CUTANEOUS ADVERSE DRUG REACTION Name Ng Wan Nah











![REFERENCES SNayak S, Acharjya B. Adverse cutaneous drug reaction. Indian J Dermatol [serial online] REFERENCES SNayak S, Acharjya B. Adverse cutaneous drug reaction. Indian J Dermatol [serial online]](https://slidetodoc.com/presentation_image/fb45df4b8722ae01132d97e240831ce9/image-26.jpg)
![National Pharmaceutical Control Bureau. Adverse skin reactions to drugs. [online] 1998 [cited 2012 June National Pharmaceutical Control Bureau. Adverse skin reactions to drugs. [online] 1998 [cited 2012 June](https://slidetodoc.com/presentation_image/fb45df4b8722ae01132d97e240831ce9/image-27.jpg)
- Slides: 27

CUTANEOUS ADVERSE DRUG REACTION Name: Ng Wan Nah 29 June 2012 Staff Number: F 0159

CUTANEOUS ADVERSE DRUG REACTION (CADR) According to Biro Pengawalan Farmaseutikal Kebangsaan (BPFK), the most common adverse drug reaction (ADR) reported is cutaneous adverse drug reaction. The present of CADR need to report with the ADR Report Form.


CUTANEOUS ADVERSE DRUG REACTION Caused by a drug Any undesirable change in the structure or function of the skin, its appendages or mucous membranes Encompass all adverse events related to drug eruption, regardless of the etiology

COMMON OFFENDING DRUG GROUPS Antimicrobials Anticonvulsant Anti-Inflammatory Less frequent such as antipsychotics, antihypertensives, oral contraceptives, antidiabetic, etc.


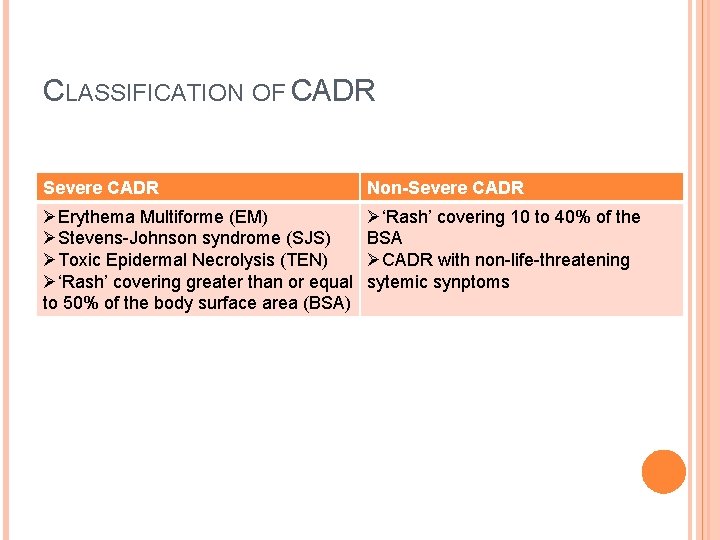
CLASSIFICATION OF CADR Severe CADR Non-Severe CADR ØErythema Multiforme (EM) ØStevens-Johnson syndrome (SJS) ØToxic Epidermal Necrolysis (TEN) Ø‘Rash’ covering greater than or equal to 50% of the body surface area (BSA) Ø‘Rash’ covering 10 to 40% of the BSA ØCADR with non-life-threatening sytemic synptoms

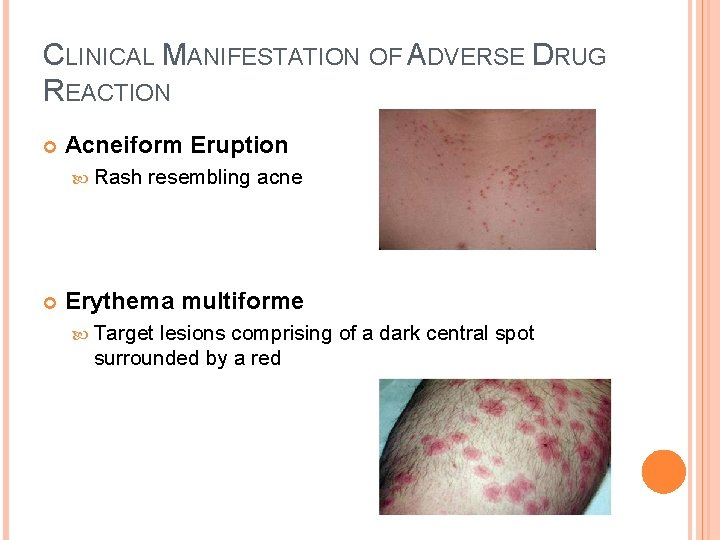
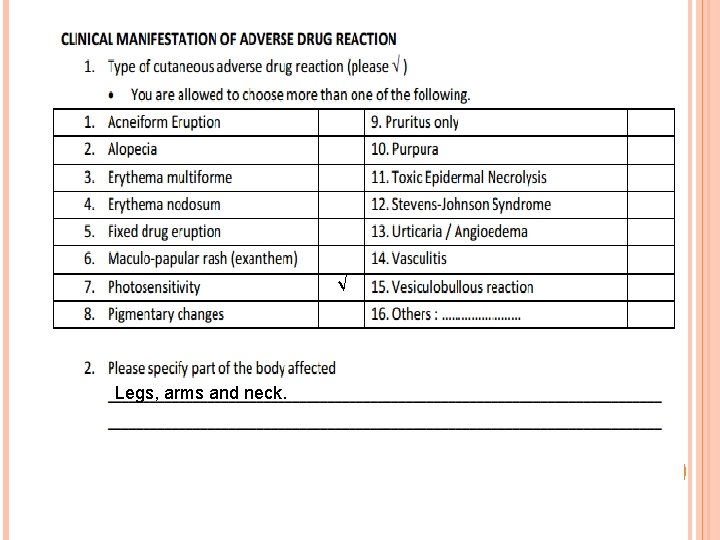
CLINICAL MANIFESTATION OF ADVERSE DRUG REACTION Acneiform Eruption Rash resembling acne Erythema multiforme Target lesions comprising of a dark central spot surrounded by a red

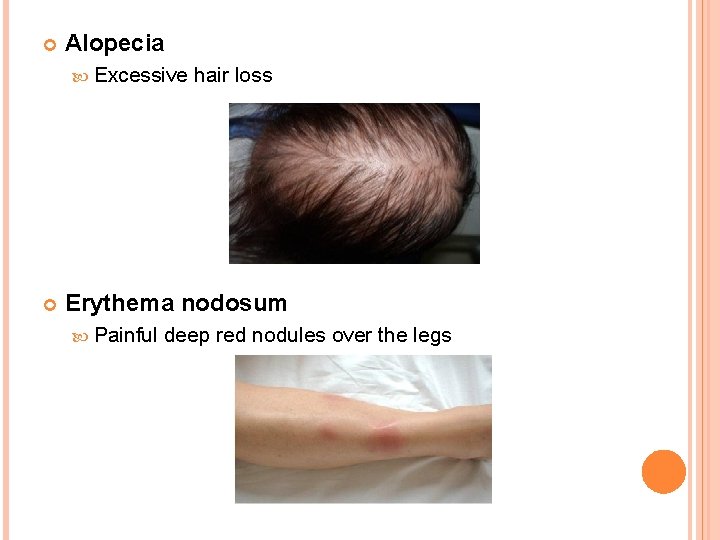
Alopecia Excessive hair loss Erythema nodosum Painful deep red nodules over the legs

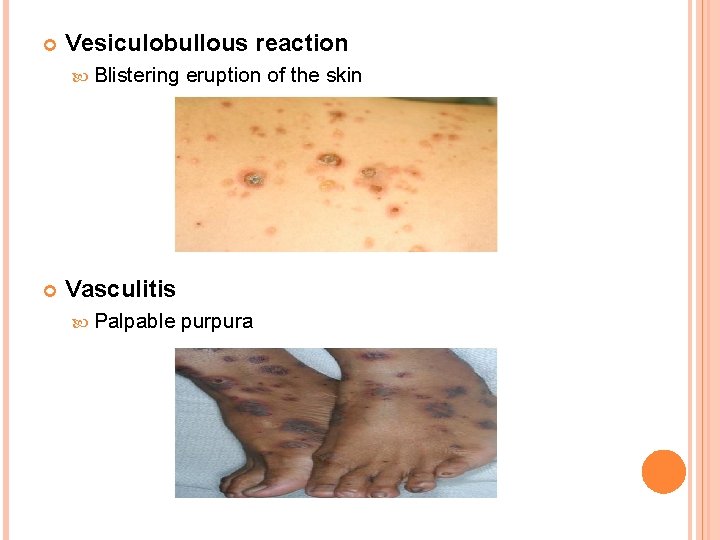
Vesiculobullous reaction Blistering eruption of the skin Vasculitis Palpable purpura

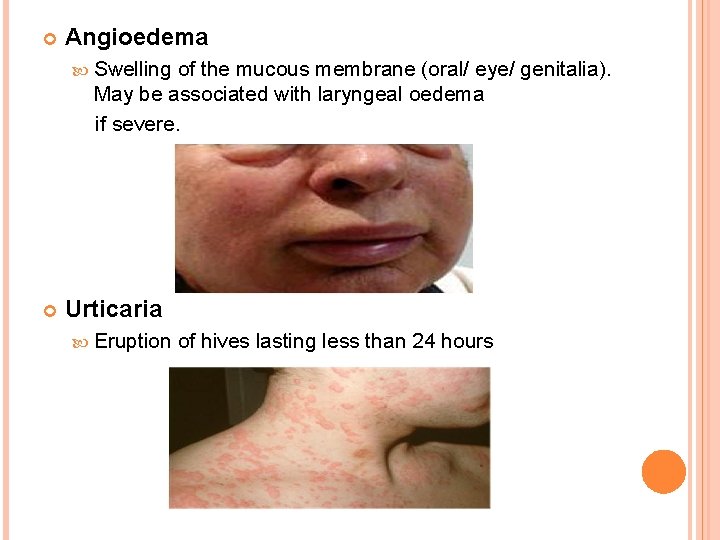
Angioedema Swelling of the mucous membrane (oral/ eye/ genitalia). May be associated with laryngeal oedema if severe. Urticaria Eruption of hives lasting less than 24 hours

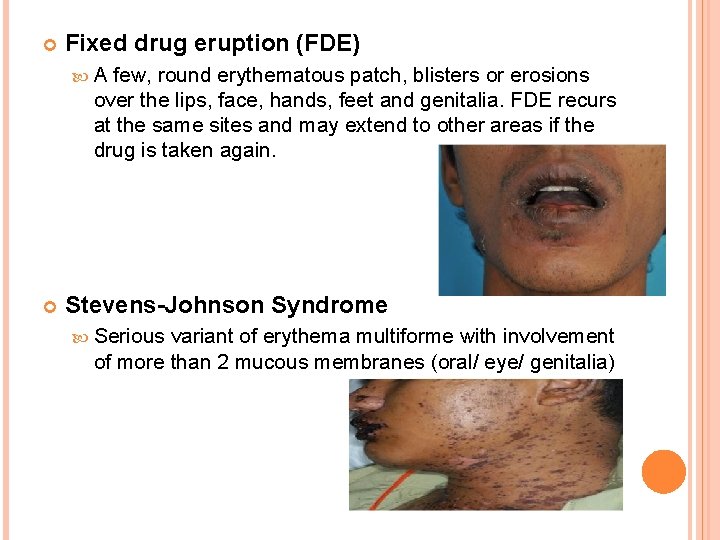
Fixed drug eruption (FDE) A few, round erythematous patch, blisters or erosions over the lips, face, hands, feet and genitalia. FDE recurs at the same sites and may extend to other areas if the drug is taken again. Stevens-Johnson Syndrome Serious variant of erythema multiforme with involvement of more than 2 mucous membranes (oral/ eye/ genitalia)

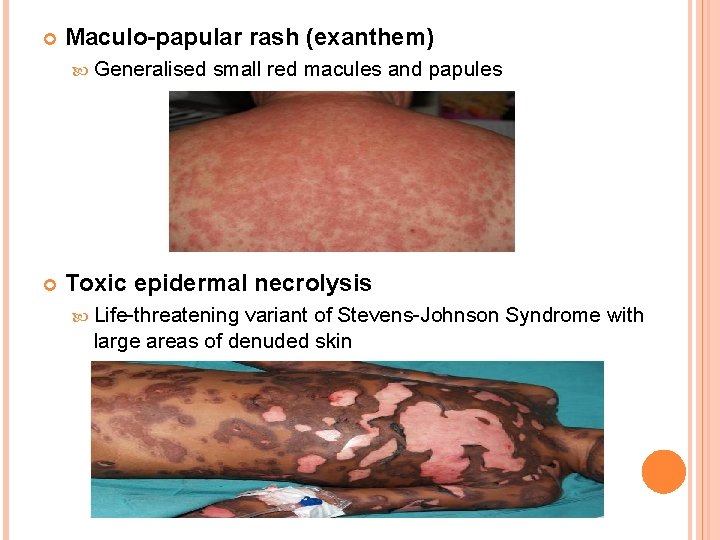
Maculo-papular rash (exanthem) Generalised small red macules and papules Toxic epidermal necrolysis Life-threatening variant of Stevens-Johnson Syndrome with large areas of denuded skin

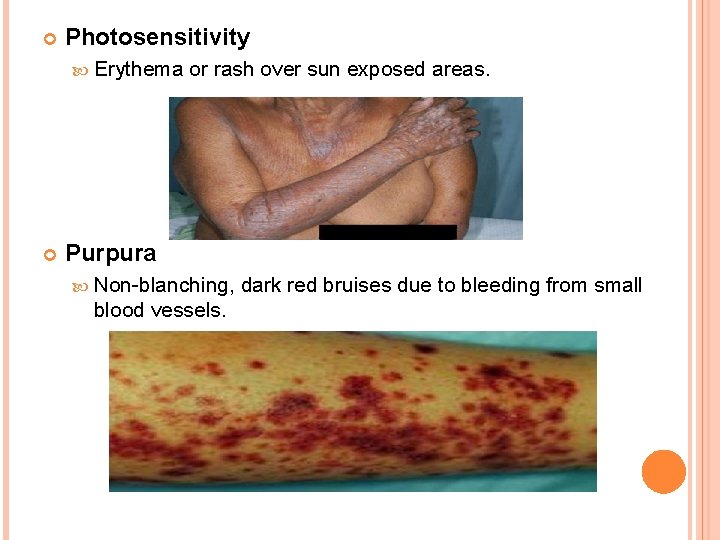
Photosensitivity Erythema or rash over sun exposed areas. Purpura Non-blanching, dark red bruises due to bleeding from small blood vessels.

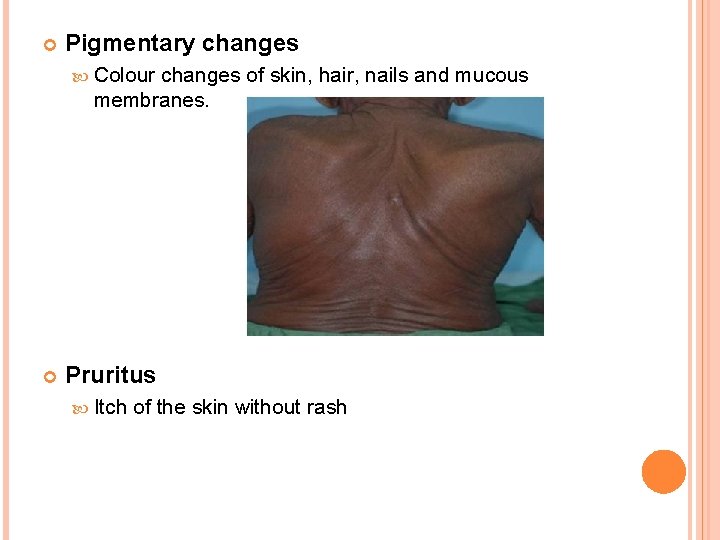
Pigmentary changes Colour changes of skin, hair, nails and mucous membranes. Pruritus Itch of the skin without rash

MANAGEMENT OF CADR The treatment are depending on the type of reaction and the severity Generally, symptoms will often disappear once stop taking the suspected drug or take it at lower dosage

The therapy for most drug eruptions is mainly supportive and treatment depends on the specific type of reaction. The therapy for exanthematous drug eruptions is supportive. First generation of antihistamine (Diphenhydramine, Chlorpheniramine, Triprolidine) is used around the clock. Mild topical steroids (hydrocortisone) and moisturizing lotions (Aqueous cream, Calamine lotion) are also used.

Severe reactions, such as Steven Johnson syndrome and toxic epidermal necrolysis, warrant hospital admission. Timing of withdrawal has been linked to outcome.

Must be directed to fluid replacement and electrolyte correction Treated with special attention to airway and hemodynamic stability, fluid stability, wound/burn care and pain control. Oral lesions can be managed with mouthwashes Topical anesthetics are useful in reducing pain.

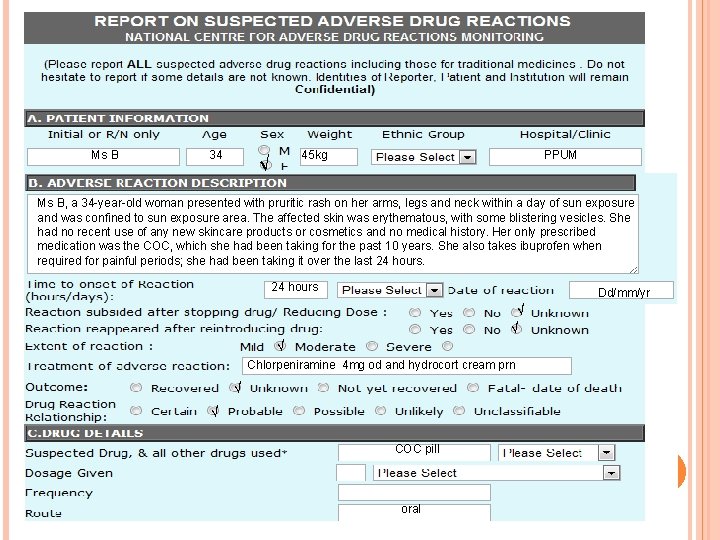
CASE REPORT EXAMPLE Ms B, a 34 -year-old woman, presented to her GP with a painful pruritic rash on her arms, legs and neck. The rash had begun within a day or so of sun exposure and was completely confined to sunexposed areas. The affected skin was erythematous, with some blistering vesicles. Ms B had no recent use of any new skincare products or cosmetics. There was no significant medical history. Her only prescribed medication was the combined oral contraceptive pill, which she had been taking for the past 10 years. Ms B also reported taking ibuprofen when required for painful periods; she had been taking it over the last 24 hours.

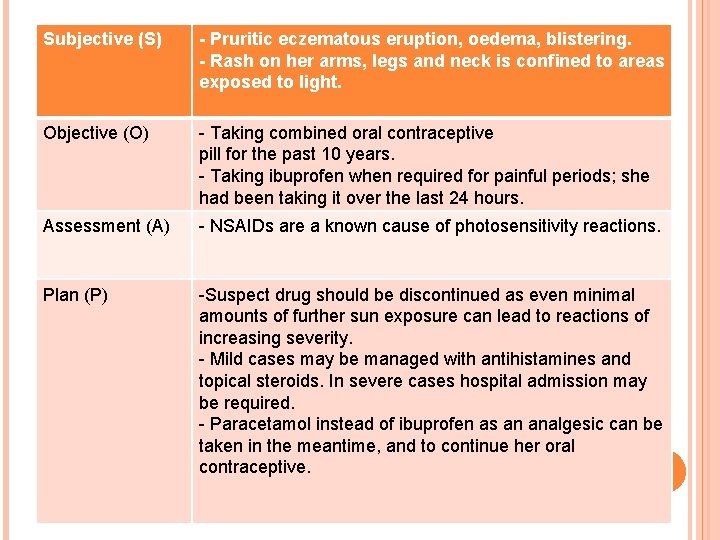
Subjective (S) - Pruritic eczematous eruption, oedema, blistering. - Rash on her arms, legs and neck is confined to areas exposed to light. Objective (O) - Taking combined oral contraceptive pill for the past 10 years. - Taking ibuprofen when required for painful periods; she had been taking it over the last 24 hours. Assessment (A) - NSAIDs are a known cause of photosensitivity reactions. Plan (P) -Suspect drug should be discontinued as even minimal amounts of further sun exposure can lead to reactions of increasing severity. - Mild cases may be managed with antihistamines and topical steroids. In severe cases hospital admission may be required. - Paracetamol instead of ibuprofen as an analgesic can be taken in the meantime, and to continue her oral contraceptive.

Ms B 34 √ 45 kg PPUM Ms B, a 34 -year-old woman presented with pruritic rash on her arms, legs and neck within a day of sun exposure and was confined to sun exposure area. The affected skin was erythematous, with some blistering vesicles. She had no recent use of any new skincare products or cosmetics and no medical history. Her only prescribed medication was the COC, which she had been taking for the past 10 years. She also takes ibuprofen when required for painful periods; she had been taking it over the last 24 hours Dd/mm/yr √ √ √ Chlorpeniramine 4 mg od and hydrocort cream prn √ √ COC pill oral

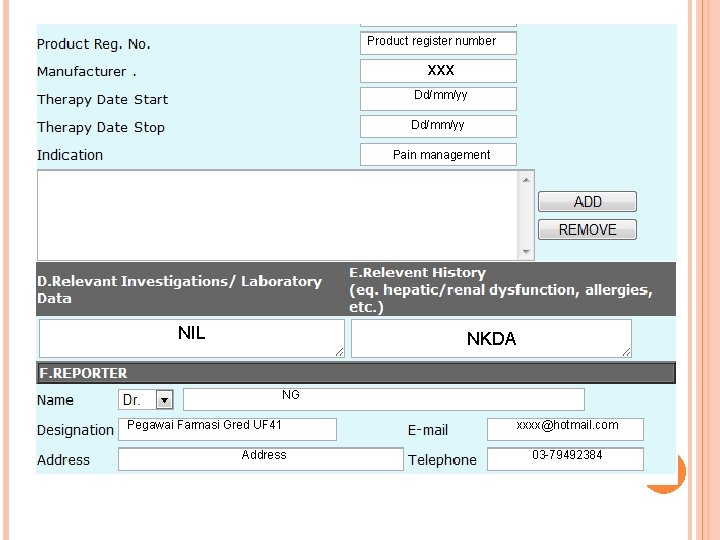
Product register number xxx Dd/mm/yy Pain management NIL NKDA NG Pegawai Farmasi Gred UF 41 Address xxxx@hotmail. com 03 -79492384

√ Legs, arms and neck.

![REFERENCES SNayak S Acharjya B Adverse cutaneous drug reaction Indian J Dermatol serial online REFERENCES SNayak S, Acharjya B. Adverse cutaneous drug reaction. Indian J Dermatol [serial online]](https://slidetodoc.com/presentation_image/fb45df4b8722ae01132d97e240831ce9/image-26.jpg)
REFERENCES SNayak S, Acharjya B. Adverse cutaneous drug reaction. Indian J Dermatol [serial online] 2008 [cited 2012 June 11]; 53: 2 -8. Available from: http: //www. eijd. org/text. asp? 2008/53/1/2/39732 Svensson CK, Cowen EW, Gaspari AA. Cutaneous Drug Reactions. Pharmacological Review. September 1, 2001 vol. 53 no. 3 357 -379. Castro-Pastrana LI, Ghannadan R, Rieder MJ, Dahlke E, Hayden M. Cutaneous Adverse Drug Reactions in Children: An Analysis of Reports from the Canadian Pharmacogenomics Network for Drug Safety. J Popul Ther Clin Pharmacol Vol 18 (1): e 106 -e 120. Annual Report of the Malaysian Adverse Drug Reactions Advisory Committee (MADRAC) 2011. [online] 2010 [cited 2012 June 11]; Available from: URL: http: //portal. bpfk. gov. my/aeimages//File/MADRAC_ Annual_Report_2010 -edited_version. pdf
![National Pharmaceutical Control Bureau Adverse skin reactions to drugs online 1998 cited 2012 June National Pharmaceutical Control Bureau. Adverse skin reactions to drugs. [online] 1998 [cited 2012 June](https://slidetodoc.com/presentation_image/fb45df4b8722ae01132d97e240831ce9/image-27.jpg)
National Pharmaceutical Control Bureau. Adverse skin reactions to drugs. [online] 1998 [cited 2012 June 11]; Available from: URL: http: //portal. bpfk. gov. my/index. cfm? menuid=2 6&parentid=16&subid=33 Anne L, Thompson J. Drug-induced skin reactions. Adverse Drug Reactions, 2 nd ed. Pharmaceutical Press. 2006. pg. 151 -2. Royal Pharmaceutical Society. Adverse drug reaction reporting by pharmacists. 2003 Sept. pg. 23.
 Azalastyna
Azalastyna Seht, die gute zeit ist nah text
Seht, die gute zeit ist nah text Nah polar or nonpolar
Nah polar or nonpolar Private wan infrastructure
Private wan infrastructure Adverse reaction definition
Adverse reaction definition Adverse reaction definition
Adverse reaction definition Adverse reaction definition
Adverse reaction definition An example of crude drug adulterated with exhausted drug
An example of crude drug adulterated with exhausted drug Type 1 cutaneous mechanoreceptors
Type 1 cutaneous mechanoreceptors Lateral side of arm
Lateral side of arm 7 functions of skin
7 functions of skin Five layer of skin
Five layer of skin Cutaneous innervation of scalp
Cutaneous innervation of scalp Cutaneous supply of lower limb
Cutaneous supply of lower limb Tibial nerve innervation
Tibial nerve innervation Cutaneous reflex
Cutaneous reflex Cutaneous reflex
Cutaneous reflex Scalp innervation
Scalp innervation Ventral vs dorsal
Ventral vs dorsal Cutaneous membrane structure
Cutaneous membrane structure Dermatomes lower limb
Dermatomes lower limb Free edge
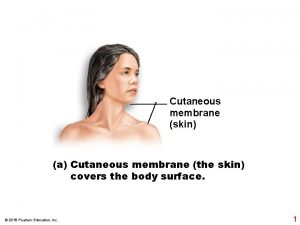
Free edge Cutaneous membrane
Cutaneous membrane Nishan silva
Nishan silva Median nerve innervation
Median nerve innervation Polychromasia
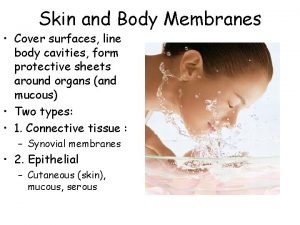
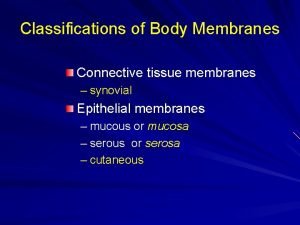
Polychromasia Types of body membranes
Types of body membranes Cutaneous amebiasis
Cutaneous amebiasis