY® 1 32% ASA alone 1. 9% clopidogrel alone 4. 5% received DAPT ROCKET-AF 2 J-ROCKET AF 3 36% ASA alone 0% DAPT not permitted ARISTOTLE 4 ENGAGE AF 5 31% ASA alone 29% ASA alone 1. 9% clopidogrel alone 0% DAPT not permitted 2. 3 % clopidogrel alone 0% DAPT not permitted Only in RE-LY® trial concomitant ASA + clopidogrel was not contraindicated 1, 2, 4, 5 Concomitant antiplatelet drugs appeared to increase the risk of major bleeding in RE-LY® without affecting the advantages of dabigatran over warfarin 1 ASA, acetylsalicylic acid; DAPT, dual antiplatelet therapy 1. Dans et al. Circulation 2013; 2. Patel et al. N Engl J Med 2011; 3. Hori et al. Circ J 2012; 4. Granger et al. N Engl J Med 2011; 5. Giugliano et al. N Engl J Med 2013

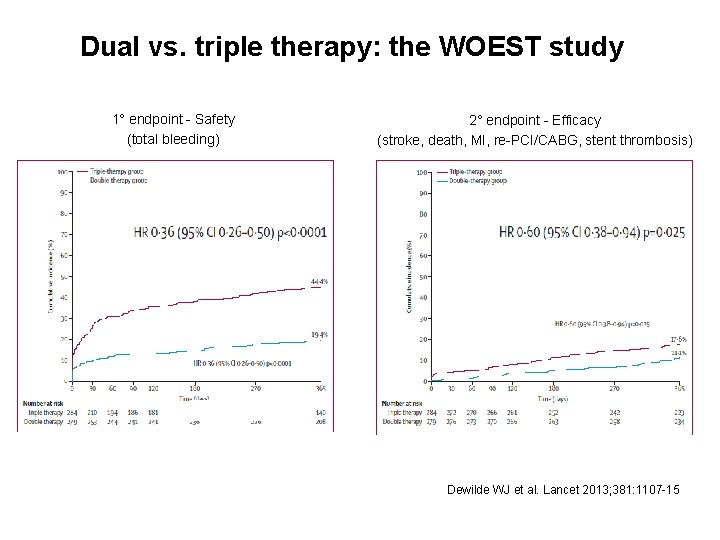
Dual vs. triple therapy: the WOEST study 1° endpoint - Safety (total bleeding) 2° endpoint - Efficacy (stroke, death, MI, re-PCI/CABG, stent thrombosis) Dewilde WJ et al. Lancet 2013; 381: 1107 -15

Clinical trials in patients with AF undergoing PCI • Augustus (Apixaban) ongoing • Redual PCI (Dabigatran) • Entrust AF-PCI (Edoxaban) ongoing • Pioneer AF-PCI Study (Rivaroxaban)

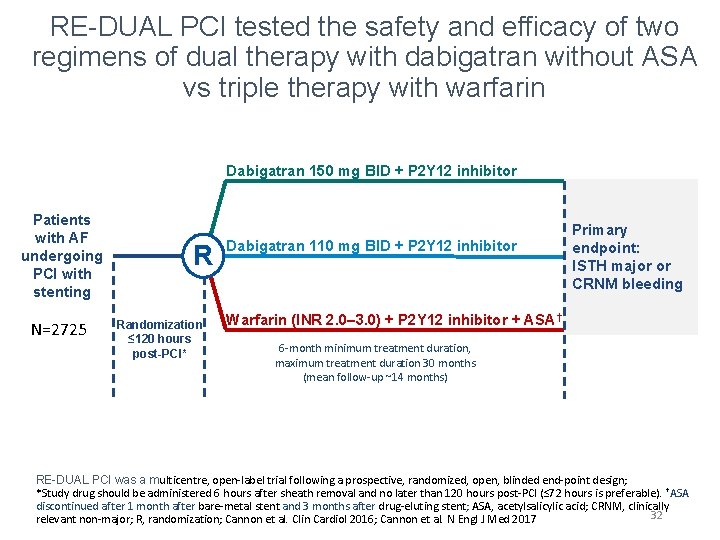
RE-DUAL PCI tested the safety and efficacy of two regimens of dual therapy with dabigatran without ASA vs triple therapy with warfarin Dabigatran 150 mg BID + P 2 Y 12 inhibitor Patients with AF undergoing PCI with stenting N=2725 R Randomization ≤ 120 hours post-PCI* Dabigatran 110 mg BID + P 2 Y 12 inhibitor Primary endpoint: ISTH major or CRNM bleeding Warfarin (INR 2. 0– 3. 0) + P 2 Y 12 inhibitor + ASA† 6 -month minimum treatment duration, maximum treatment duration 30 months (mean follow-up ~14 months) RE-DUAL PCI was a multicentre, open-label trial following a prospective, randomized, open, blinded end-point design; *Study drug should be administered 6 hours after sheath removal and no later than 120 hours post-PCI (≤ 72 hours is preferable). †ASA discontinued after 1 month after bare-metal stent and 3 months after drug-eluting stent; ASA, acetylsalicylic acid; CRNM, clinically 32 relevant non-major; R, randomization; Cannon et al. Clin Cardiol 2016; Cannon et al. N Engl J Med 2017

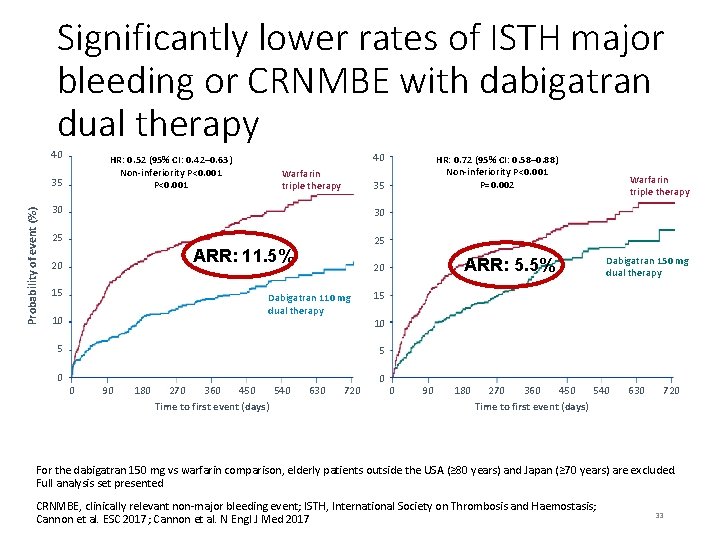
Significantly lower rates of ISTH major bleeding or CRNMBE with dabigatran dual therapy 40 HR: 0. 52 (95% CI: 0. 42– 0. 63) Non-inferiority P<0. 001 Probability of event (%) 35 40 Warfarin triple therapy 35 30 30 25 25 ARR: 11. 5% 20 15 5 0 ARR: 5. 5% 20 Dabigatran 110 mg dual therapy 10 HR: 0. 72 (95% CI: 0. 58– 0. 88) Non-inferiority P<0. 001 P=0. 002 Warfarin triple therapy Dabigatran 150 mg dual therapy 15 10 5 0 90 180 270 450 540 360 Time to first event (days) 630 720 0 0 90 180 270 450 540 360 Time to first event (days) 630 720 For the dabigatran 150 mg vs warfarin comparison, elderly patients outside the USA (≥ 80 years) and Japan (≥ 70 years) are excluded. Full analysis set presented CRNMBE, clinically relevant non-major bleeding event; ISTH, International Society on Thrombosis and Haemostasis; Cannon et al. ESC 2017; Cannon et al. N Engl J Med 2017 33

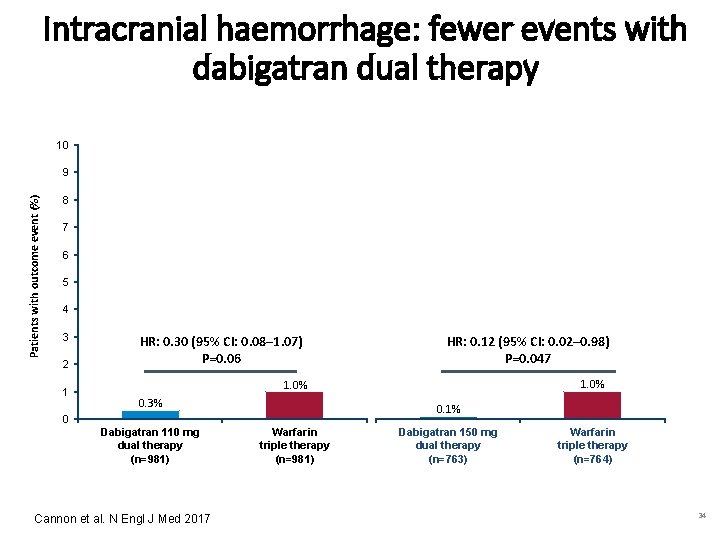
Intracranial haemorrhage: fewer events with dabigatran dual therapy 10 Patients with outcome event (%) 9 8 7 6 5 4 3 2 1 0 HR: 0. 30 (95% CI: 0. 08– 1. 07) P=0. 06 0. 3% Dabigatran 110 mg dual therapy (n=981) Cannon et al. N Engl J Med 2017 1. 0% Warfarin triple therapy (n=981) HR: 0. 12 (95% CI: 0. 02– 0. 98) P=0. 047 0. 1% Dabigatran 150 mg dual therapy (n=763) 1. 0% Warfarin triple therapy (n=764) 34

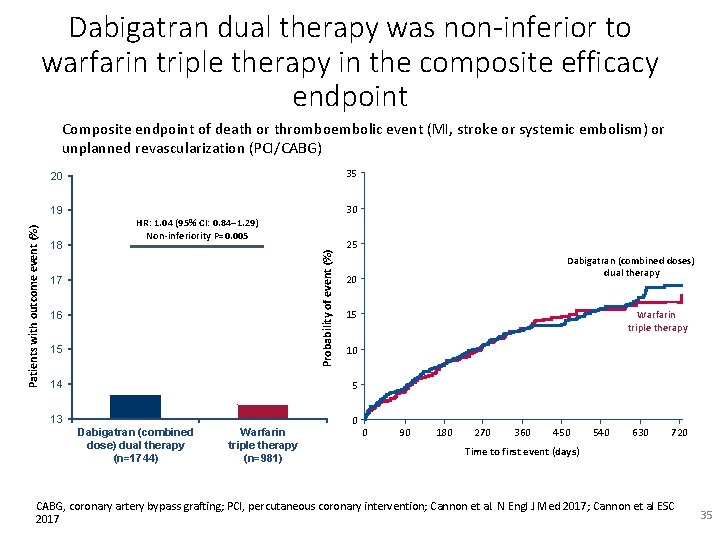
Dabigatran dual therapy was non-inferior to warfarin triple therapy in the composite efficacy endpoint Composite endpoint of death or thromboembolic event (MI, stroke or systemic embolism) or unplanned revascularization (PCI/CABG) 35 20 18 30 HR: 1. 04 (95% CI: 0. 84– 1. 29) Non-inferiority P=0. 005 13. 7% 17 13. 4% 16 15 14 Probability of event (%) Patients with outcome event (%) 19 25 Dabigatran (combined doses) dual therapy 20 15 Warfarin triple therapy 10 5 13 Dabigatran (combined dose) dual therapy (n=1744) Warfarin triple therapy (n=981) 0 0 90 180 270 360 450 540 630 720 Time to first event (days) CABG, coronary artery bypass grafting; PCI, percutaneous coronary intervention; Cannon et al. N Engl J Med 2017; Cannon et al ESC 2017 35

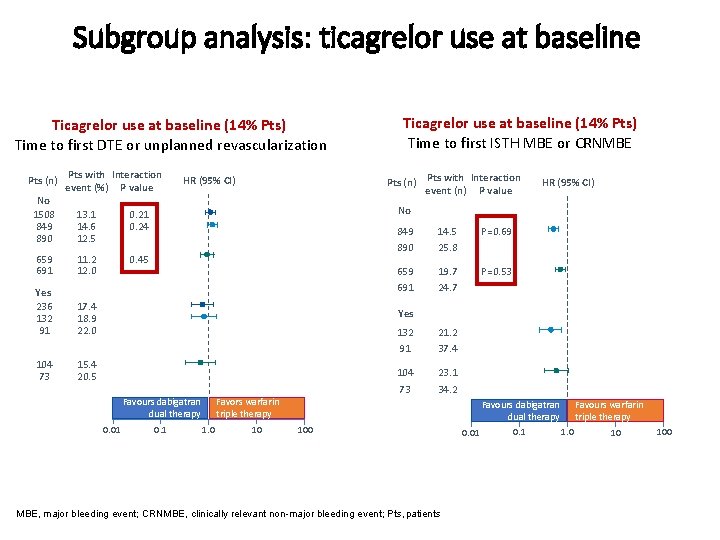
Subgroup analysis: ticagrelor use at baseline Ticagrelor use at baseline (14% Pts) Time to first DTE or unplanned revascularization Pts (n) Pts with Interaction event (%) P value No 1508 13. 1 0. 21 849 14. 6 0. 24 890 12. 5 659 691 11. 2 12. 0 Yes 236 132 91 17. 4 18. 9 22. 0 104 73 15. 4 20. 5 Ticagrelor use at baseline (14% Pts) Time to first ISTH MBE or CRNMBE Pts (n) Pts with Interaction event (n) P value HR (95% CI) No 0. 45 849 890 14. 5 25. 8 P=0. 69 659 691 19. 7 24. 7 P=0. 53 Yes Favours dabigatran dual therapy 0. 01 0. 1 1. 0 132 91 21. 2 37. 4 104 73 23. 1 34. 2 Favors warfarin triple therapy 10 Favours dabigatran dual therapy 100 MBE, major bleeding event; CRNMBE, clinically relevant non-major bleeding event; Pts, patients 0. 01 0. 1 1. 0 Favours warfarin triple therapy 10 100

Agenda • Perché un DOAC • Differenze metodologiche trials DOACs • Differenze farmacocinetiche e farmacodinamiche • Aderenza e compliance • Malattia coronarica • In tutte le situazioni

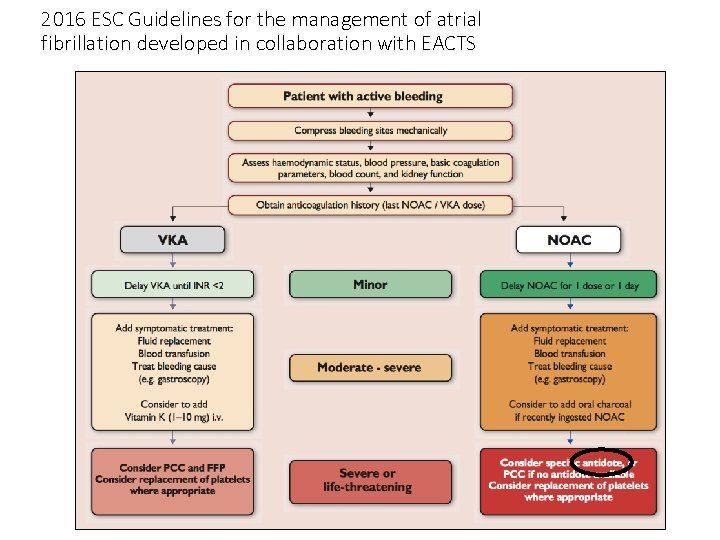
2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS

N Engl J Med, 11 July 2017; 377: 431 -

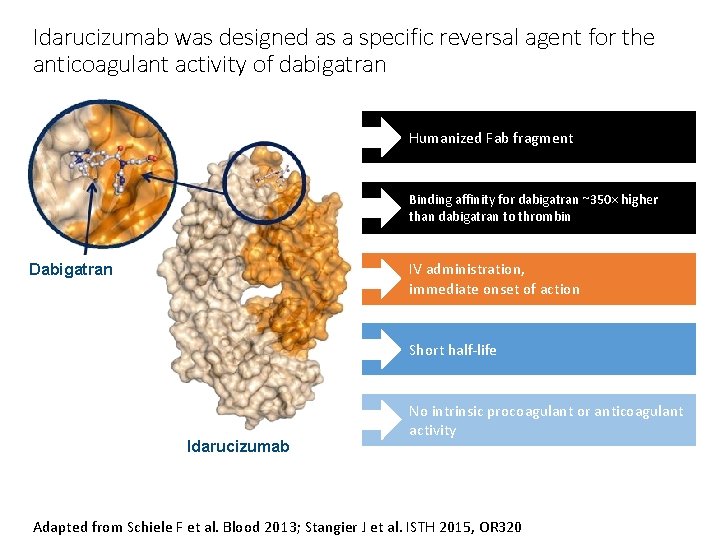
Idarucizumab was designed as a specific reversal agent for the anticoagulant activity of dabigatran Humanized Fab fragment Binding affinity for dabigatran ~350× higher than dabigatran to thrombin IV administration, immediate onset of action Dabigatran Short half-life Idarucizumab No intrinsic procoagulant or anticoagulant activity Adapted from Schiele F et al. Blood 2013; Stangier J et al. ISTH 2015, OR 320

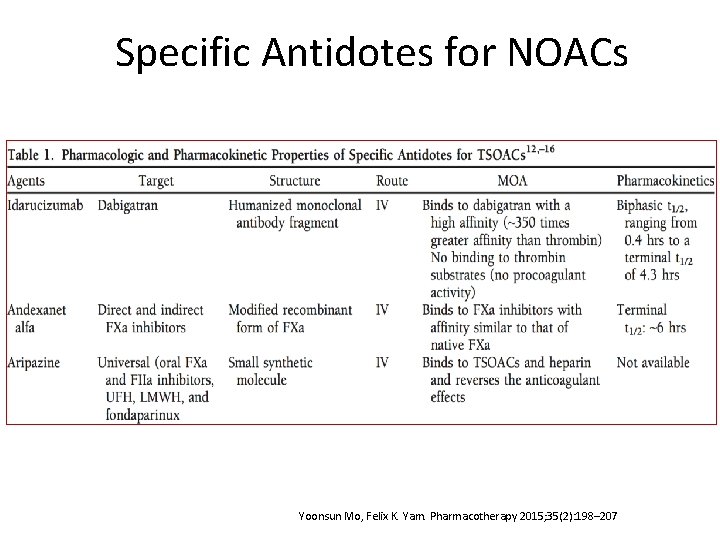
Specific Antidotes for NOACs Yoonsun Mo, Felix K. Yam. Pharmacotherapy 2015; 35(2): 198– 207

Conclusioni • Differenze metodologiche degli studi (disegno studio, popolazione arruolata, outcomes) • Differenza farmacocinetiche e farmacodinamiche • Necessità di conoscere le differenze tra i DOACs • Utilizzo appropriato nei diversi pazienti • Garantire maggiore efficacia e sicurezza

GRAZIE PER L’ATTENZIONE www. arcaliguria. it
 Sintassi significato
Sintassi significato Trasforma le frasi dalla forma attiva alla forma passiva
Trasforma le frasi dalla forma attiva alla forma passiva Dalla preistoria alla storia scuola primaria
Dalla preistoria alla storia scuola primaria Soluzioni posca fiorani
Soluzioni posca fiorani Soluzioni dalla mole alla nomenclatura capitolo 14
Soluzioni dalla mole alla nomenclatura capitolo 14 Soluzioni chimica capitolo 14
Soluzioni chimica capitolo 14 Soluzioni chimica più capitolo 17
Soluzioni chimica più capitolo 17 Chimica più soluzioni capitolo 20
Chimica più soluzioni capitolo 20 Il passaggio dalla preistoria alla storia
Il passaggio dalla preistoria alla storia Dalla monarchia alla repubblica romana
Dalla monarchia alla repubblica romana Elementi di geometria analitica
Elementi di geometria analitica Dalla monarchia alla repubblica romana
Dalla monarchia alla repubblica romana Ho tanta fede in te montale
Ho tanta fede in te montale Para que mi amor no sea un sentimiento letra
Para que mi amor no sea un sentimiento letra Vinillo de rioja
Vinillo de rioja Toc toc quelqu'un frappe à ma porte
Toc toc quelqu'un frappe à ma porte Nulla ha senso
Nulla ha senso Nao se queixe do mundo o mundo nao e mau
Nao se queixe do mundo o mundo nao e mau Analise do poema perdigão perdeu a pena
Analise do poema perdigão perdeu a pena Nao tive filhos não transmiti a nenhuma criatura
Nao tive filhos não transmiti a nenhuma criatura Uma ostra que não foi ferida não produz pérola
Uma ostra que não foi ferida não produz pérola Uma ostra que não foi ferida não produz pérola
Uma ostra que não foi ferida não produz pérola Classificazione fonti storiche
Classificazione fonti storiche Figure sovrapponibili
Figure sovrapponibili Io sono il signore dio tuo non avrai altro dio
Io sono il signore dio tuo non avrai altro dio Equivalenza ed equiscomponibilità
Equivalenza ed equiscomponibilità Le cose importanti della vita non sono cose
Le cose importanti della vita non sono cose Chaplin einstein frase
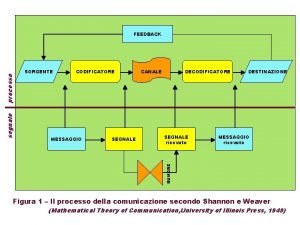
Chaplin einstein frase Messaggio pronunciato dalla sorgente
Messaggio pronunciato dalla sorgente Nomi derivati di mare
Nomi derivati di mare Dalla materia prima al prodotto finito scuola primaria
Dalla materia prima al prodotto finito scuola primaria Nave che non governa
Nave che non governa Nomi derivati della parola pane
Nomi derivati della parola pane Dalla lana school of public health
Dalla lana school of public health Dalla vita ho imparato che per ogni tramonto
Dalla vita ho imparato che per ogni tramonto Le cose che ho imparato nella vita
Le cose che ho imparato nella vita Carlo alberto dalla chiesa afragola
Carlo alberto dalla chiesa afragola Gianfranco dalla betta
Gianfranco dalla betta Fuga dalla tv
Fuga dalla tv Mappa concettuale trasformazioni chimiche e fisiche
Mappa concettuale trasformazioni chimiche e fisiche Tentacin
Tentacin Liturgia de este domingo
Liturgia de este domingo Verbo inseguire forma attiva
Verbo inseguire forma attiva Padre antonio geraldo dalla costa
Padre antonio geraldo dalla costa