Current Landscape and Future Directions of Docetaxel in

























- Slides: 109


Current Landscape and Future Directions of Docetaxel in Advanced Breast Cancer John Crown Current Landscape and Future Directions of Docetaxel in Early-Stage Breast Cancer John Mackey Taxotere® Summary of Product Characteristics

Current Landscape and Future Directions of Docetaxel in Advanced Breast Cancer John Crown, MD St. Vincent’s University Hospital Dublin, Ireland Main Menu

Metastatic Breast Cancer: Overview • Metastatic breast cancer is incurable with current conventional hormonal therapy and chemotherapy • Median survival after development of metastases is approximately 3 years • Treatment of metastatic breast cancer has the potential to provide: – – Control and regression of disease Palliation of symptoms Improvement in quality of life Prolongation of life Harris J et al. Cancer: Principles & Practice of Oncology. 1997; 1557 -1616. Hortobagyi GN. N Engl J Med. 1998; 339: 974 -984. Lindley C. Pharmacotherapy: A Pathophysiologic Approach. 1997; 2467 -2497.

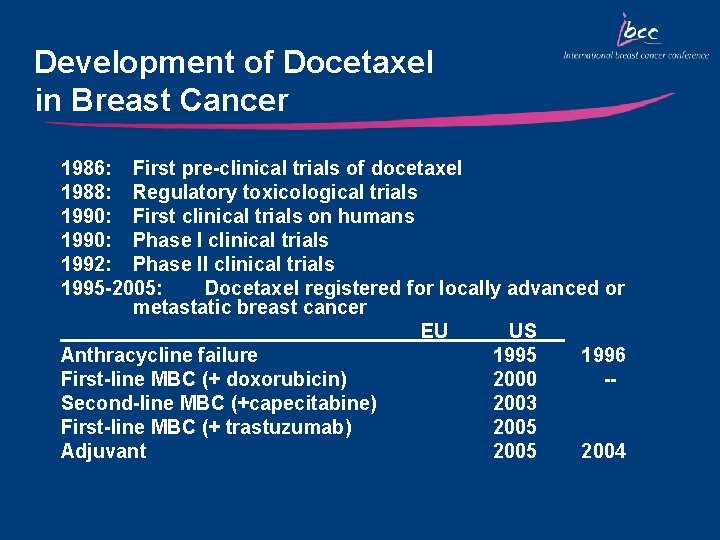
Development of Docetaxel in Breast Cancer 1986: First pre-clinical trials of docetaxel 1988: Regulatory toxicological trials 1990: First clinical trials on humans 1990: Phase I clinical trials 1992: Phase II clinical trials 1995 -2005: Docetaxel registered for locally advanced or metastatic breast cancer EU US Anthracycline failure 1995 1996 First-line MBC (+ doxorubicin) 2000 -Second-line MBC (+capecitabine) 2003 First-line MBC (+ trastuzumab) 2005 Adjuvant 2005 2004

Single-Agent Docetaxel as Second-Line Therapy in MBC

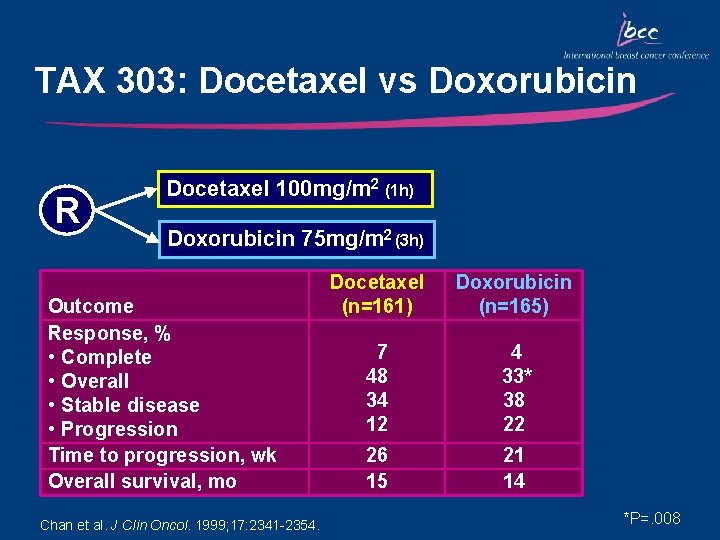
TAX 303: Docetaxel vs Doxorubicin R Docetaxel 100 mg/m 2 (1 h) Doxorubicin 75 mg/m 2 (3 h) Outcome Response, % • Complete • Overall • Stable disease • Progression Time to progression, wk Overall survival, mo Chan et al. J Clin Oncol. 1999; 17: 2341 -2354. Docetaxel (n=161) Doxorubicin (n=165) 7 48 34 12 4 33* 38 22 26 15 21 14 *P=. 008

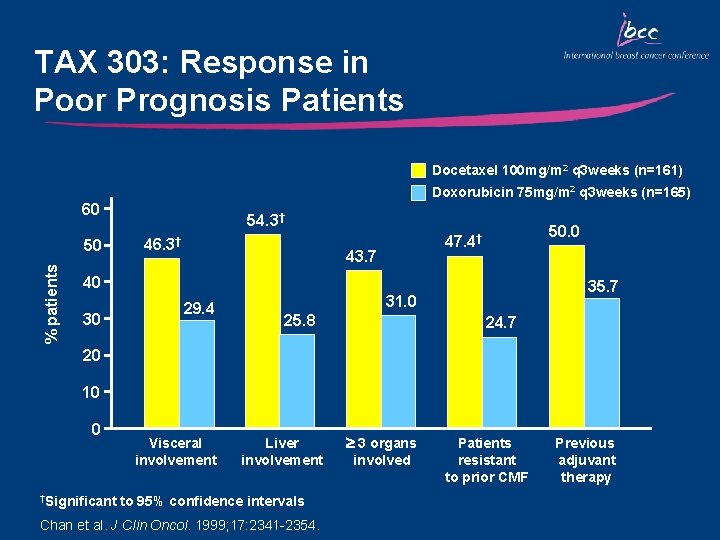
TAX 303: Response in Poor Prognosis Patients Docetaxel 100 mg/m 2 q 3 weeks (n=161) Doxorubicin 75 mg/m 2 q 3 weeks (n=165) 60 % patients 50 54. 3† 46. 3† 50. 0 47. 4† 43. 7 40 30 29. 4 35. 7 31. 0 25. 8 24. 7 20 10 0 †Significant Visceral involvement Liver involvement to 95% confidence intervals Chan et al. J Clin Oncol. 1999; 17: 2341 -2354. 3 organs involved Patients resistant to prior CMF Previous adjuvant therapy

Key Outcomes: TAX 303 • Single-agent docetaxel in MBC patients pretreated with alkylating agents was more active than doxorubicin in terms of overall response including that in patients with poor prognostic factors • TTP was longer with docetaxel than with doxorubicin (26 wk vs 21 wk) – This difference was not statistically significant – Overall survival was similar between groups Chan et al. J Clin Oncol. 1999; 17: 2341 -2354.

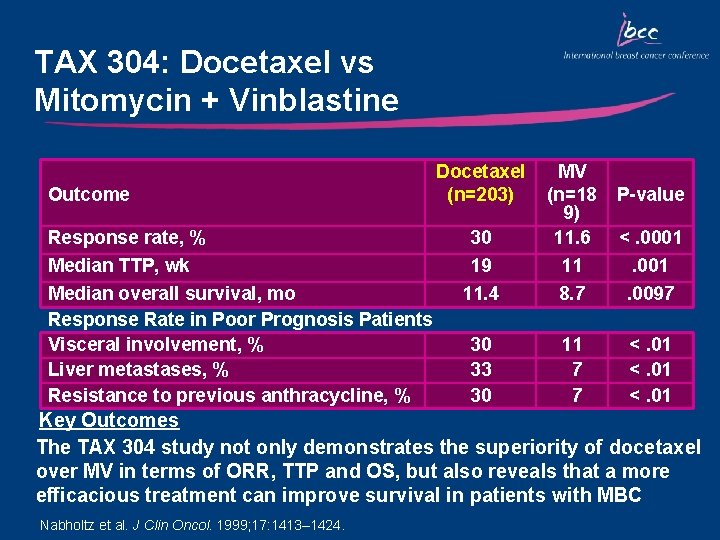
TAX 304: Docetaxel vs Mitomycin + Vinblastine Outcome Response rate, % Median TTP, wk Median overall survival, mo Response Rate in Poor Prognosis Patients Visceral involvement, % Liver metastases, % Resistance to previous anthracycline, % Docetaxel (n=203) 30 19 11. 4 MV (n=18 9) 11. 6 11 8. 7 30 33 30 11 7 7 P-value <. 0001. 0097 <. 01 Key Outcomes The TAX 304 study not only demonstrates the superiority of docetaxel over MV in terms of ORR, TTP and OS, but also reveals that a more efficacious treatment can improve survival in patients with MBC Nabholtz et al. J Clin Oncol. 1999; 17: 1413– 1424.

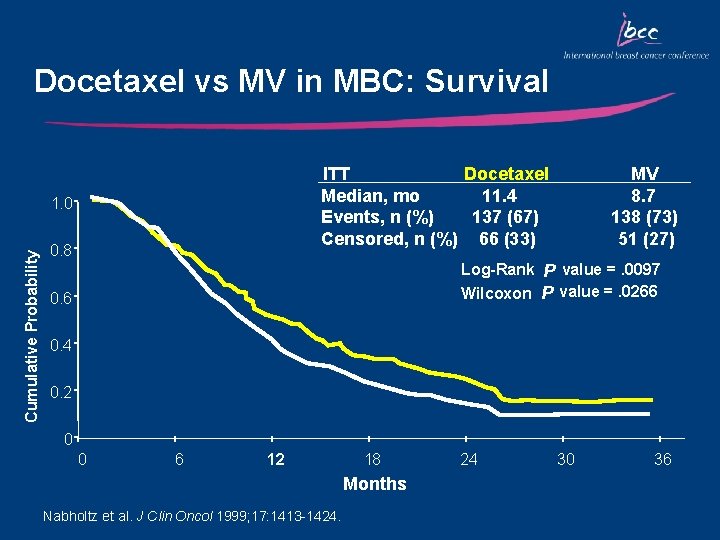
Docetaxel vs MV in MBC: Survival ITT Docetaxel Median, mo 11. 4 Events, n (%) 137 (67) Censored, n (%) 66 (33) Cumulative Probability 1. 0 0. 8 MV 8. 7 138 (73) 51 (27) Log-Rank P value =. 0097 Wilcoxon P value =. 0266 0. 4 0. 2 0 0 6 12 18 Months Nabholtz et al. J Clin Oncol 1999; 17: 1413 -1424. 24 30 36

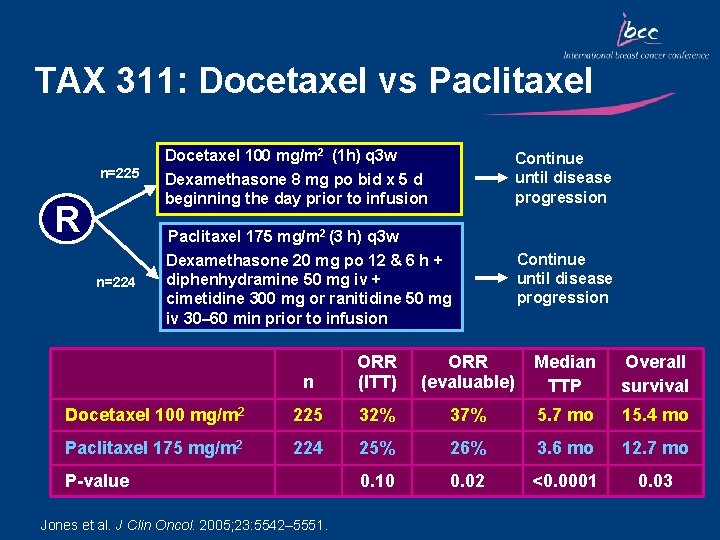
TAX 311: Docetaxel vs Paclitaxel Docetaxel 100 mg/m 2 (1 h) q 3 w n=225 R Continue until disease progression Dexamethasone 8 mg po bid x 5 d beginning the day prior to infusion Paclitaxel 175 mg/m 2 (3 h) q 3 w n=224 Dexamethasone 20 mg po 12 & 6 h + diphenhydramine 50 mg iv + cimetidine 300 mg or ranitidine 50 mg iv 30– 60 min prior to infusion Continue until disease progression n ORR (ITT) ORR (evaluable) Median TTP Overall survival Docetaxel 100 mg/m 2 225 32% 37% 5. 7 mo 15. 4 mo Paclitaxel 175 mg/m 2 224 25% 26% 3. 6 mo 12. 7 mo 0. 10 0. 02 <0. 0001 0. 03 P-value Jones et al. J Clin Oncol. 2005; 23: 5542– 5551.

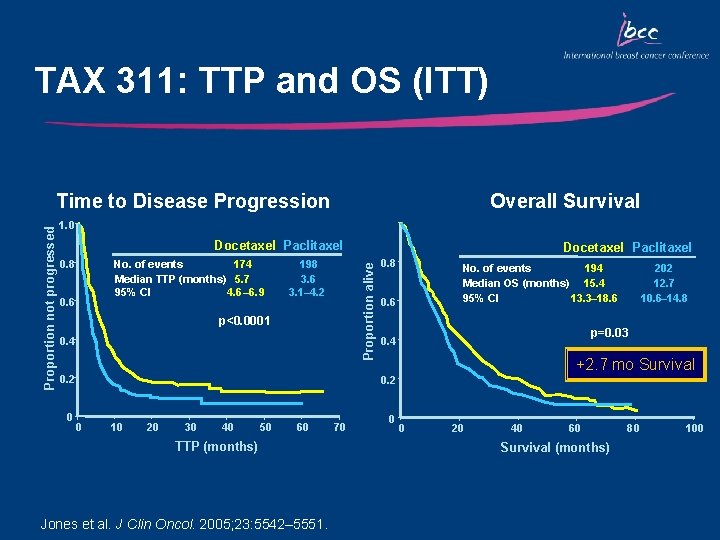
TAX 311: TTP and OS (ITT) Overall Survival 1. 0 Docetaxel Paclitaxel 0. 8 No. of events 174 Median TTP (months) 5. 7 95% CI 4. 6– 6. 9 0. 6 198 3. 6 3. 1– 4. 2 p<0. 0001 0. 4 0. 2 0 Docetaxel Paclitaxel Proportion alive Proportion not progressed Time to Disease Progression 0. 8 194 No. of events Median OS (months) 15. 4 95% CI 13. 3– 18. 6 0. 6 p=0. 03 0. 4 +2. 7 mo Survival 0. 2 0 10 20 30 40 50 60 TTP (months) Jones et al. J Clin Oncol. 2005; 23: 5542– 5551. 70 0 202 12. 7 10. 6– 14. 8 0 20 40 60 Survival (months) 80 100

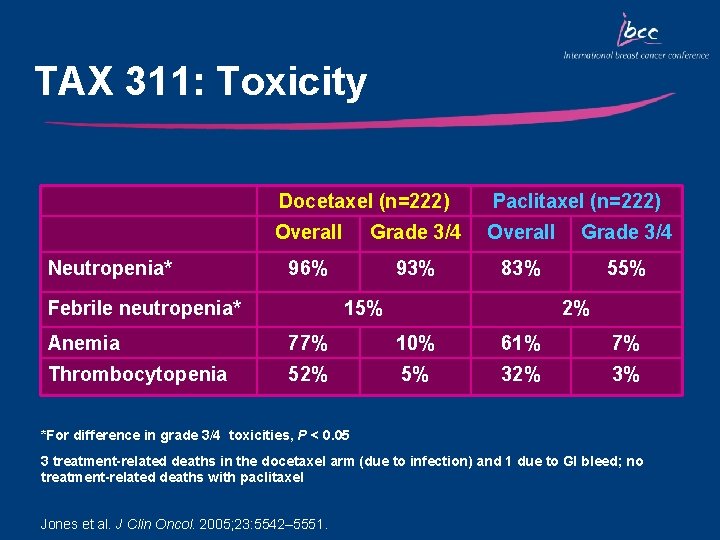
TAX 311: Toxicity Neutropenia* Docetaxel (n=222) Paclitaxel (n=222) Overall Grade 3/4 96% 93% 83% 55% Febrile neutropenia* 15% 2% Anemia 77% 10% 61% 7% Thrombocytopenia 52% 5% 32% 3% *For difference in grade 3/4 toxicities, P < 0. 05 3 treatment-related deaths in the docetaxel arm (due to infection) and 1 due to GI bleed; no treatment-related deaths with paclitaxel Jones et al. J Clin Oncol. 2005; 23: 5542– 5551.

Key Outcomes: TAX 311 • Overall survival was superior for docetaxel compared with paclitaxel: 15. 4 vs 12. 7 months (p=0. 03) • TTP was superior for docetaxel vs paclitaxel: 5. 7 vs 3. 6 months (p<0. 0001) • ORR was improved with docetaxel vs paclitaxel – ITT analysis: 32% vs 25%, p=0. 10 – Evaluable population: 37% vs 26%, p<0. 02 • Docetaxel was associated with an increased incidence of grade 3/4 hematologic and non-hematologic toxicity • There were no statistically significant differences in modified FACT-B-assessed global Qo. L scores between treatments over time Jones et al. J Clin Oncol. 2005; 23: 5542– 5551.

Docetaxel Combinations as Second-Line Therapy in MBC

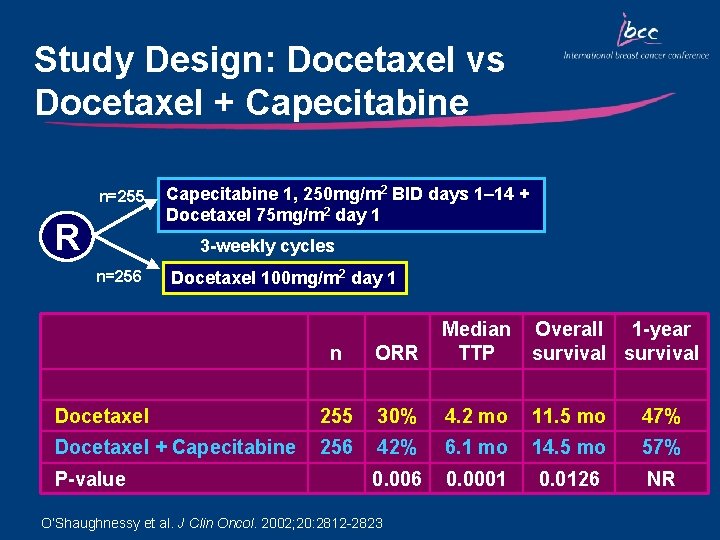
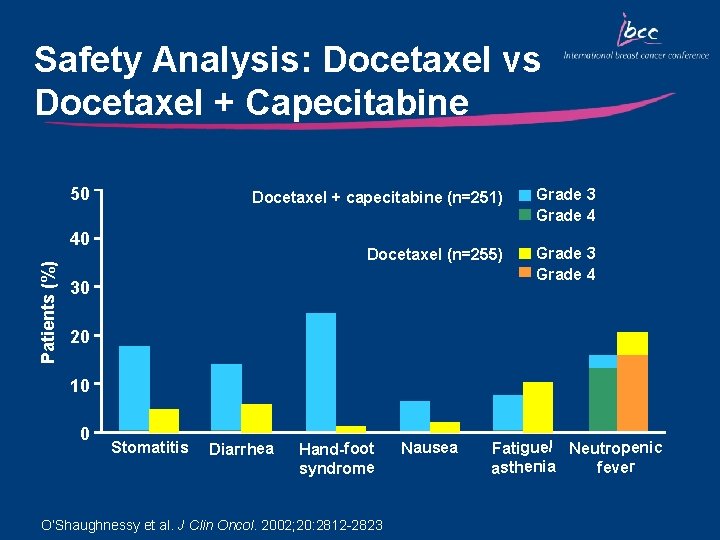
Study Design: Docetaxel vs Docetaxel + Capecitabine n=255 R Capecitabine 1, 250 mg/m 2 BID days 1– 14 + Docetaxel 75 mg/m 2 day 1 3 -weekly cycles n=256 Docetaxel 100 mg/m 2 day 1 n ORR Median TTP Docetaxel 255 30% 4. 2 mo 11. 5 mo 47% Docetaxel + Capecitabine 256 42% 6. 1 mo 14. 5 mo 57% 0. 006 0. 0001 0. 0126 NR P-value O’Shaughnessy et al. J Clin Oncol. 2002; 20: 2812 -2823 Overall 1 -year survival

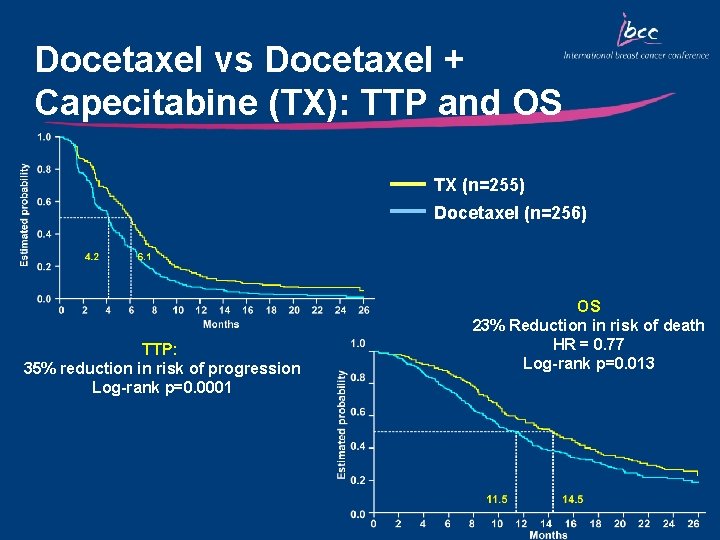
Docetaxel vs Docetaxel + Capecitabine (TX): TTP and OS TX (n=255) Docetaxel (n=256) TTP: 35% reduction in risk of progression Log-rank p=0. 0001 OS 23% Reduction in risk of death HR = 0. 77 Log-rank p=0. 013

Safety Analysis: Docetaxel vs Docetaxel + Capecitabine 50 Docetaxel + capecitabine (n=251) Grade 3 Grade 4 Docetaxel (n=255) Grade 3 Grade 4 Patients (%) 40 30 20 10 0 Stomatitis Diarrhea Hand-foot syndrome O’Shaughnessy et al. J Clin Oncol. 2002; 20: 2812 -2823 Nausea Fatigue/ Neutropenic fever asthenia

Key Outcomes: Docetaxel vs Docetaxel + Capecitabine • Docetaxel + capecitabine is an effective option for patients with – rapidly progressing disease – visceral metastases – good performance status • Compared with single-agent docetaxel, combination therapy produced significant increases in median time to progression, overall survival, and objective response

Docetaxel Combinations as First-Line Therapy in MBC

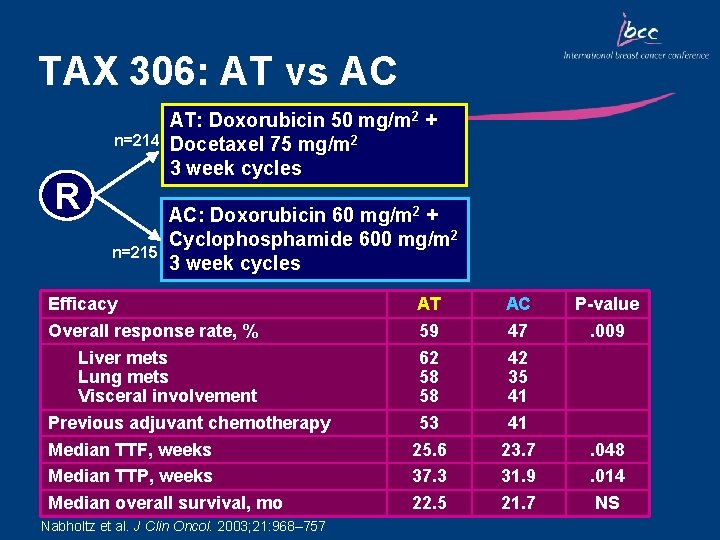
TAX 306: AT vs AC n=214 R n=215 AT: Doxorubicin 50 mg/m 2 + Docetaxel 75 mg/m 2 3 week cycles AC: Doxorubicin 60 mg/m 2 + Cyclophosphamide 600 mg/m 2 3 week cycles Efficacy Overall response rate, % Liver mets Lung mets Visceral involvement Previous adjuvant chemotherapy Median TTF, weeks Median TTP, weeks Median overall survival, mo Nabholtz et al. J Clin Oncol. 2003; 21: 968– 757 AT 59 62 58 58 53 25. 6 37. 3 22. 5 AC 47 42 35 41 41 23. 7 31. 9 21. 7 P-value. 009 . 048. 014 NS

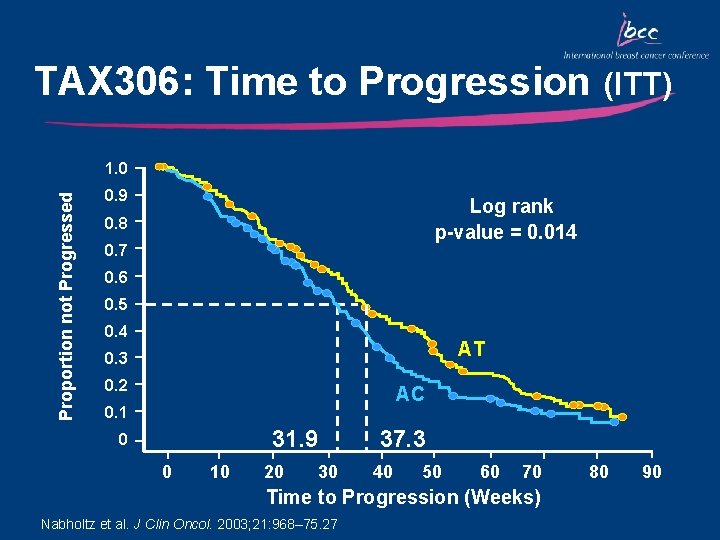
TAX 306: Time to Progression (ITT) Proportion not Progressed 1. 0 0. 9 Log rank p-value = 0. 014 0. 8 0. 7 0. 6 0. 5 0. 4 AT 0. 3 0. 2 AC 0. 1 31. 9 0 0 10 20 30 37. 3 40 50 60 70 Time to Progression (Weeks) Nabholtz et al. J Clin Oncol. 2003; 21: 968– 75. 27 80 90

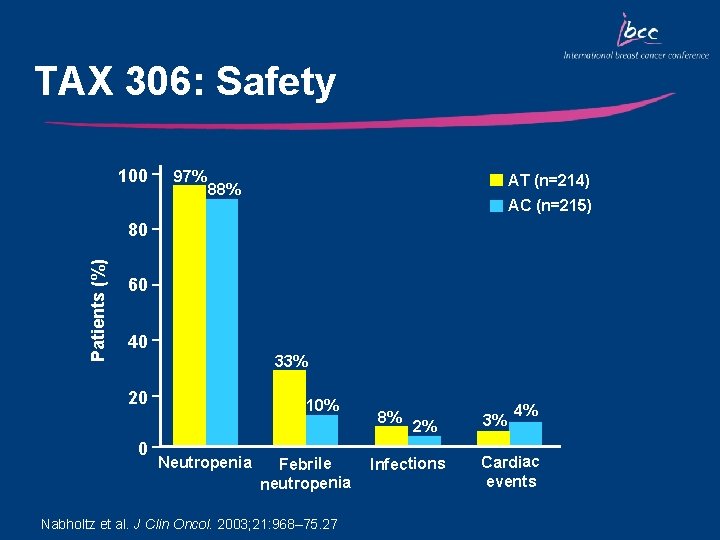
TAX 306: Safety 100 97% AT (n=214) 88% AC (n=215) Patients (%) 80 60 40 33% 20 0 10% Neutropenia Febrile neutropenia Nabholtz et al. J Clin Oncol. 2003; 21: 968– 75. 27 8% 2% Infections 3% 4% Cardiac events

Key Outcomes: TAX 306 • Compared with AC, AT chemotherapy significantly increased the overall response rate, median time to disease progression and median time to treatment failure • OS was, however, comparable between the two treatment groups • AT chemotherapy resulted in manageable short term side effects • AT is the first regimen to show superiority over a standard anthracycline combination in the first-line treatment of MBC

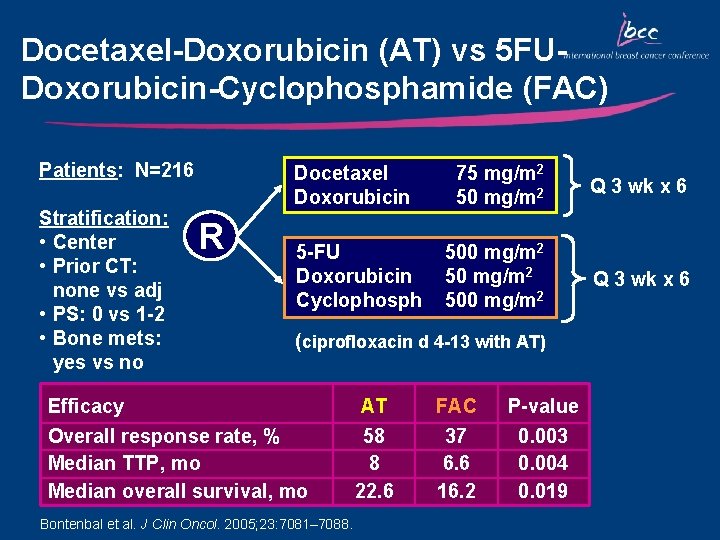
Docetaxel-Doxorubicin (AT) vs 5 FUDoxorubicin-Cyclophosphamide (FAC) Patients: N=216 Stratification: • Center • Prior CT: none vs adj • PS: 0 vs 1 -2 • Bone mets: yes vs no Docetaxel Doxorubicin R 5 -FU Doxorubicin Cyclophosph 75 mg/m 2 50 mg/m 2 Q 3 wk x 6 500 mg/m 2 Q 3 wk x 6 (ciprofloxacin d 4 -13 with AT) Efficacy Overall response rate, % Median TTP, mo Median overall survival, mo Bontenbal et al. J Clin Oncol. 2005; 23: 7081– 7088. AT 58 8 22. 6 FAC 37 6. 6 16. 2 P-value 0. 003 0. 004 0. 019

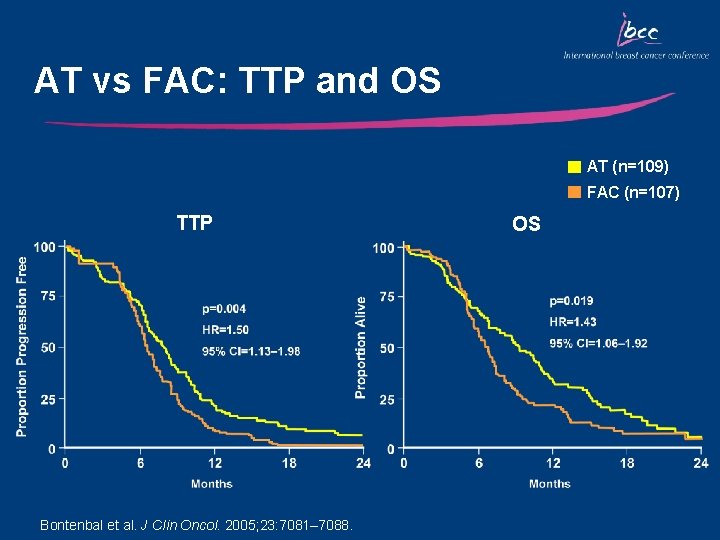
AT vs FAC: TTP and OS AT (n=109) FAC (n=107) TTP Bontenbal et al. J Clin Oncol. 2005; 23: 7081– 7088. OS

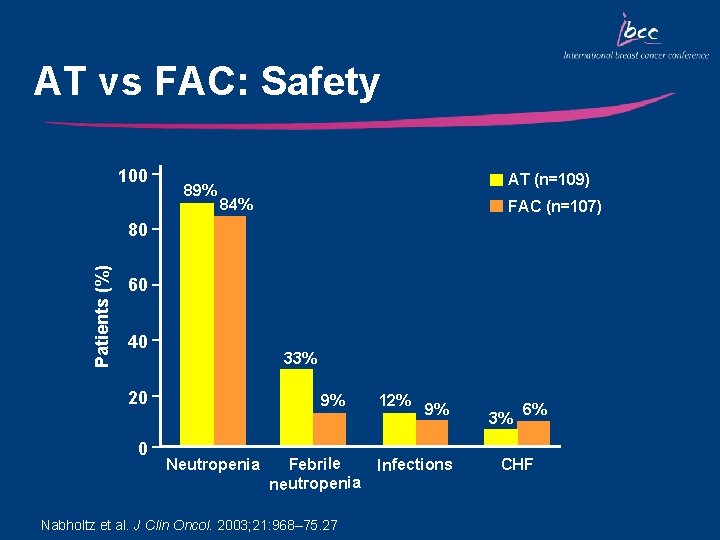
AT vs FAC: Safety 100 89% AT (n=109) 84% FAC (n=107) Patients (%) 80 60 40 33% 20 0 9% Neutropenia Febrile neutropenia Nabholtz et al. J Clin Oncol. 2003; 21: 968– 75. 27 12% 9% Infections 3% 6% CHF

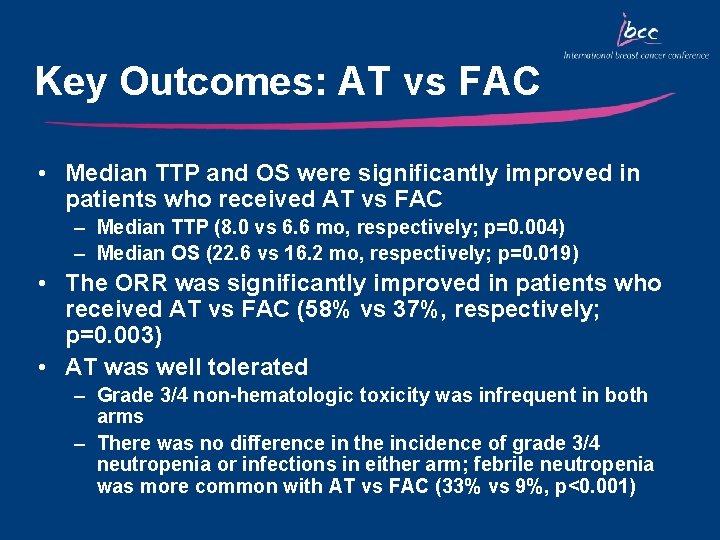
Key Outcomes: AT vs FAC • Median TTP and OS were significantly improved in patients who received AT vs FAC – Median TTP (8. 0 vs 6. 6 mo, respectively; p=0. 004) – Median OS (22. 6 vs 16. 2 mo, respectively; p=0. 019) • The ORR was significantly improved in patients who received AT vs FAC (58% vs 37%, respectively; p=0. 003) • AT was well tolerated – Grade 3/4 non-hematologic toxicity was infrequent in both arms – There was no difference in the incidence of grade 3/4 neutropenia or infections in either arm; febrile neutropenia was more common with AT vs FAC (33% vs 9%, p<0. 001)

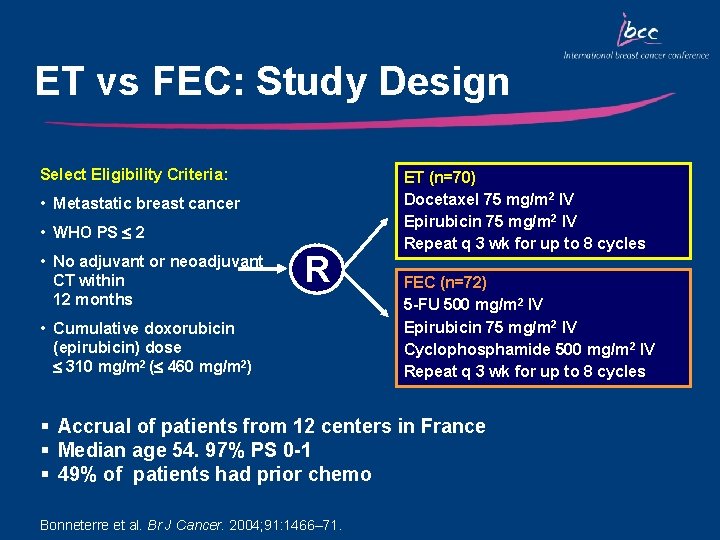
ET vs FEC: Study Design Select Eligibility Criteria: • Metastatic breast cancer • WHO PS 2 • No adjuvant or neoadjuvant CT within 12 months R • Cumulative doxorubicin (epirubicin) dose 310 mg/m 2 ( 460 mg/m 2) ET (n=70) Docetaxel 75 mg/m 2 IV Epirubicin 75 mg/m 2 IV Repeat q 3 wk for up to 8 cycles FEC (n=72) 5 -FU 500 mg/m 2 IV Epirubicin 75 mg/m 2 IV Cyclophosphamide 500 mg/m 2 IV Repeat q 3 wk for up to 8 cycles § Accrual of patients from 12 centers in France § Median age 54. 97% PS 0 -1 § 49% of patients had prior chemo Bonneterre et al. Br J Cancer. 2004; 91: 1466– 71.

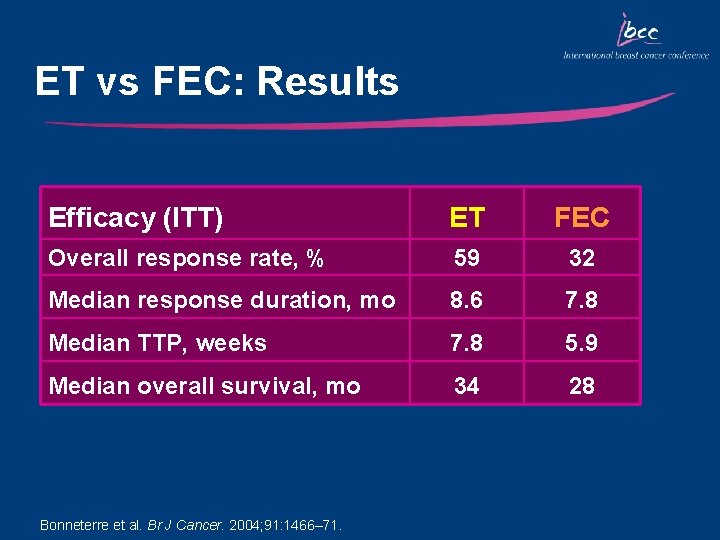
ET vs FEC: Results Efficacy (ITT) ET FEC Overall response rate, % 59 32 Median response duration, mo 8. 6 7. 8 Median TTP, weeks 7. 8 5. 9 Median overall survival, mo 34 28 Bonneterre et al. Br J Cancer. 2004; 91: 1466– 71.

Key Outcomes: ET vs FEC • ORR was improved with ET vs FEC, after a median of 7 and 6 cycles, respectively (59% vs 32%) • Similarly, TTP and OS were improved with ET vs FEC • ET was well tolerated – Infrequent nonhematologic toxicities in both arms • ET is a highly active first-line regimen for MBC

Docetaxel in the Treatment of HER 2+ MBC

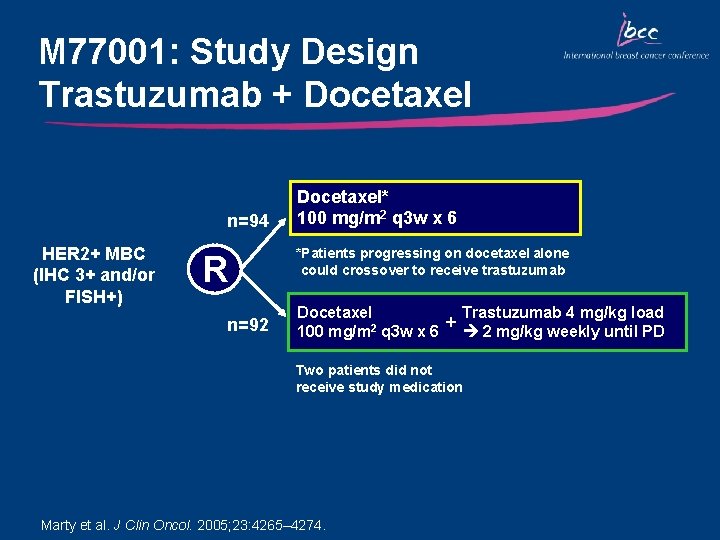
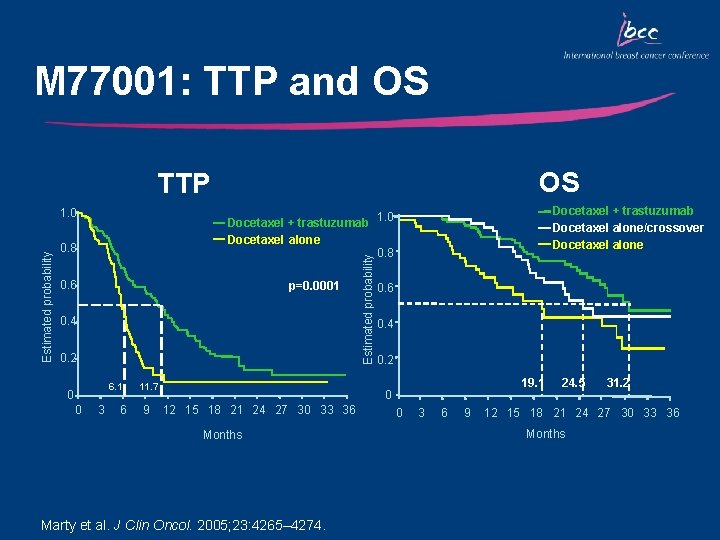
M 77001: Study Design Trastuzumab + Docetaxel n=94 HER 2+ MBC (IHC 3+ and/or FISH+) R n=92 Docetaxel* 100 mg/m 2 q 3 w x 6 *Patients progressing on docetaxel alone could crossover to receive trastuzumab Trastuzumab 4 mg/kg load Docetaxel + 100 mg/m 2 q 3 w x 6 2 mg/kg weekly until PD Two patients did not receive study medication Marty et al. J Clin Oncol. 2005; 23: 4265– 4274.

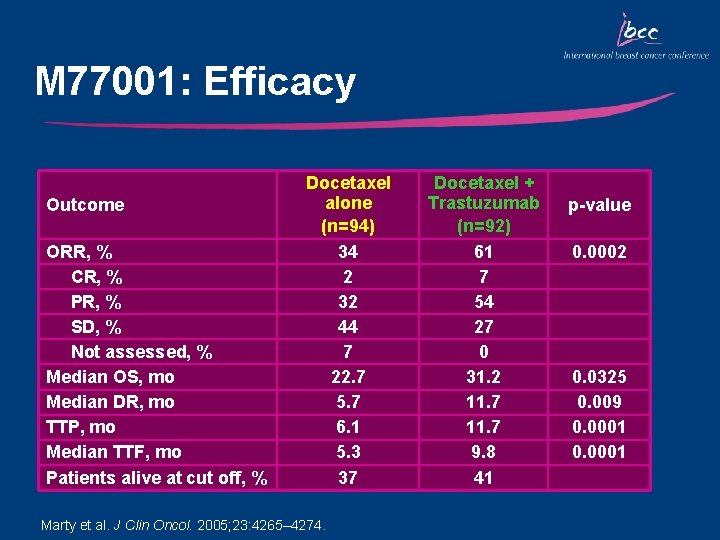
M 77001: Efficacy Outcome ORR, % CR, % PR, % SD, % Not assessed, % Median OS, mo Median DR, mo TTP, mo Median TTF, mo Patients alive at cut off, % Docetaxel alone (n=94) 34 2 32 44 7 22. 7 5. 7 6. 1 5. 3 37 Marty et al. J Clin Oncol. 2005; 23: 4265– 4274. Docetaxel + Trastuzumab (n=92) 61 7 54 27 0 31. 2 11. 7 9. 8 41 p-value 0. 0002 0. 0325 0. 009 0. 0001

M 77001: TTP and OS OS TTP 0. 8 0. 6 p=0. 0001 0. 4 0. 2 6. 1 0 0 Docetaxel + trastuzumab Docetaxel alone/crossover Docetaxel alone Docetaxel + trastuzumab 1. 0 Docetaxel alone 0. 8 3 6 11. 7 9 Estimated probability 1. 0 0. 6 0. 4 0. 2 19. 1 0 12 15 18 21 24 27 30 33 36 Months Marty et al. J Clin Oncol. 2005; 23: 4265– 4274. 0 3 6 9 24. 5 31. 2 12 15 18 21 24 27 30 33 36 Months

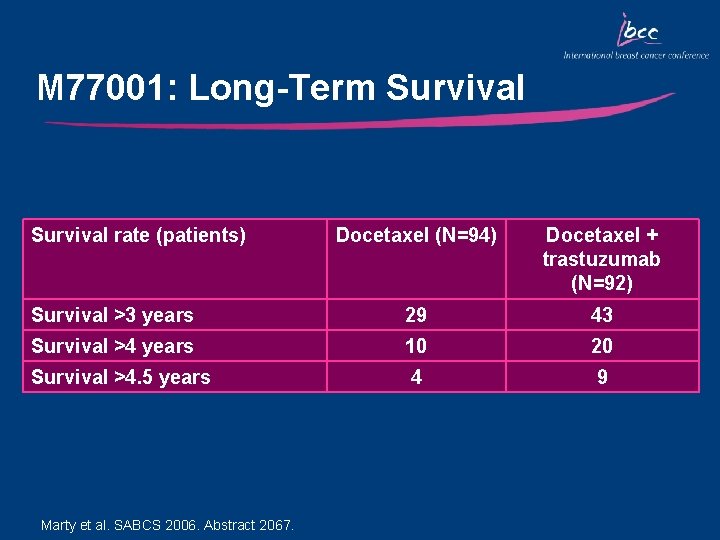
M 77001: Long-Term Survival rate (patients) Docetaxel (N=94) Docetaxel + trastuzumab (N=92) Survival >3 years 29 43 Survival >4 years 10 20 Survival >4. 5 years 4 9 Marty et al. SABCS 2006. Abstract 2067.

M 77001: Safety • Grade 3/4 neutropenia and febrile neutropenia were more common with docetaxel-trastuzumab vs singleagent docetaxel – Neutropenia: 32% vs 22% – Febrile neutropenia: 23% vs 17% M 77001: Key Outcomes • Docetaxel + trastuzumab combination therapy produced an OS of 31. 2 months when used as a firstline treatment in patients with HER 2+ MBC Marty et al. J Clin Oncol. 2005; 23: 4265– 4274.

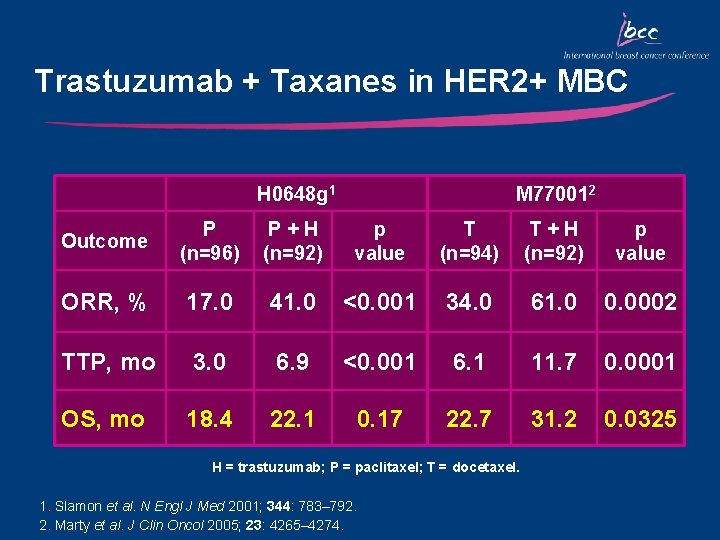
Trastuzumab + Taxanes in HER 2+ MBC H 0648 g 1 M 770012 Outcome P (n=96) P+H (n=92) p value T (n=94) T+H (n=92) p value ORR, % 17. 0 41. 0 <0. 001 34. 0 61. 0 0. 0002 TTP, mo 3. 0 6. 9 <0. 001 6. 1 11. 7 0. 0001 OS, mo 18. 4 22. 1 0. 17 22. 7 31. 2 0. 0325 H = trastuzumab; P = paclitaxel; T = docetaxel. 1. Slamon et al. N Engl J Med 2001; 344: 783– 792. 2. Marty et al. J Clin Oncol 2005; 23: 4265– 4274.

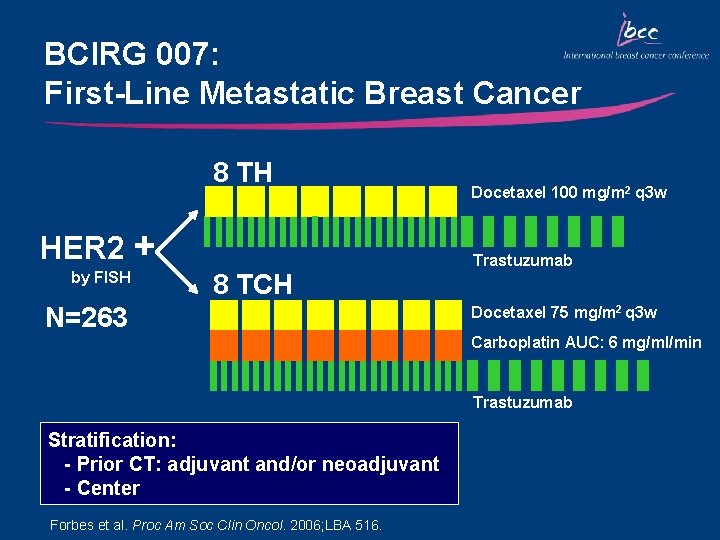
BCIRG 007: First-Line Metastatic Breast Cancer 8 TH HER 2 + by FISH 8 TCH N=263 Docetaxel 100 mg/m 2 q 3 w Trastuzumab Docetaxel 75 mg/m 2 q 3 w Carboplatin AUC: 6 mg/ml/min Trastuzumab Stratification: - Prior CT: adjuvant and/or neoadjuvant - Center Forbes et al. Proc Am Soc Clin Oncol. 2006; LBA 516.

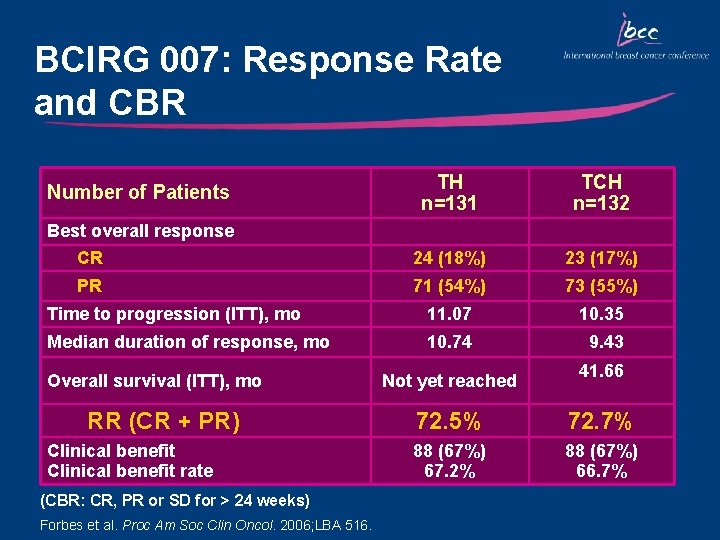
BCIRG 007: Response Rate and CBR TH n=131 TCH n=132 24 (18%) 23 (17%) 71 (54%) 73 (55%) Time to progression (ITT), mo 11. 07 10. 35 Median duration of response, mo 10. 74 9. 43 Number of Patients Best overall response CR PR Overall survival (ITT), mo RR (CR + PR) Clinical benefit rate (CBR: CR, PR or SD for > 24 weeks) Forbes et al. Proc Am Soc Clin Oncol. 2006; LBA 516. Not yet reached 41. 66 72. 5% 72. 7% 88 (67%) 67. 2% 88 (67%) 66. 7%

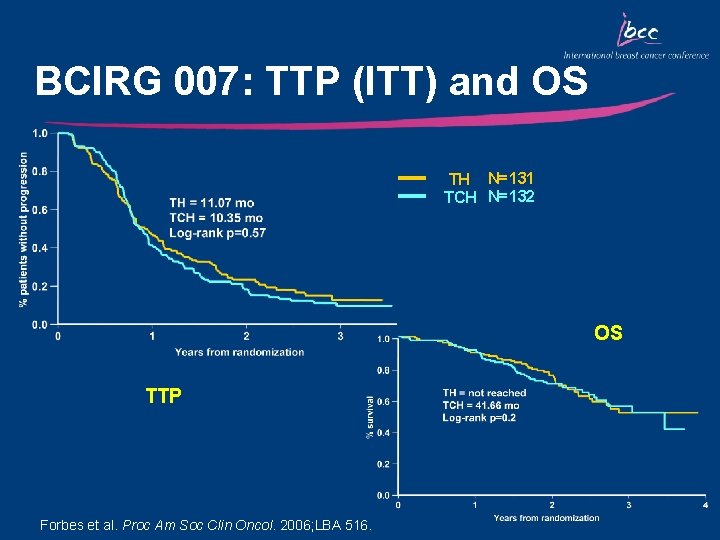
BCIRG 007: TTP (ITT) and OS TH N=131 TCH N=132 OS TTP Forbes et al. Proc Am Soc Clin Oncol. 2006; LBA 516.

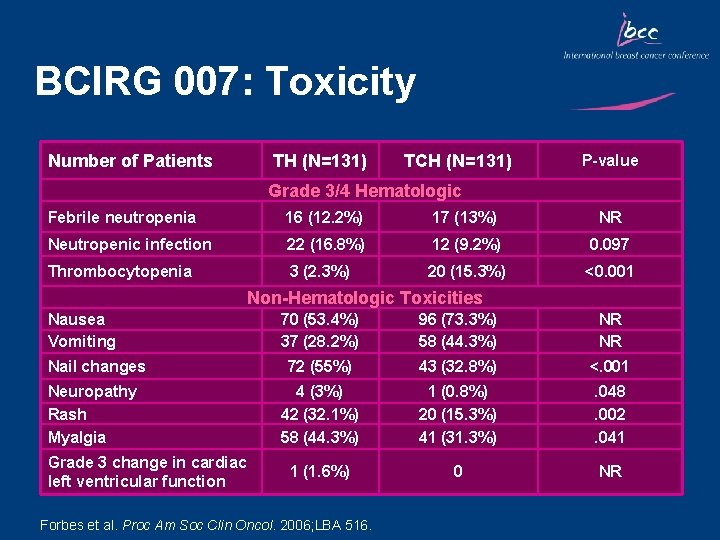
BCIRG 007: Toxicity Number of Patients TH (N=131) TCH (N=131) P-value Grade 3/4 Hematologic Febrile neutropenia 16 (12. 2%) 17 (13%) NR Neutropenic infection 22 (16. 8%) 12 (9. 2%) 0. 097 Thrombocytopenia 3 (2. 3%) 20 (15. 3%) <0. 001 Non-Hematologic Toxicities Nausea Vomiting Nail changes Neuropathy Rash Myalgia Grade 3 change in cardiac left ventricular function 70 (53. 4%) 37 (28. 2%) 96 (73. 3%) 58 (44. 3%) NR NR 72 (55%) 43 (32. 8%) <. 001 4 (3%) 42 (32. 1%) 58 (44. 3%) 1 (0. 8%) 20 (15. 3%) 41 (31. 3%) . 048. 002. 041 1 (1. 6%) 0 NR Forbes et al. Proc Am Soc Clin Oncol. 2006; LBA 516.

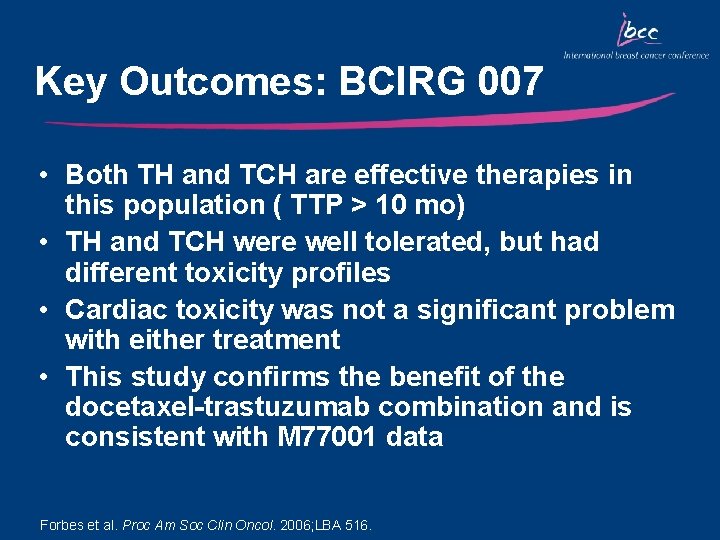
Key Outcomes: BCIRG 007 • Both TH and TCH are effective therapies in this population ( TTP > 10 mo) • TH and TCH were well tolerated, but had different toxicity profiles • Cardiac toxicity was not a significant problem with either treatment • This study confirms the benefit of the docetaxel-trastuzumab combination and is consistent with M 77001 data Forbes et al. Proc Am Soc Clin Oncol. 2006; LBA 516.

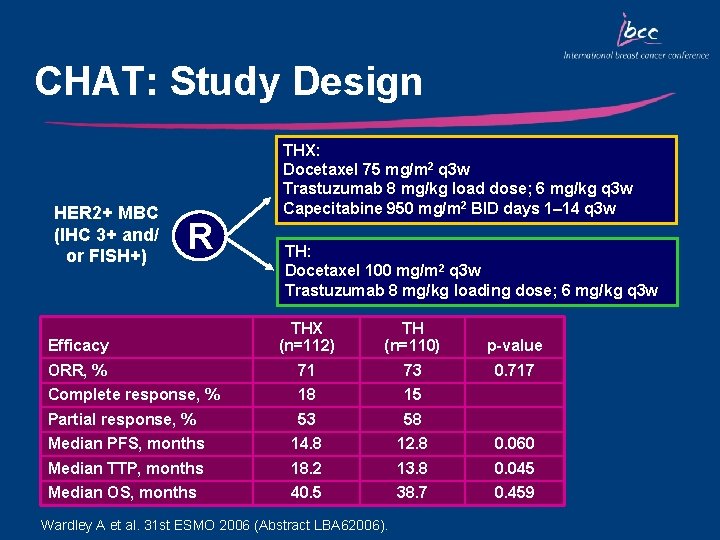
CHAT: Study Design HER 2+ MBC (IHC 3+ and/ or FISH+) R THX: Docetaxel 75 mg/m 2 q 3 w Trastuzumab 8 mg/kg load dose; 6 mg/kg q 3 w Capecitabine 950 mg/m 2 BID days 1– 14 q 3 w TH: Docetaxel 100 mg/m 2 q 3 w Trastuzumab 8 mg/kg loading dose; 6 mg/kg q 3 w Efficacy THX (n=112) TH (n=110) p-value ORR, % 71 73 0. 717 Complete response, % 18 15 Partial response, % 53 58 Median PFS, months 14. 8 12. 8 0. 060 Median TTP, months 18. 2 13. 8 0. 045 Median OS, months 40. 5 38. 7 0. 459 Wardley A et al. 31 st ESMO 2006 (Abstract LBA 62006).

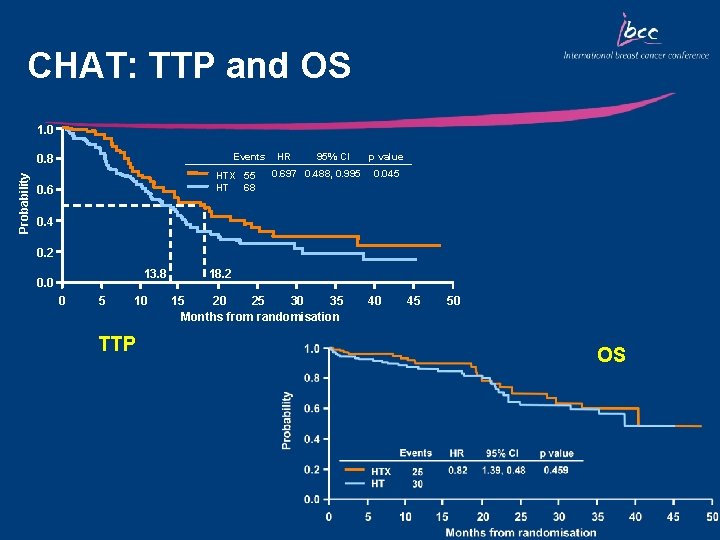
CHAT: TTP and OS 1. 0 Events Probability 0. 8 HTX 55 68 HT 0. 6 HR 95% CI 0. 697 0. 488, 0. 995 p value 0. 045 0. 4 0. 2 13. 8 0. 0 0 5 10 TTP 18. 2 15 20 25 30 35 Months from randomisation 40 45 50 OS

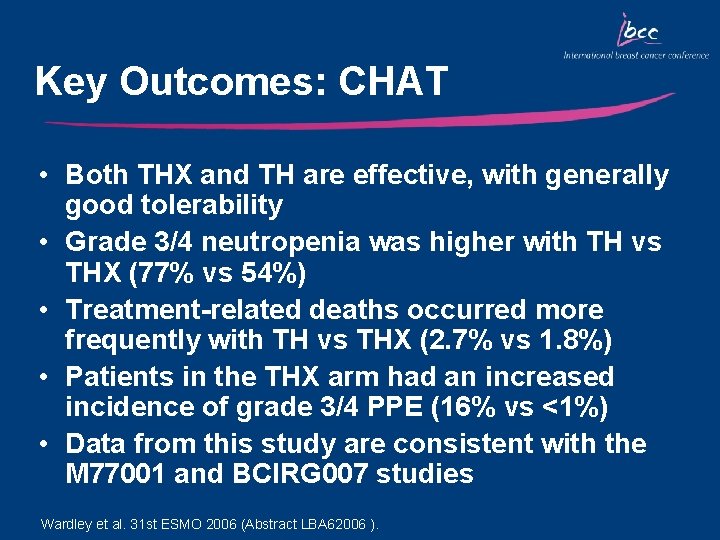
Key Outcomes: CHAT • Both THX and TH are effective, with generally good tolerability • Grade 3/4 neutropenia was higher with TH vs THX (77% vs 54%) • Treatment-related deaths occurred more frequently with TH vs THX (2. 7% vs 1. 8%) • Patients in the THX arm had an increased incidence of grade 3/4 PPE (16% vs <1%) • Data from this study are consistent with the M 77001 and BCIRG 007 studies Wardley et al. 31 st ESMO 2006 (Abstract LBA 62006 ).

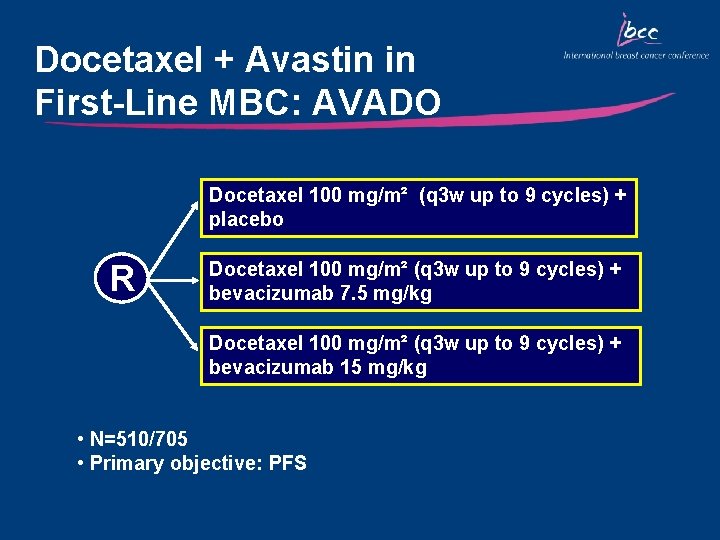
Docetaxel + Avastin in First-Line MBC: AVADO Docetaxel 100 mg/m² (q 3 w up to 9 cycles) + placebo R Docetaxel 100 mg/m² (q 3 w up to 9 cycles) + bevacizumab 7. 5 mg/kg Docetaxel 100 mg/m² (q 3 w up to 9 cycles) + bevacizumab 15 mg/kg • N=510/705 • Primary objective: PFS

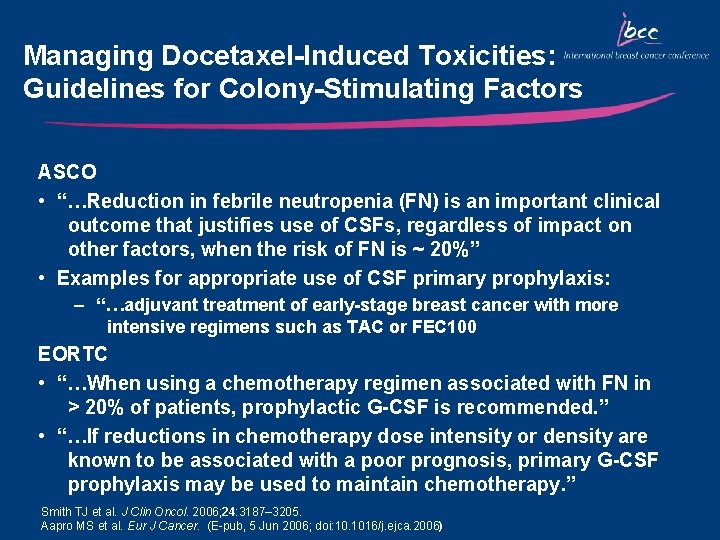
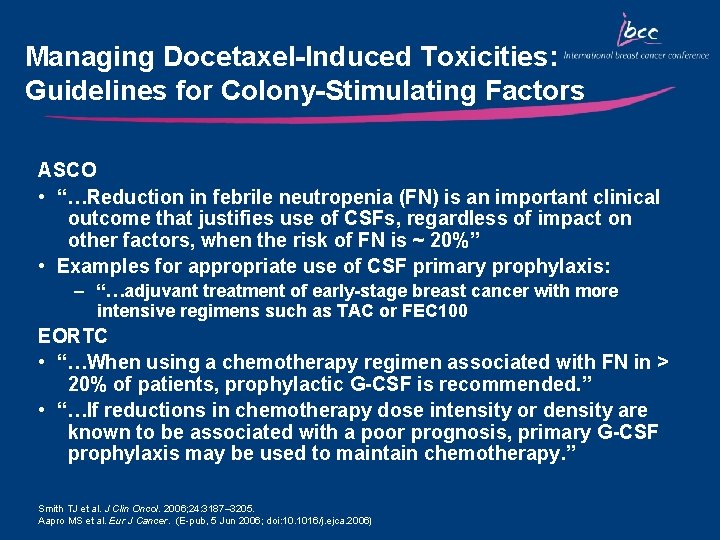
Managing Docetaxel-Induced Toxicities: Guidelines for Colony-Stimulating Factors ASCO • “…Reduction in febrile neutropenia (FN) is an important clinical outcome that justifies use of CSFs, regardless of impact on other factors, when the risk of FN is ~ 20%” • Examples for appropriate use of CSF primary prophylaxis: – “…adjuvant treatment of early-stage breast cancer with more intensive regimens such as TAC or FEC 100 EORTC • “…When using a chemotherapy regimen associated with FN in > 20% of patients, prophylactic G-CSF is recommended. ” • “…If reductions in chemotherapy dose intensity or density are known to be associated with a poor prognosis, primary G-CSF prophylaxis may be used to maintain chemotherapy. ” Smith TJ et al. J Clin Oncol. 2006; 24: 3187– 3205. Aapro MS et al. Eur J Cancer. (E-pub, 5 Jun 2006; doi: 10. 1016/j. ejca. 2006)

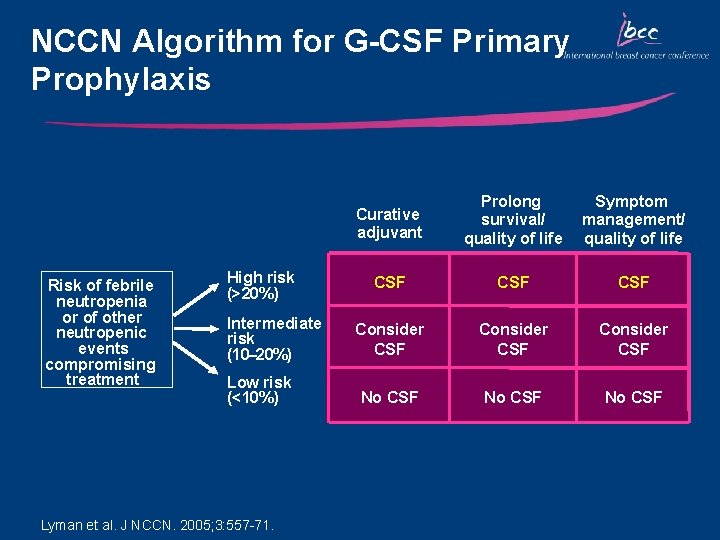
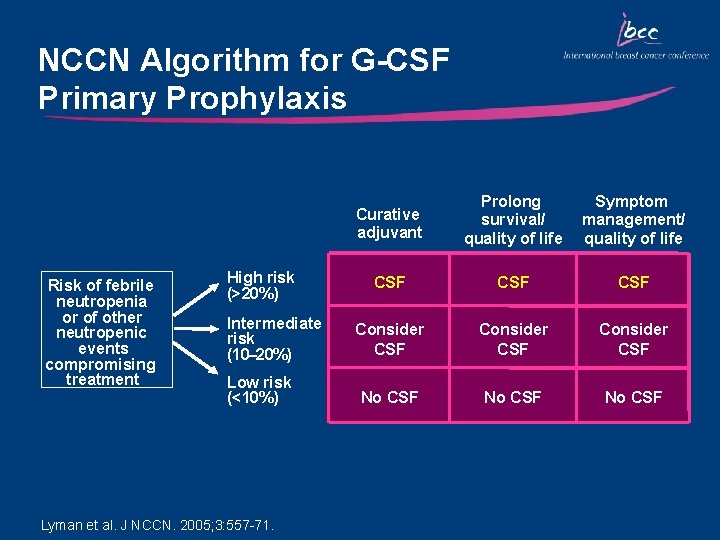
NCCN Algorithm for G-CSF Primary Prophylaxis Risk of febrile neutropenia or of other neutropenic events compromising treatment High risk (>20%) Intermediate risk (10– 20%) Low risk (<10%) Lyman et al. J NCCN. 2005; 3: 557 -71. Curative adjuvant Prolong survival/ quality of life Symptom management/ quality of life CSF CSF Consider CSF No CSF

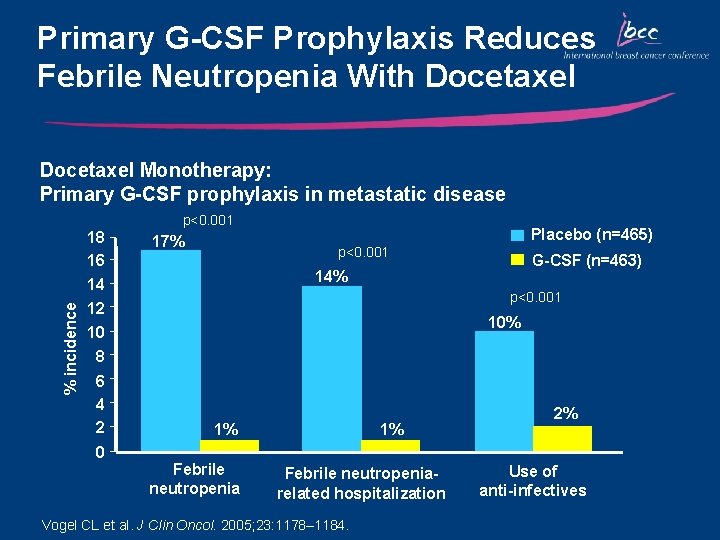
Primary G-CSF Prophylaxis Reduces Febrile Neutropenia With Docetaxel Monotherapy: Primary G-CSF prophylaxis in metastatic disease p<0. 001 % incidence 18 16 17% Placebo (n=465) p<0. 001 G-CSF (n=463) 14% 14 12 p<0. 001 10% 10 8 6 4 2 0 1% Febrile neutropeniarelated hospitalization Vogel CL et al. J Clin Oncol. 2005; 23: 1178– 1184. 2% Use of anti-infectives

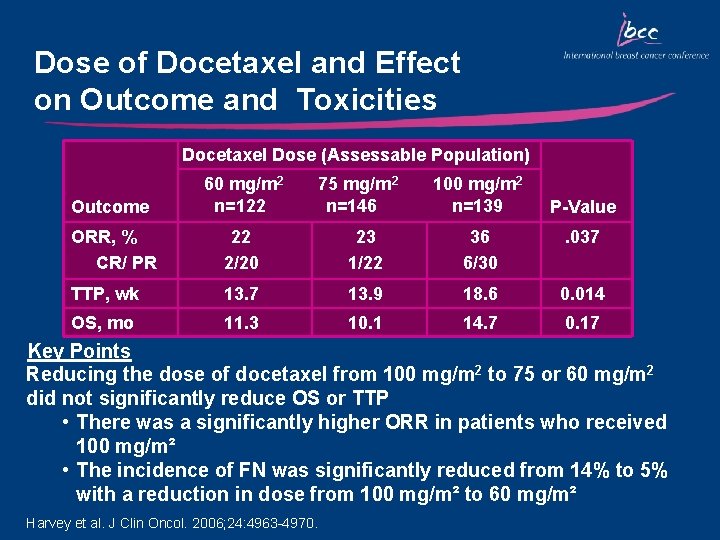
Dose of Docetaxel and Effect on Outcome and Toxicities Docetaxel Dose (Assessable Population) Outcome 60 mg/m 2 n=122 75 mg/m 2 n=146 100 mg/m 2 n=139 ORR, % CR/ PR 22 2/20 23 1/22 36 6/30 . 037 TTP, wk 13. 7 13. 9 18. 6 0. 014 OS, mo 11. 3 10. 1 14. 7 0. 17 P-Value Key Points Reducing the dose of docetaxel from 100 mg/m 2 to 75 or 60 mg/m 2 did not significantly reduce OS or TTP • There was a significantly higher ORR in patients who received 100 mg/m² • The incidence of FN was significantly reduced from 14% to 5% with a reduction in dose from 100 mg/m² to 60 mg/m² Harvey et al. J Clin Oncol. 2006; 24: 4963 -4970.

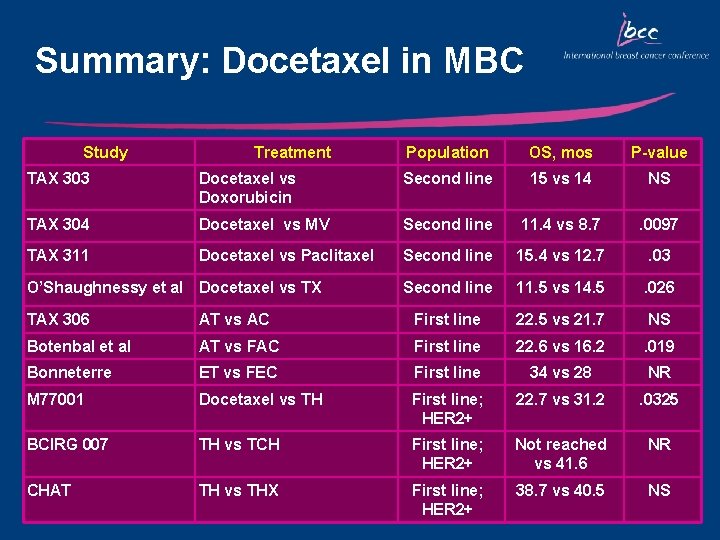
Summary: Docetaxel in MBC Study Treatment Population OS, mos P-value TAX 303 Docetaxel vs Doxorubicin Second line 15 vs 14 NS TAX 304 Docetaxel vs MV Second line 11. 4 vs 8. 7 . 0097 TAX 311 Docetaxel vs Paclitaxel Second line 15. 4 vs 12. 7 . 03 Second line 11. 5 vs 14. 5 . 026 O’Shaughnessy et al Docetaxel vs TX TAX 306 AT vs AC First line 22. 5 vs 21. 7 NS Botenbal et al AT vs FAC First line 22. 6 vs 16. 2 . 019 Bonneterre ET vs FEC First line 34 vs 28 NR M 77001 Docetaxel vs TH First line; HER 2+ 22. 7 vs 31. 2 . 0325 BCIRG 007 TH vs TCH First line; HER 2+ Not reached vs 41. 6 NR CHAT TH vs THX First line; HER 2+ 38. 7 vs 40. 5 NS

Docetaxel in Breast Cancer: Conclusions • Docetaxel is one of the most, if not the most, active chemotherapy agents for breast cancer patients – Five studies have demonstrated a survival benefit in MBC (monotherapy, or in combination with doxorubicin, capecitabine, or trastuzumab) – Three studies have demonstrated the benefit of the docetaxel –Herceptin combination in MBC (M 77001, BCIRG 007 and CHAT) • Primary prophylaxis with G-CSF improves the safety profile of docetaxel, making it a very tolerable regimen Main Menu

Current Landscape and Future Directions of Docetaxel in Early-Stage Breast Cancer John Mackey, MD Cross Cancer Institute Edmonton, Alberta, Canada Main Menu

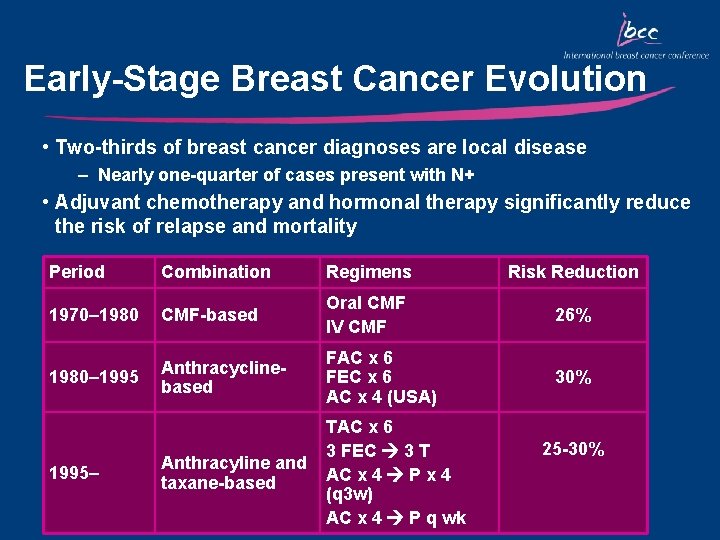
Early-Stage Breast Cancer Evolution • Two-thirds of breast cancer diagnoses are local disease – Nearly one-quarter of cases present with N+ • Adjuvant chemotherapy and hormonal therapy significantly reduce the risk of relapse and mortality Period Combination Regimens Risk Reduction 1970– 1980 CMF-based Oral CMF IV CMF 26% 1980– 1995 Anthracyclinebased FAC x 6 FEC x 6 AC x 4 (USA) 30% Anthracyline and taxane-based TAC x 6 3 FEC 3 T AC x 4 P x 4 (q 3 w) AC x 4 P q wk 1995– 25 -30%

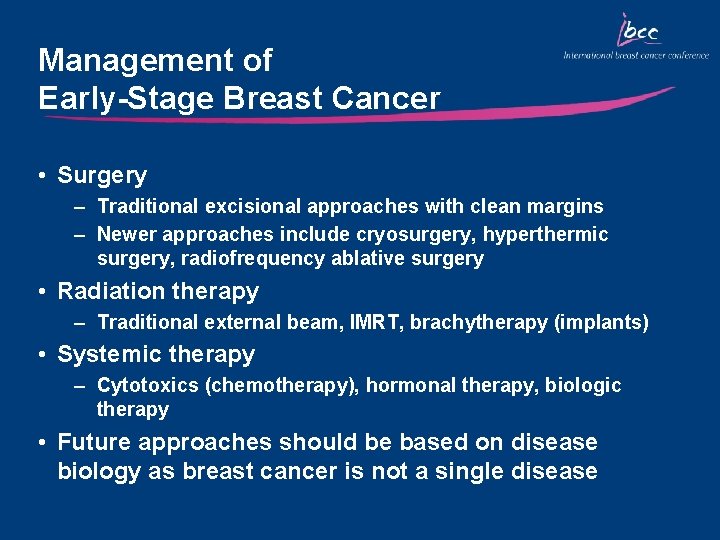
Management of Early-Stage Breast Cancer • Surgery – Traditional excisional approaches with clean margins – Newer approaches include cryosurgery, hyperthermic surgery, radiofrequency ablative surgery • Radiation therapy – Traditional external beam, IMRT, brachytherapy (implants) • Systemic therapy – Cytotoxics (chemotherapy), hormonal therapy, biologic therapy • Future approaches should be based on disease biology as breast cancer is not a single disease

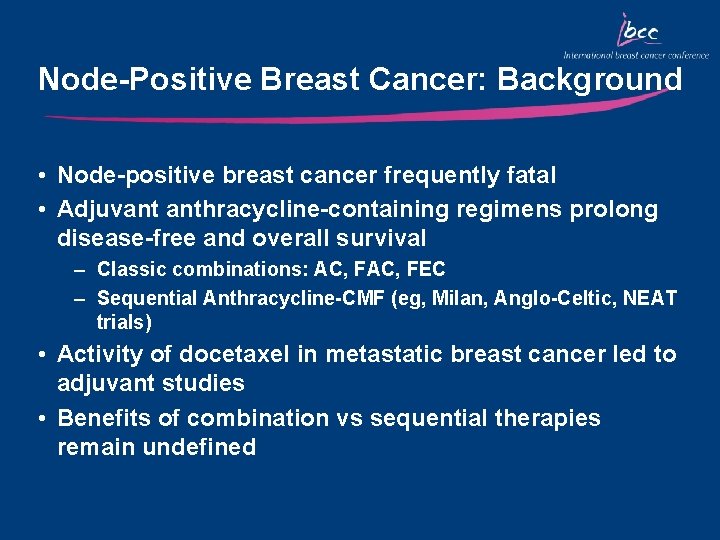
Node-Positive Breast Cancer: Background • Node-positive breast cancer frequently fatal • Adjuvant anthracycline-containing regimens prolong disease-free and overall survival – Classic combinations: AC, FEC – Sequential Anthracycline-CMF (eg, Milan, Anglo-Celtic, NEAT trials) • Activity of docetaxel in metastatic breast cancer led to adjuvant studies • Benefits of combination vs sequential therapies remain undefined

Docetaxel Combination Therapies in Early-Stage Breast Cancer

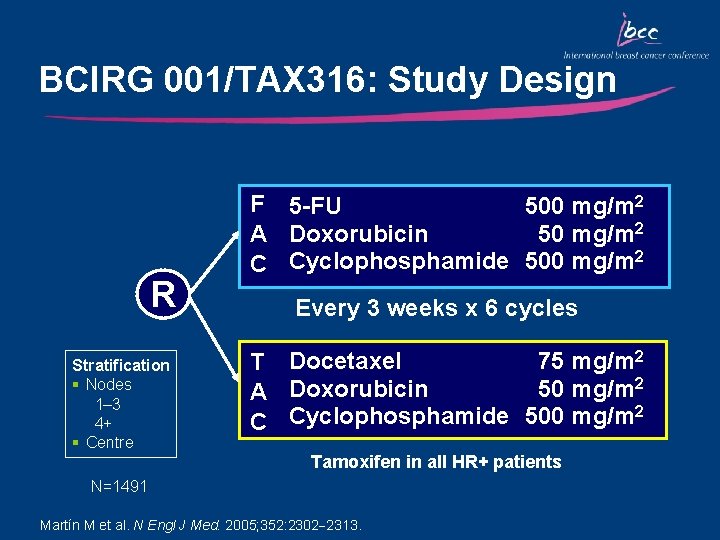
BCIRG 001/TAX 316: Study Design R Stratification § Nodes 1– 3 4+ § Centre F 5 -FU 500 mg/m 2 50 mg/m 2 A Doxorubicin C Cyclophosphamide 500 mg/m 2 Every 3 weeks x 6 cycles 75 mg/m 2 T Docetaxel 50 mg/m 2 A Doxorubicin 2 C Cyclophosphamide 500 mg/m Tamoxifen in all HR+ patients N=1491 Martín M et al. N Engl J Med. 2005; 352: 2302 2313.

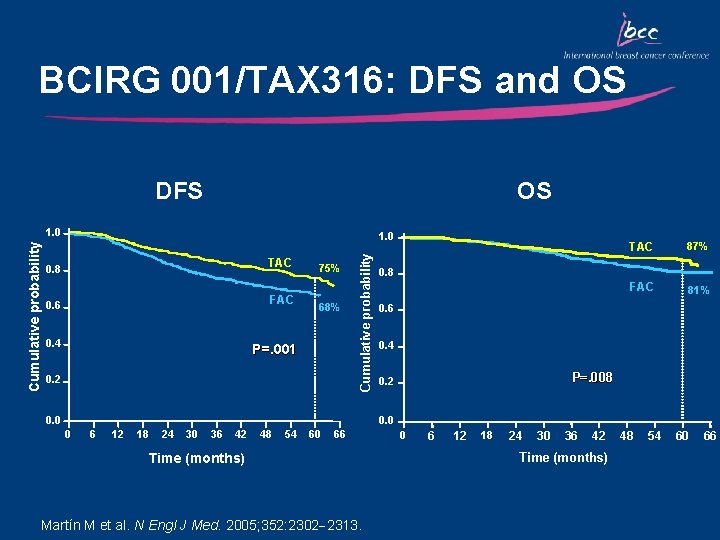
BCIRG 001/TAX 316: DFS and OS DFS OS 1. 0 TAC 0. 8 FAC 0. 6 0. 4 75% 68% P=. 001 0. 2 Cumulative probability 1. 0 0. 0 TAC 87% FAC 81% 0. 8 0. 6 0. 4 P=. 008 0. 2 0. 0 0 6 12 18 24 30 36 42 48 54 60 66 Time (months) Martín M et al. N Engl J Med. 2005; 352: 2302 2313. 0 6 12 18 24 30 36 42 Time (months) 48 54 60 66

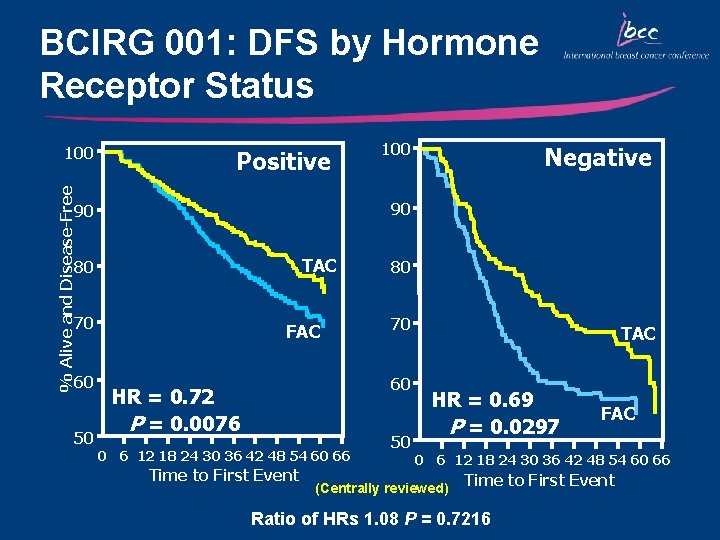
BCIRG 001: DFS by Hormone Receptor Status % Alive and Disease-Free 100 Positive TAC 80 70 50 Negative 90 90 60 100 FAC 80 70 60 HR = 0. 72 P = 0. 0076 0 6 12 18 24 30 36 42 48 54 60 66 Time to First Event 50 TAC HR = 0. 69 P = 0. 0297 FAC 0 6 12 18 24 30 36 42 48 54 60 66 (Centrally reviewed) Time to First Event Ratio of HRs 1. 08 P = 0. 7216

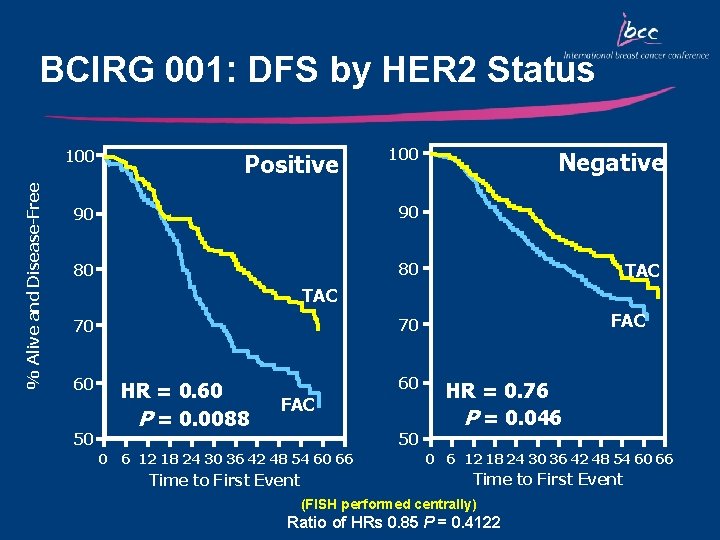
BCIRG 001: DFS by HER 2 Status % Alive and Disease-Free 100 Positive 100 90 90 80 80 Negative TAC 60 50 FAC 70 70 HR = 0. 60 P = 0. 0088 60 FAC 50 HR = 0. 76 P = 0. 046 0 6 12 18 24 30 36 42 48 54 60 66 Time to First Event (FISH performed centrally) Ratio of HRs 0. 85 P = 0. 4122

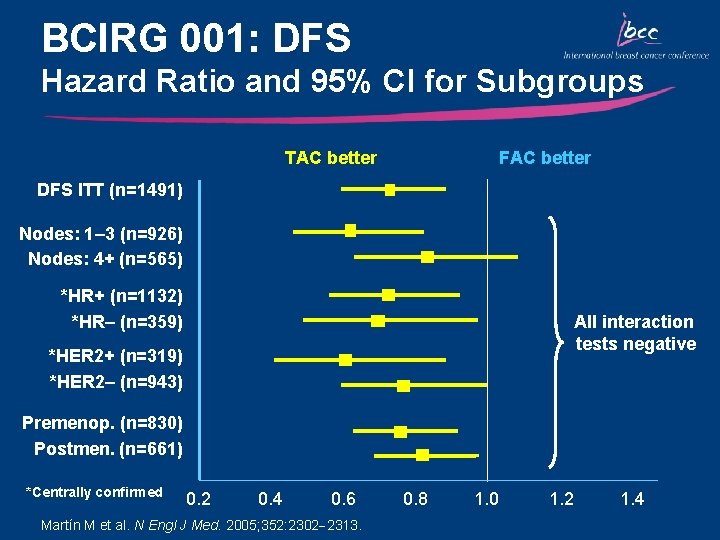
BCIRG 001: DFS Hazard Ratio and 95% CI for Subgroups TAC better FAC better DFS ITT (n=1491) Nodes: 1– 3 (n=926) Nodes: 4+ (n=565) *HR+ (n=1132) *HR– (n=359) All interaction tests negative *HER 2+ (n=319) *HER 2– (n=943) Premenop. (n=830) Postmen. (n=661) *Centrally confirmed 0. 2 0. 4 0. 6 Martín M et al. N Engl J Med. 2005; 352: 2302 2313. 0. 8 1. 0 1. 2 1. 4

Key Outcomes: BCIRG 001/TAX 316 • TAC significantly improved DFS and OS compared with FAC irrespective of nodal, HER 2, menopausal, and hormonal status – DFS: 28% relative reduction in the risk of relapse – OS: 30% relative reduction in the risk of mortality • Primary prophylaxis with G-CSF is recommended for TAC due to the risk of neutropenia • The absolute increase in the magnitude of benefit for DFS and OS with TAC is the highest reported in adjuvant studies

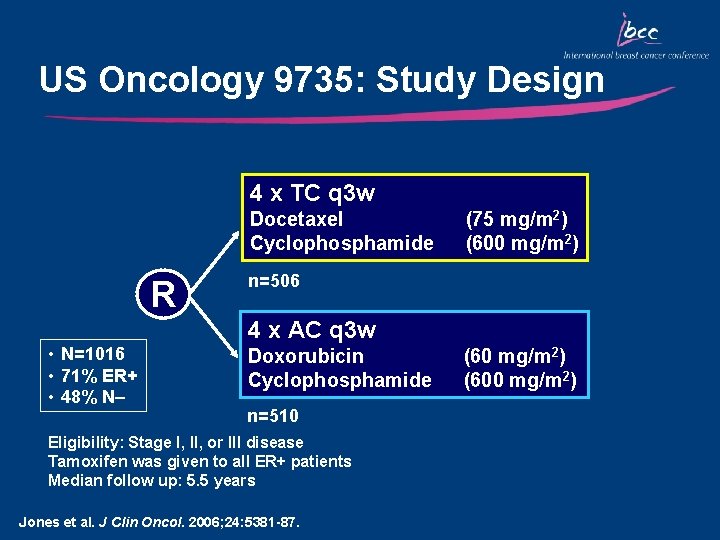
US Oncology 9735: Study Design 4 x TC q 3 w Docetaxel Cyclophosphamide R (75 mg/m 2) (600 mg/m 2) n=506 4 x AC q 3 w • N=1016 • 71% ER+ • 48% N– Doxorubicin Cyclophosphamide n=510 Eligibility: Stage I, II, or III disease Tamoxifen was given to all ER+ patients Median follow up: 5. 5 years Jones et al. J Clin Oncol. 2006; 24: 5381 -87. (60 mg/m 2) (600 mg/m 2)

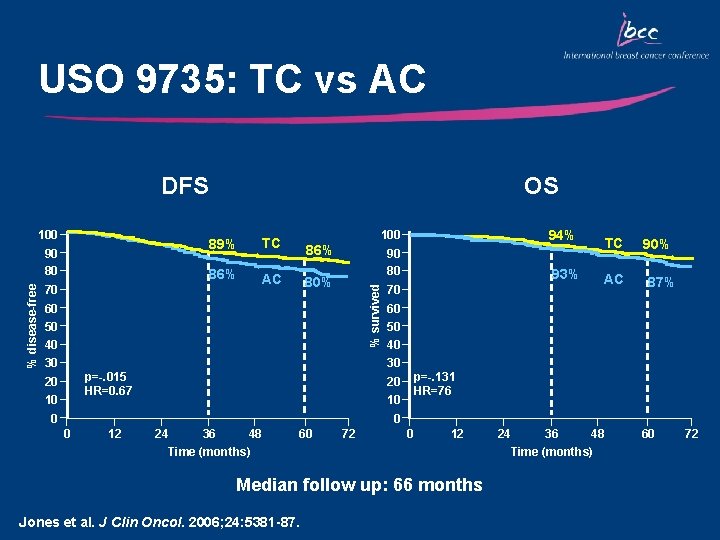
USO 9735: TC vs AC DFS % disease-free 90 80 70 89% TC 86% AC 100 94% 90 80 93% 86% 80% % survived 100 OS 60 50 40 30 70 TC AC 90% 87% 60 50 40 30 p=-. 015 HR=0. 67 20 10 p=-. 131 HR=76 20 10 0 12 24 36 48 Time (months) 60 72 0 12 Median follow up: 66 months Jones et al. J Clin Oncol. 2006; 24: 5381 -87. 24 36 48 Time (months) 60 72

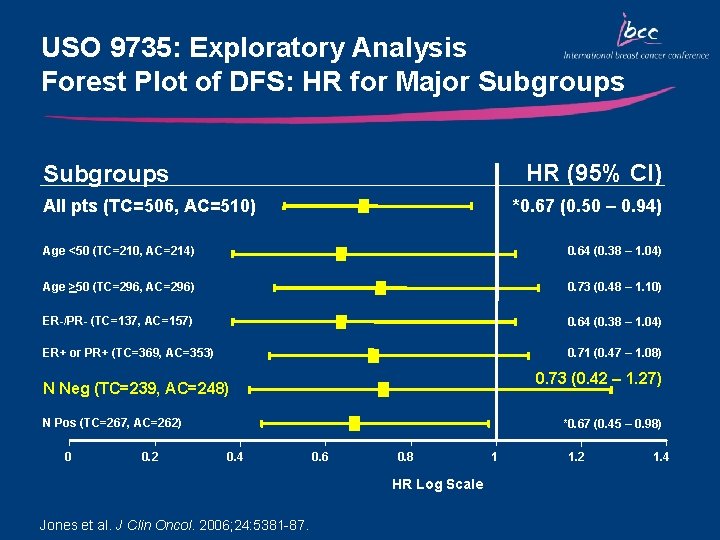
USO 9735: Exploratory Analysis Forest Plot of DFS: HR for Major Subgroups HR (95% CI) Subgroups All pts (TC=506, AC=510) *0. 67 (0. 50 – 0. 94) Age <50 (TC=210, AC=214) 0. 64 (0. 38 – 1. 04) Age >50 (TC=296, AC=296) 0. 73 (0. 48 – 1. 10) ER-/PR- (TC=137, AC=157) 0. 64 (0. 38 – 1. 04) ER+ or PR+ (TC=369, AC=353) 0. 71 (0. 47 – 1. 08) 0. 73 (0. 42 – 1. 27) N Neg (TC=239, AC=248) N Pos (TC=267, AC=262) 0 0. 2 *0. 67 (0. 45 – 0. 98) 0. 4 0. 6 0. 8 HR Log Scale Jones et al. J Clin Oncol. 2006; 24: 5381 -87. 1 1. 2 1. 4

Key Outcomes: USO 9735 • TC was associated with superior DFS and improved OS vs AC • At a median follow-up of 66 months: • 5 -year DFS rate was significantly increased with TC compared with AC (86% vs 80%; p=0. 015) • 33% reduction in the risk of recurrence • 24% reduction in the risk of mortality (p=0. 13) • FN occurs more frequently with TC vs AC (6% vs 3%) • Nausea and vomiting occurs more frequently with AC vs TC • TC is an alternative, non-anthracycline-based regimen • Findings of this trial are fundamentally significant, particularly for node-negative patients

Sequential Administration of Docetaxel in Early. Stage Breast Cancer

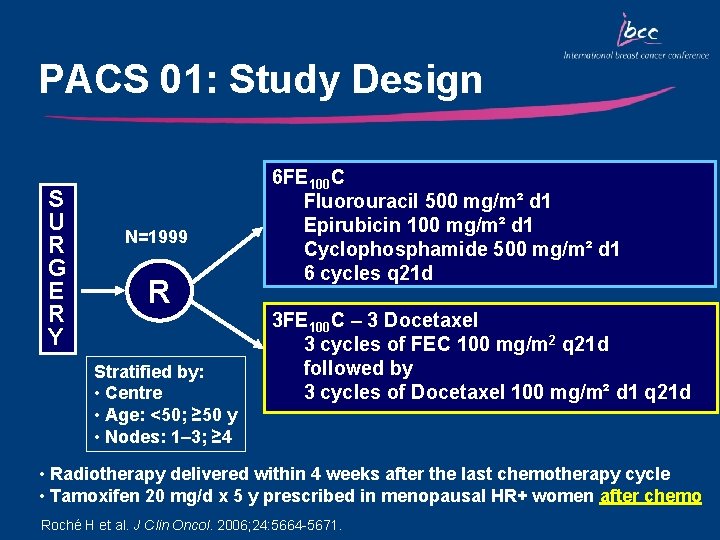
PACS 01: Study Design S U R G E R Y N=1999 R Stratified by: • Centre • Age: <50; ≥ 50 y • Nodes: 1– 3; ≥ 4 6 FE 100 C Fluorouracil 500 mg/m² d 1 Epirubicin 100 mg/m² d 1 Cyclophosphamide 500 mg/m² d 1 6 cycles q 21 d 3 FE 100 C – 3 Docetaxel 3 cycles of FEC 100 mg/m 2 q 21 d followed by 3 cycles of Docetaxel 100 mg/m² d 1 q 21 d • Radiotherapy delivered within 4 weeks after the last chemotherapy cycle • Tamoxifen 20 mg/d x 5 y prescribed in menopausal HR+ women after chemo Roché H et al. J Clin Oncol. 2006; 24: 5664 -5671.

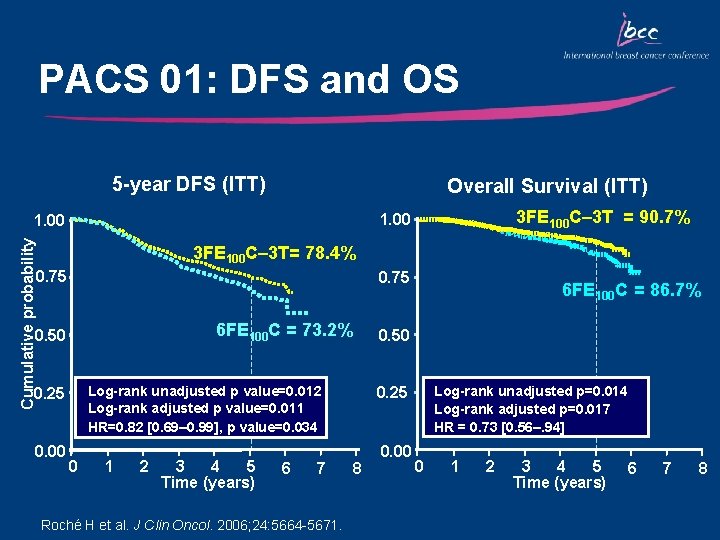
PACS 01: DFS and OS 5 -year DFS (ITT) Overall Survival (ITT) 3 FE 100 C– 3 T = 90. 7% 1. 00 Cumulative probability 1. 00 3 FE 100 C– 3 T= 78. 4% 0. 75 6 FE 100 C = 73. 2% 0. 50 0. 00 0. 50 Log-rank unadjusted p value=0. 012 Log-rank adjusted p value=0. 011 HR=0. 82 [0. 69– 0. 99], p value=0. 034 0. 25 0 1 2 3 4 5 Time (years) 6 7 Roché H et al. J Clin Oncol. 2006; 24: 5664 -5671. 6 FE 100 C = 86. 7% Log-rank unadjusted p=0. 014 Log-rank adjusted p=0. 017 HR = 0. 73 [0. 56–. 94] 0. 25 8 0. 00 0 1 2 3 4 5 Time (years) 6 7 8

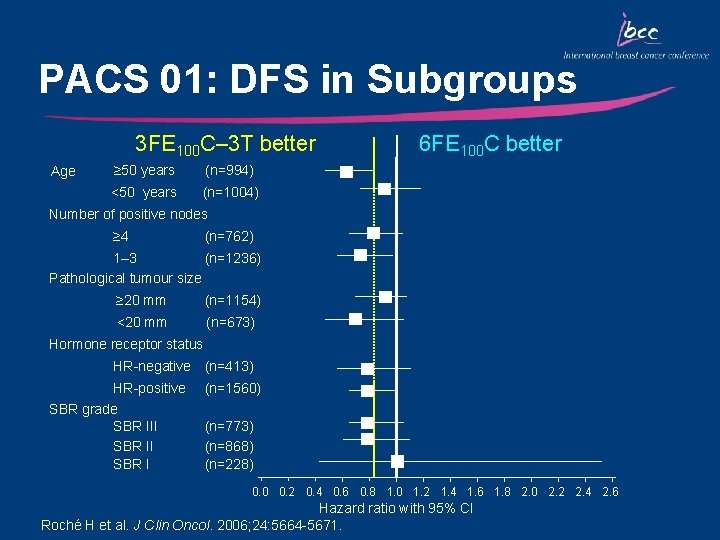
PACS 01: DFS in Subgroups 3 FE 100 C– 3 T better Age ≥ 50 years (n=994) <50 years (n=1004) 6 FE 100 C better Number of positive nodes ≥ 4 (n=762) 1– 3 (n=1236) Pathological tumour size ≥ 20 mm (n=1154) <20 mm (n=673) Hormone receptor status HR-negative (n=413) HR-positive SBR grade SBR III SBR I (n=1560) (n=773) (n=868) (n=228) 0. 0 0. 2 0. 4 0. 6 0. 8 1. 0 1. 2 1. 4 1. 6 1. 8 2. 0 2. 2 2. 4 2. 6 Hazard ratio with 95% CI Roché H et al. J Clin Oncol. 2006; 24: 5664 -5671.

Key Outcomes: PACS 01 • 3 FEC → 3 docetaxel demonstrated superior DFS and OS irrespective of the number of nodes or hormone-receptor status – 18% reduction of risk of relapse – 23% reduction of risk of death • 3 FEC → 3 docetaxel is feasible without systemic antibiotics and/or G-CSF – Incidence of FN with FEC → docetaxel: 11. 2% (vs 24. 7% with the TAC combination regimen) • Toxicity related to docetaxel is easily manageable • Reduced risks of acute and delayed cardiac events because of 50% less exposure to anthracyclines • 3 FEC → 3 docetaxel is an alternative to combination therapy in patients ≥ 50 years of age

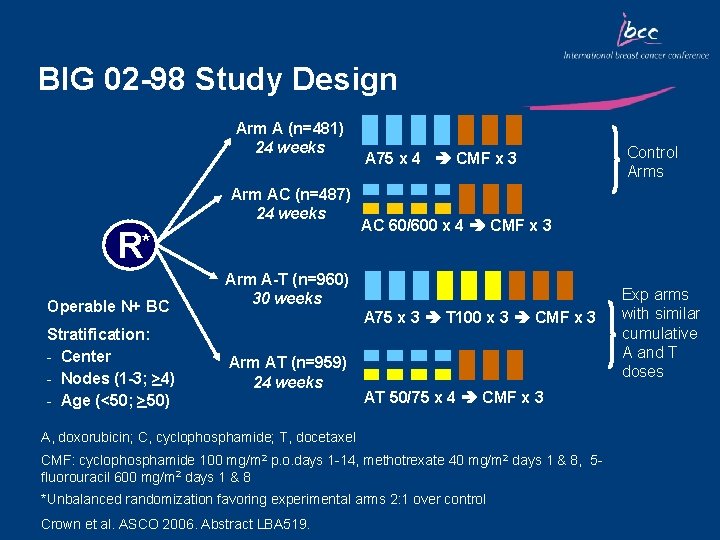
BIG 02 -98 Study Design Arm A (n=481) 24 weeks Arm AC (n=487) 24 weeks R* Operable N+ BC Stratification: - Center - Nodes (1 -3; >4) - Age (<50; >50) A 75 x 4 CMF x 3 AC 60/600 x 4 CMF x 3 Arm A-T (n=960) 30 weeks A 75 x 3 T 100 x 3 CMF x 3 Arm AT (n=959) 24 weeks AT 50/75 x 4 CMF x 3 A, doxorubicin; C, cyclophosphamide; T, docetaxel CMF: cyclophosphamide 100 mg/m 2 p. o. days 1 -14, methotrexate 40 mg/m 2 days 1 & 8, 5 fluorouracil 600 mg/m 2 days 1 & 8 *Unbalanced randomization favoring experimental arms 2: 1 over control Crown et al. ASCO 2006. Abstract LBA 519. Control Arms Exp arms with similar cumulative A and T doses

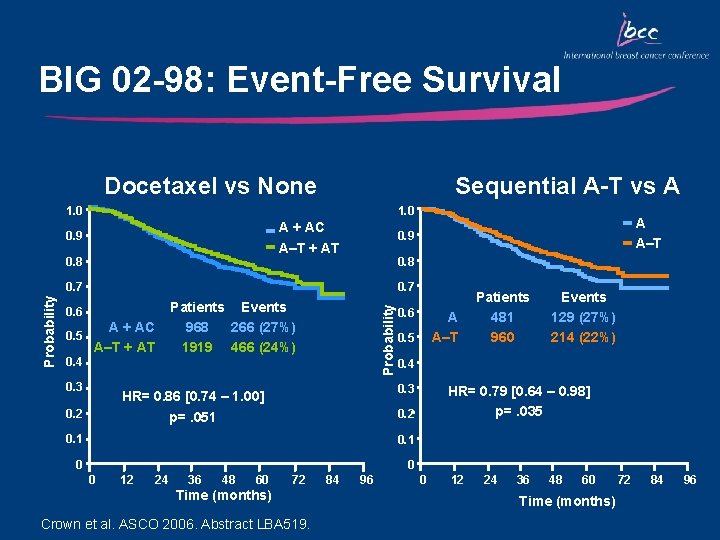
BIG 02 -98: Event-Free Survival Docetaxel vs None Sequential A-T vs A 1. 0 A + AC A–T + AT 0. 9 0. 8 0. 7 0. 6 A + AC 0. 5 A–T + AT 0. 4 0. 3 Patients Events 968 266 (27%) 1919 466 (24%) Probability A A–T 0. 9 0. 5 0. 1 0 0 12 24 36 48 60 72 Time (months) Crown et al. ASCO 2006. Abstract LBA 519. 84 96 Events 129 (27%) 214 (22%) 0. 4 HR= 0. 79 [0. 64 – 0. 98] p=. 035 0. 2 p=. 051 0 A A–T 0. 3 HR= 0. 86 [0. 74 – 1. 00] 0. 2 0. 6 Patients 481 960 0 12 24 36 48 60 Time (months) 72 84 96

Key Outcomes: BIG 02 -98 • There was a lower than expected relapse rate – Control groups had 73% EFS at 5 years, despite 46% having > 4 nodes • Addition of docetaxel improved EFS, which was of borderline significance (p=0. 051), however • Sequential A-T-CMF produced superior EFS vs both combination AT-CMF and A-CMF • This is the first trial to demonstrate improved outcome for sequential over combination anthracycline/taxane chemotherapy Crown et al. ASCO 2006. Abstract LBA 519.

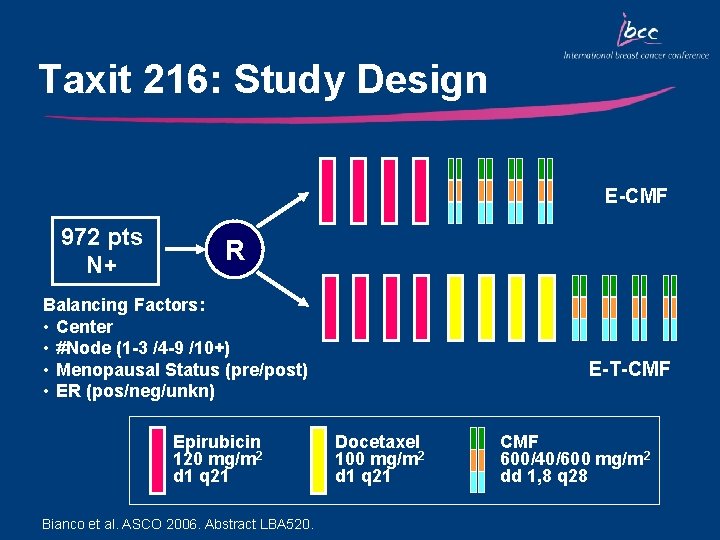
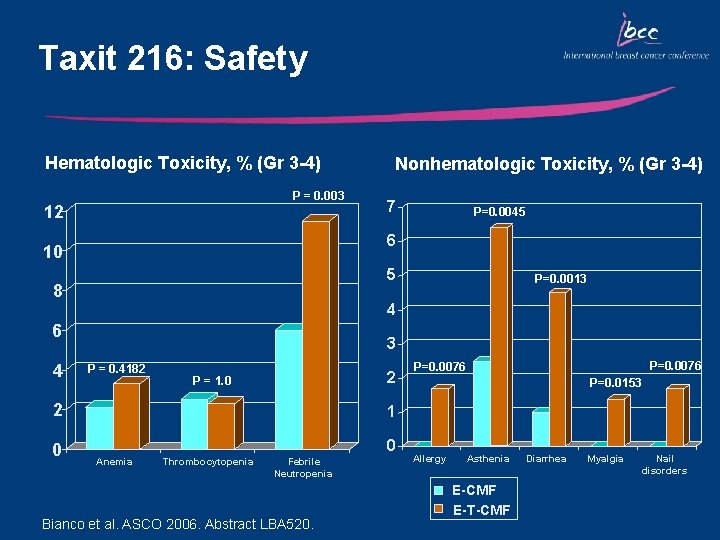
Taxit 216: Study Design E-CMF 972 pts N+ R Balancing Factors: • Center • #Node (1 -3 /4 -9 /10+) • Menopausal Status (pre/post) • ER (pos/neg/unkn) Epirubicin 120 mg/m 2 d 1 q 21 Bianco et al. ASCO 2006. Abstract LBA 520. E-T-CMF Docetaxel 100 mg/m 2 d 1 q 21 CMF 600/40/600 mg/m 2 dd 1, 8 q 28

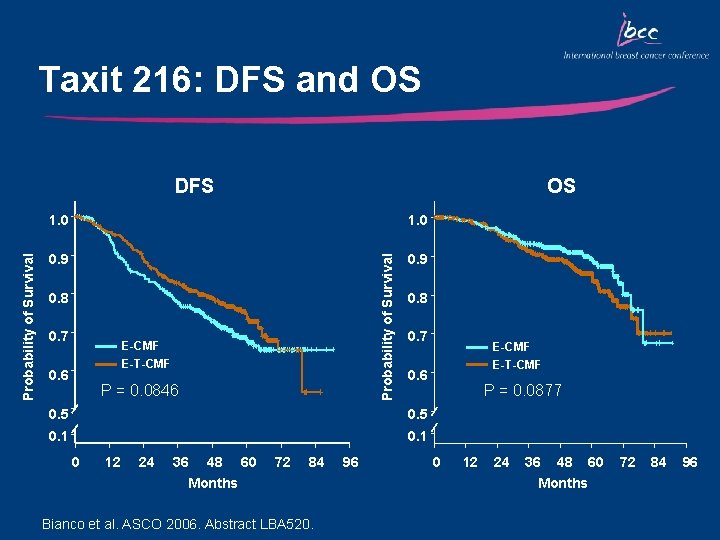
Taxit 216: DFS and OS OS 1. 0 0. 9 Probability of Survival DFS 0. 8 0. 7 E-CMF E-T-CMF 0. 6 P = 0. 0846 0. 8 0. 7 0. 6 0. 5 0. 1 0 12 24 36 48 60 Months 72 84 Bianco et al. ASCO 2006. Abstract LBA 520. 96 E-CMF E-T-CMF P = 0. 0877 0 12 24 36 48 60 Months 72 84 96

Taxit 216: Safety Hematologic Toxicity, % (Gr 3 -4) P = 0. 003 12 7 P=0. 0045 6 10 5 8 P=0. 0013 4 6 4 Nonhematologic Toxicity, % (Gr 3 -4) 3 P = 0. 4182 2 P = 1. 0 2 1 0 0 Anemia Thrombocytopenia Febrile Neutropenia Bianco et al. ASCO 2006. Abstract LBA 520. P=0. 0076 P=0. 0153 Allergy Asthenia E-CMF E-T-CMF Diarrhea Myalgia Nail disorders

Key Outcomes: TAXIT 216 Sequential E T CMF vs E CMF: • Produced a 21% reduction in the risk of relapse (borderline statistical significance) • Produced a 28% reduction in the risk of death • Induced more febrile neutropenia (11% vs 6%) • Induced more G 3 -4 toxicities including: arthralgia/ myalgia, allergy, asthenia, skin/nail disorders, and diarrhea • Cumulative incidence of non-hematologic adverse events was clinically acceptable (<4. 5% of pts)

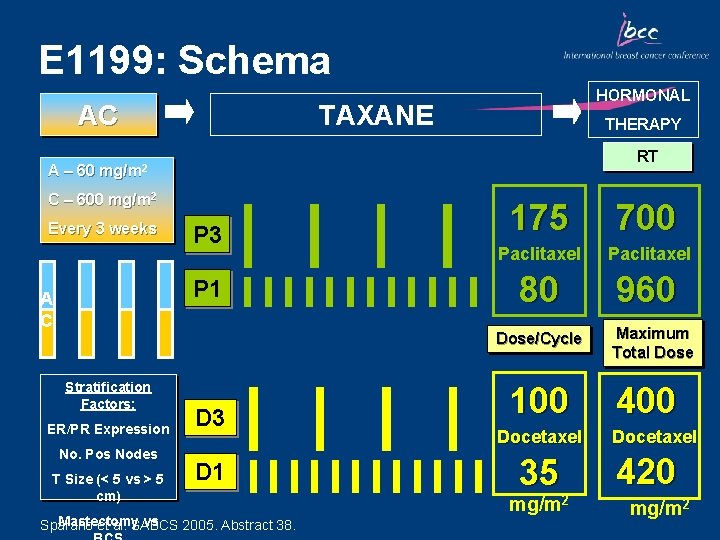
E 1199: Schema AC A – 60 HORMONAL TAXANE THERAPY RT mg/m 2 C – 600 mg/m 2 Every 3 weeks P 3 P 1 A C 175 700 Paclitaxel 80 960 Dose/Cycle Stratification Factors: ER/PR Expression No. Pos Nodes T Size (< 5 vs > 5 cm) D 3 D 1 Mastectomy vs Sparano et al. SABCS 2005. Abstract 38. 100 Docetaxel 35 mg/m 2 Maximum Total Dose 400 Docetaxel 420 mg/m 2

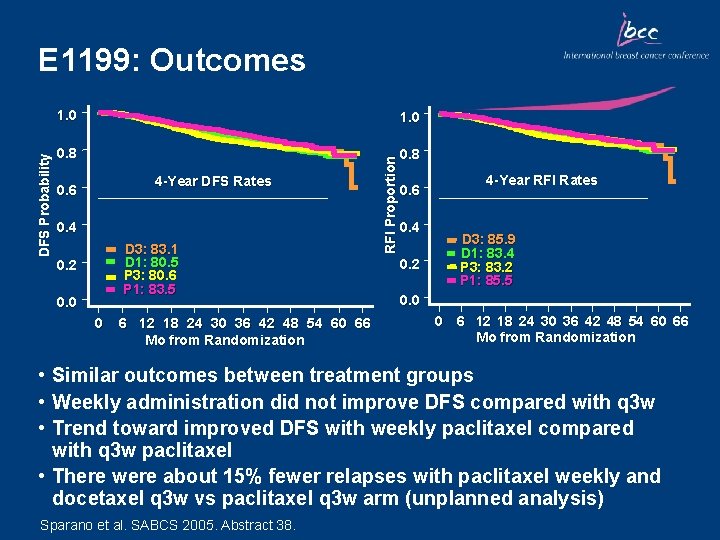
1. 0 0. 8 4 -Year DFS Rates 0. 6 0. 4 D 3: 83. 1 D 1: 80. 5 P 3: 80. 6 P 1: 83. 5 0. 2 0. 0 0 6 12 18 24 30 36 42 48 54 60 66 Mo from Randomization RFI Proportion DFS Probability E 1199: Outcomes 0. 6 0. 4 0. 2 4 -Year RFI Rates D 3: 85. 9 D 1: 83. 4 P 3: 83. 2 P 1: 85. 5 0. 0 0 6 12 18 24 30 36 42 48 54 60 66 Mo from Randomization • Similar outcomes between treatment groups • Weekly administration did not improve DFS compared with q 3 w • Trend toward improved DFS with weekly paclitaxel compared with q 3 w paclitaxel • There were about 15% fewer relapses with paclitaxel weekly and docetaxel q 3 w vs paclitaxel q 3 w arm (unplanned analysis) Sparano et al. SABCS 2005. Abstract 38.

Docetaxel in HER 2 -Positive Early -Stage Breast Cancer

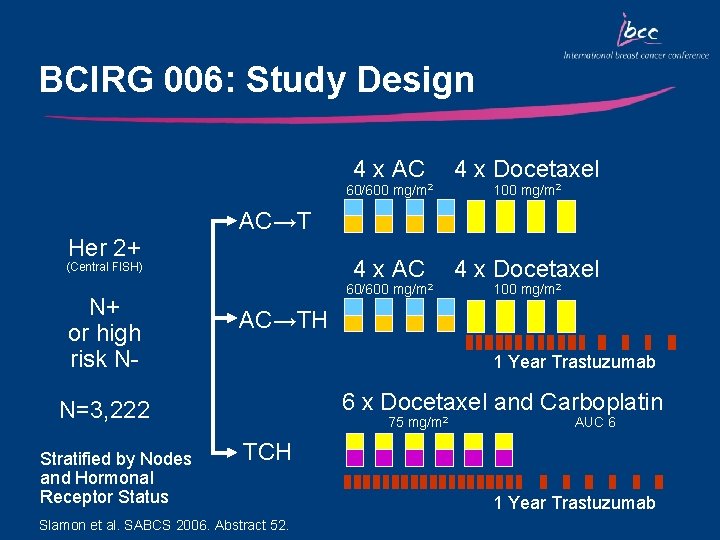
BCIRG 006: Study Design 4 x AC 4 x Docetaxel 60/600 Her 2+ 60/600 mg/m 2 100 mg/m 2 AC→TH 1 Year Trastuzumab 6 x Docetaxel and Carboplatin N=3, 222 Stratified by Nodes and Hormonal Receptor Status 100 mg/m 2 AC→T (Central FISH) N+ or high risk N- mg/m 2 75 mg/m 2 AUC 6 TCH Slamon et al. SABCS 2006. Abstract 52. 1 Year Trastuzumab

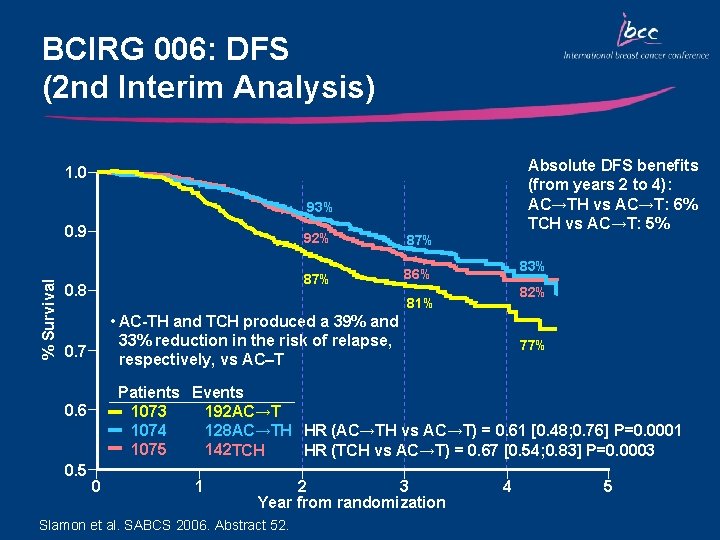
BCIRG 006: DFS (2 nd Interim Analysis) Absolute DFS benefits (from years 2 to 4): AC→TH vs AC→T: 6% TCH vs AC→T: 5% 1. 0 93% % Survival 0. 9 0. 8 87% 86% 83% 82% 81% • AC-TH and TCH produced a 39% and 33% reduction in the risk of relapse, respectively, vs AC–T 0. 7 77% Patients Events 1073 192 AC→T 1074 128 AC→TH HR (AC→TH vs AC→T) = 0. 61 [0. 48; 0. 76] P=0. 0001 1075 142 TCH HR (TCH vs AC→T) = 0. 67 [0. 54; 0. 83] P=0. 0003 0. 6 0. 5 92% 0 1 2 3 Year from randomization Slamon et al. SABCS 2006. Abstract 52. 4 5

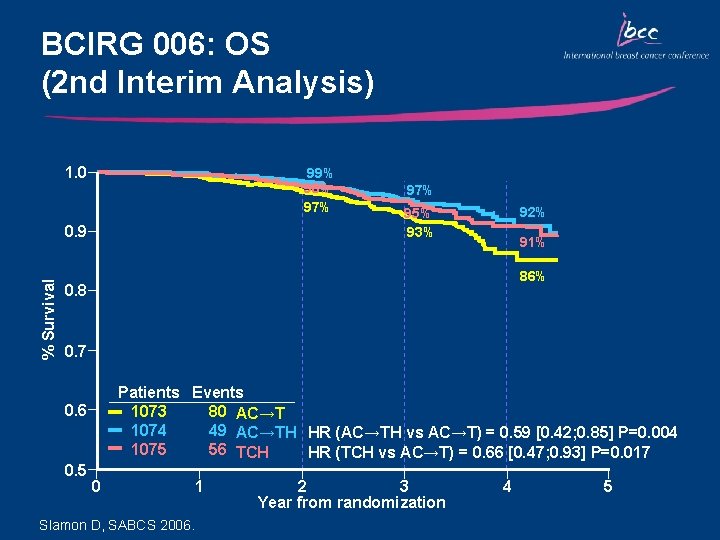
BCIRG 006: OS (2 nd Interim Analysis) 1. 0 99% 98% 97% % Survival 0. 9 97% 92% 95% 93% 91% 86% 0. 8 0. 7 Patients Events 1073 80 AC→T 1074 49 AC→TH HR (AC→TH vs AC→T) = 0. 59 [0. 42; 0. 85] P=0. 004 1075 56 TCH HR (TCH vs AC→T) = 0. 66 [0. 47; 0. 93] P=0. 017 0. 6 0. 5 0 Slamon D, SABCS 2006. 1 2 3 Year from randomization 4 5

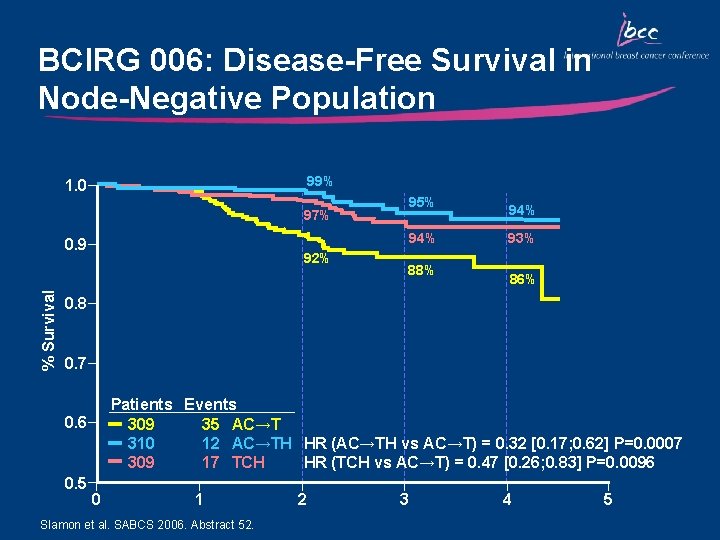
BCIRG 006: Disease-Free Survival in Node-Negative Population 99% 1. 0 95% 97% 94% % Survival 0. 9 92% 88% 94% 93% 86% 0. 8 0. 7 Patients Events 309 35 AC→T 310 12 AC→TH HR (AC→TH vs AC→T) = 0. 32 [0. 17; 0. 62] P=0. 0007 309 17 TCH HR (TCH vs AC→T) = 0. 47 [0. 26; 0. 83] P=0. 0096 0. 5 0 1 Slamon et al. SABCS 2006. Abstract 52. 2 3 4 5

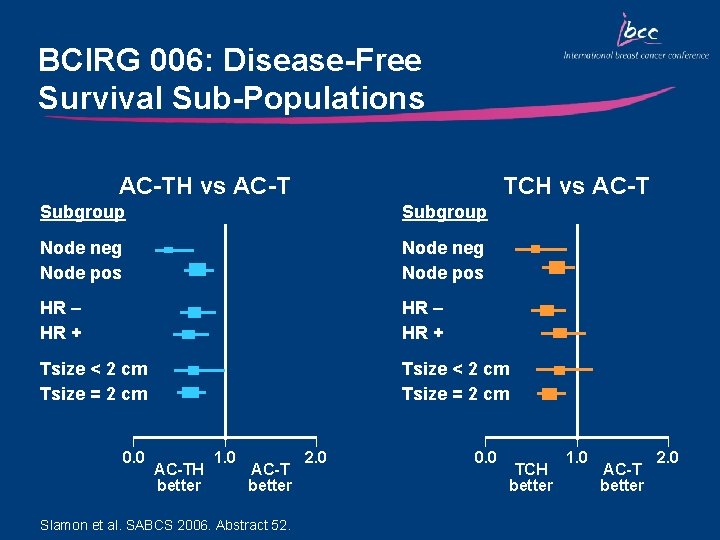
BCIRG 006: Disease-Free Survival Sub-Populations AC-TH vs AC-T TCH vs AC-T Subgroup Node neg Node pos HR – HR + Tsize < 2 cm Tsize = 2 cm 0. 0 AC-TH better 1. 0 AC-T better Slamon et al. SABCS 2006. Abstract 52. 2. 0 0. 0 TCH better 1. 0 AC-T better 2. 0

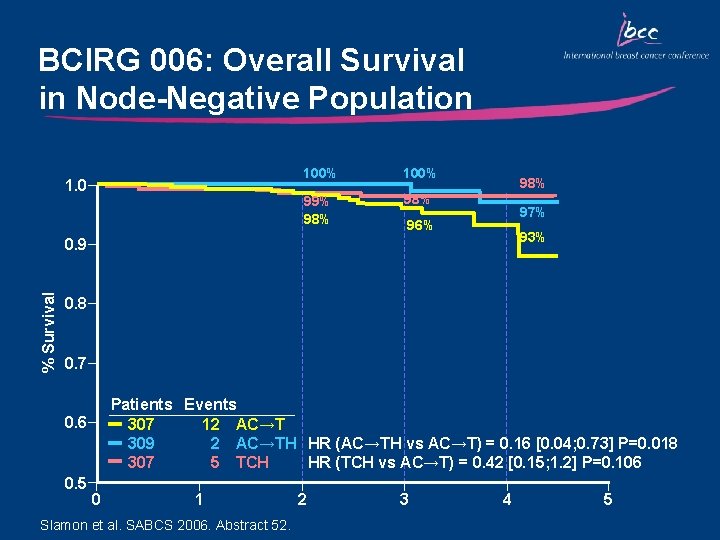
BCIRG 006: Overall Survival in Node-Negative Population 1. 0 100% 99% 98% 98% 97% 96% 93% % Survival 0. 9 0. 8 0. 7 Patients Events 307 12 AC→T 309 2 AC→TH HR (AC→TH vs AC→T) = 0. 16 [0. 04; 0. 73] P=0. 018 307 5 TCH HR (TCH vs AC→T) = 0. 42 [0. 15; 1. 2] P=0. 106 0. 5 0 1 Slamon et al. SABCS 2006. Abstract 52. 2 3 4 5

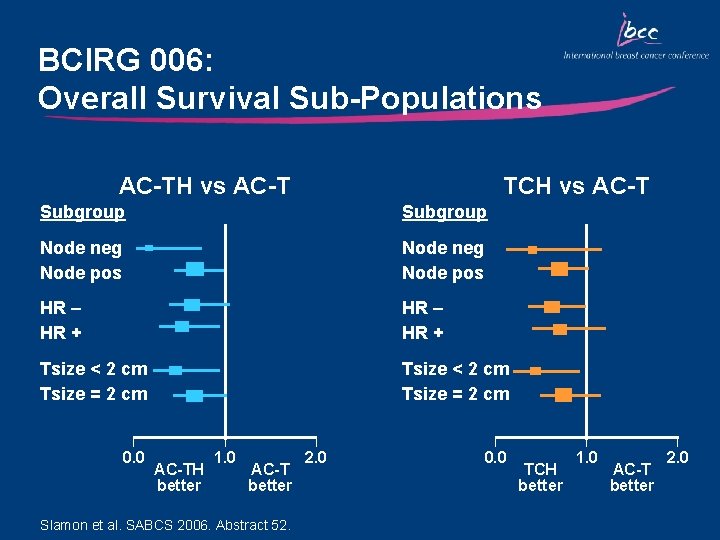
BCIRG 006: Overall Survival Sub-Populations AC-TH vs AC-T TCH vs AC-T Subgroup Node neg Node pos HR – HR + Tsize < 2 cm Tsize = 2 cm 0. 0 AC-TH better 1. 0 AC-T better Slamon et al. SABCS 2006. Abstract 52. 2. 0 0. 0 TCH better 1. 0 AC-T better 2. 0

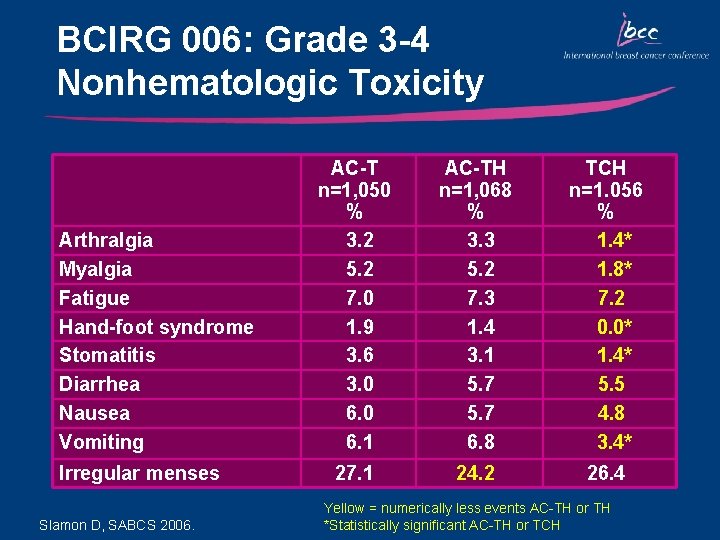
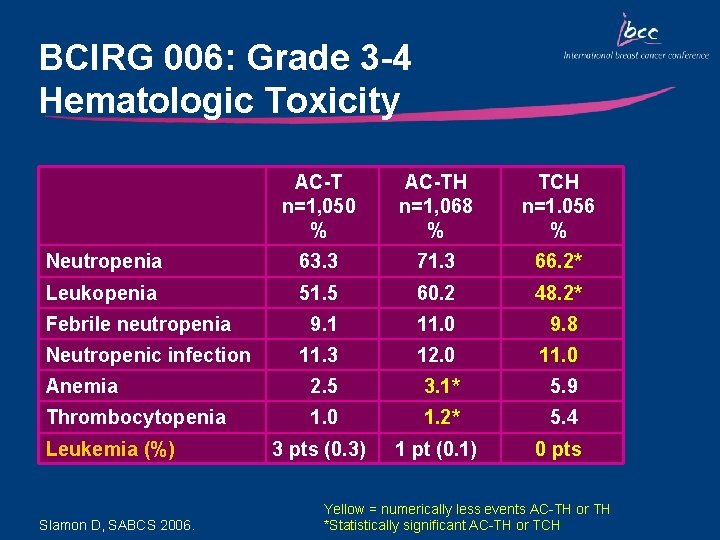
BCIRG 006: Grade 3 -4 Nonhematologic Toxicity Arthralgia Myalgia Fatigue Hand-foot syndrome Stomatitis Diarrhea Nausea Vomiting Irregular menses Slamon D, SABCS 2006. AC-T n=1, 050 % 3. 2 5. 2 7. 0 1. 9 3. 6 3. 0 6. 1 AC-TH n=1, 068 % 3. 3 5. 2 7. 3 1. 4 3. 1 5. 7 6. 8 TCH n=1. 056 % 1. 4* 1. 8* 7. 2 0. 0* 1. 4* 5. 5 4. 8 3. 4* 27. 1 24. 2 26. 4 Yellow = numerically less events AC-TH or TH *Statistically significant AC-TH or TCH

BCIRG 006: Grade 3 -4 Hematologic Toxicity AC-T n=1, 050 % AC-TH n=1, 068 % TCH n=1. 056 % Neutropenia 63. 3 71. 3 66. 2* Leukopenia 51. 5 60. 2 48. 2* 9. 1 11. 0 9. 8 11. 3 12. 0 11. 0 Anemia 2. 5 3. 1* 5. 9 Thrombocytopenia 1. 0 1. 2* 5. 4 3 pts (0. 3) 1 pt (0. 1) 0 pts Febrile neutropenia Neutropenic infection Leukemia (%) Slamon D, SABCS 2006. Yellow = numerically less events AC-TH or TH *Statistically significant AC-TH or TCH

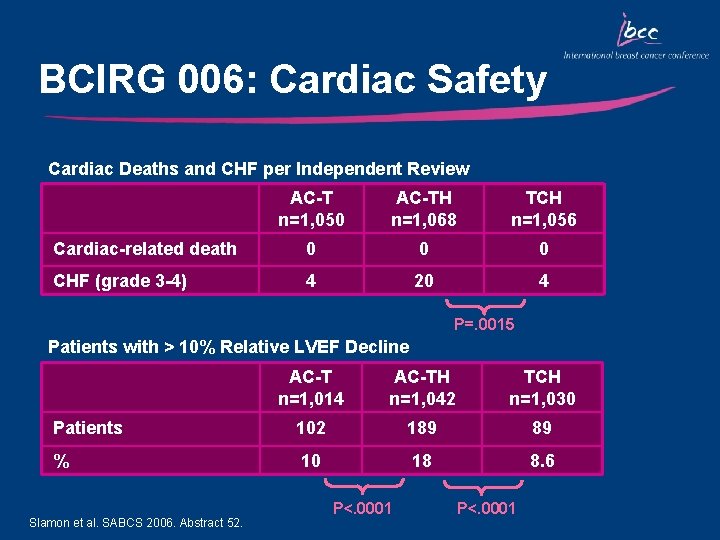
BCIRG 006: Cardiac Safety Cardiac Deaths and CHF per Independent Review AC-T n=1, 050 AC-TH n=1, 068 TCH n=1, 056 Cardiac-related death 0 0 0 CHF (grade 3 -4) 4 20 4 P=. 0015 Patients with > 10% Relative LVEF Decline AC-T n=1, 014 AC-TH n=1, 042 TCH n=1, 030 Patients 102 189 89 % 10 18 8. 6 Slamon et al. SABCS 2006. Abstract 52. P<. 0001

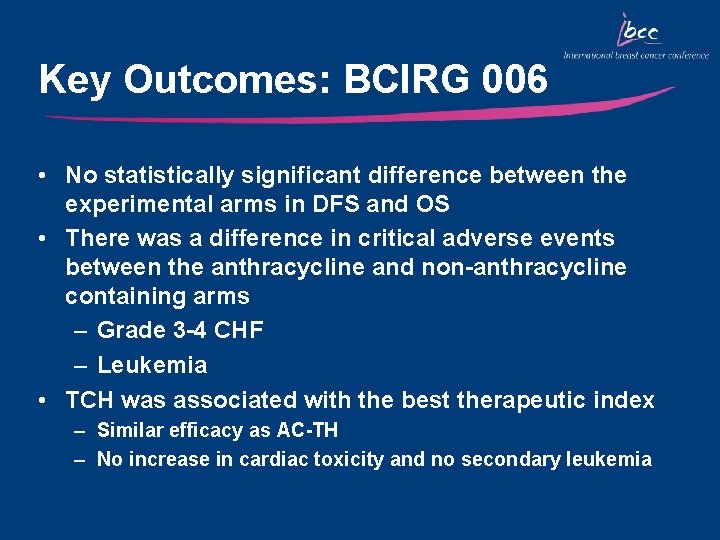
Key Outcomes: BCIRG 006 • No statistically significant difference between the experimental arms in DFS and OS • There was a difference in critical adverse events between the anthracycline and non-anthracycline containing arms – Grade 3 -4 CHF – Leukemia • TCH was associated with the best therapeutic index – Similar efficacy as AC-TH – No increase in cardiac toxicity and no secondary leukemia

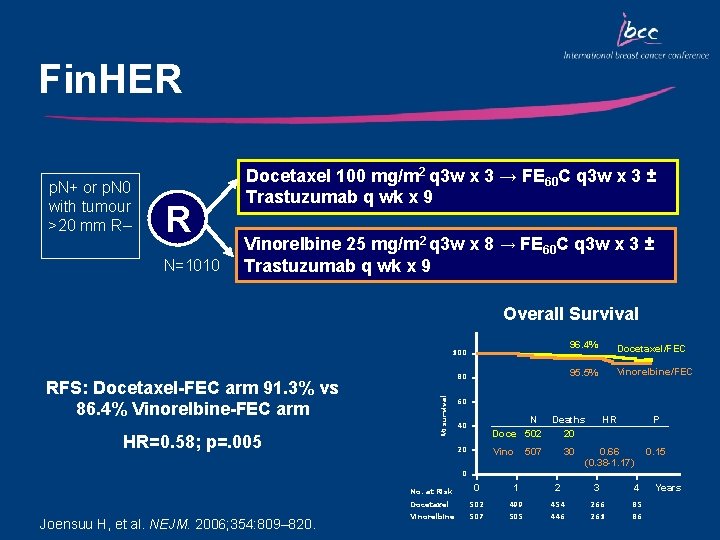
Fin. HER p. N+ or p. N 0 with tumour >20 mm R– R N=1010 Docetaxel 100 mg/m 2 q 3 w x 3 → FE 60 C q 3 w x 3 ± Trastuzumab q wk x 9 Vinorelbine 25 mg/m 2 q 3 w x 8 → FE 60 C q 3 w x 3 ± Trastuzumab q wk x 9 Overall Survival 100 HR=0. 58; p=. 005 % survival RFS: Docetaxel-FEC arm 91. 3% vs 86. 4% Vinorelbine-FEC arm 80 96. 4% Docetaxel/FEC 95. 5% Vinorelbine/FEC 60 40 N Doce 502 20 Vino Deaths 20 HR P 30 0. 66 (0. 38 -1. 17) 0. 15 507 0 Joensuu H, et al. NEJM. 2006; 354: 809– 820. No. at Risk 0 1 2 3 4 Docetaxel 502 499 454 266 85 Vinorelbine 507 505 446 261 86 Years

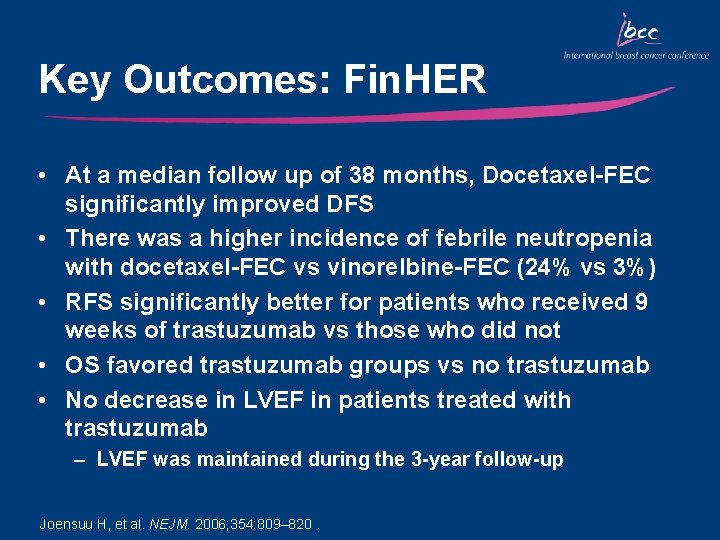
Key Outcomes: Fin. HER • At a median follow up of 38 months, Docetaxel-FEC significantly improved DFS • There was a higher incidence of febrile neutropenia with docetaxel-FEC vs vinorelbine-FEC (24% vs 3%) • RFS significantly better for patients who received 9 weeks of trastuzumab vs those who did not • OS favored trastuzumab groups vs no trastuzumab • No decrease in LVEF in patients treated with trastuzumab – LVEF was maintained during the 3 -year follow-up Joensuu H, et al. NEJM. 2006; 354: 809– 820.

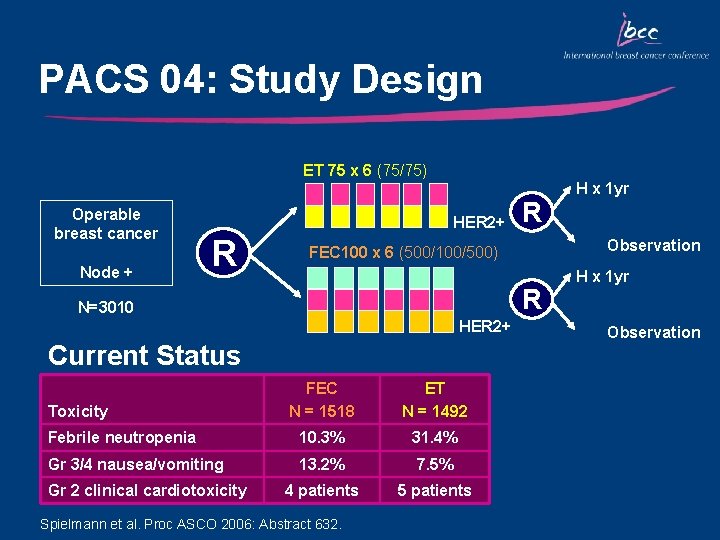
PACS 04: Study Design ET 75 x 6 (75/75) Operable breast cancer Node + HER 2+ R R HER 2+ Current Status FEC N = 1518 ET N = 1492 Febrile neutropenia 10. 3% 31. 4% Gr 3/4 nausea/vomiting 13. 2% 7. 5% 4 patients 5 patients Gr 2 clinical cardiotoxicity Observation FEC 100 x 6 (500/100/500) N=3010 Toxicity R Spielmann et al. Proc ASCO 2006: Abstract 632. H x 1 yr Observation

Ongoing Trials Evaluating Docetaxel Regimens in Early-Stage Breast Cancer

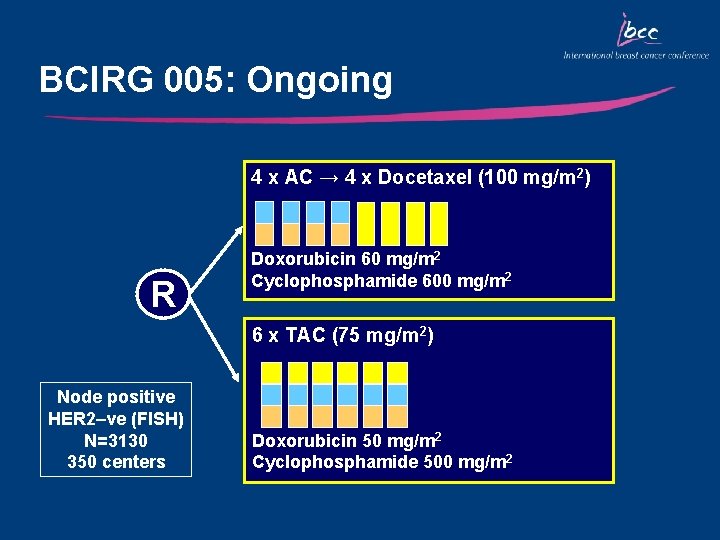
BCIRG 005: Ongoing 4 x AC → 4 x Docetaxel (100 mg/m 2) R Doxorubicin 60 mg/m 2 Cyclophosphamide 600 mg/m 2 6 x TAC (75 mg/m 2) Node positive HER 2–ve (FISH) N=3130 350 centers Doxorubicin 50 mg/m 2 Cyclophosphamide 500 mg/m 2

NSABP B-30: Ongoing • Number of positive nodes • Tamoxifen (Y/N) • Surgery and radiotherapy R 4 x AC → 4 x T q 3 w Doxorubicin Cyclophosphamide Docetaxel (60 mg/m 2) (600 mg/m 2) (100 mg/m 2) 4 x AT q 3 w Doxorubicin Docetaxel (50 mg/m 2) (75 mg/m 2) 4 x TAC q 3 w Docetaxel Doxorubicin Cyclophosphamide (75 mg/m 2) (500 mg/m 2) N=5350 pts Current Status • • The trial has closed to accrual (5351 patients enrolled) Quality of life substudy closed to accrual as of 7/20/01 First data are likely to be presented in 2007 Preliminary results of amenorrhea in the AC→T arm presented at ASCO 2005

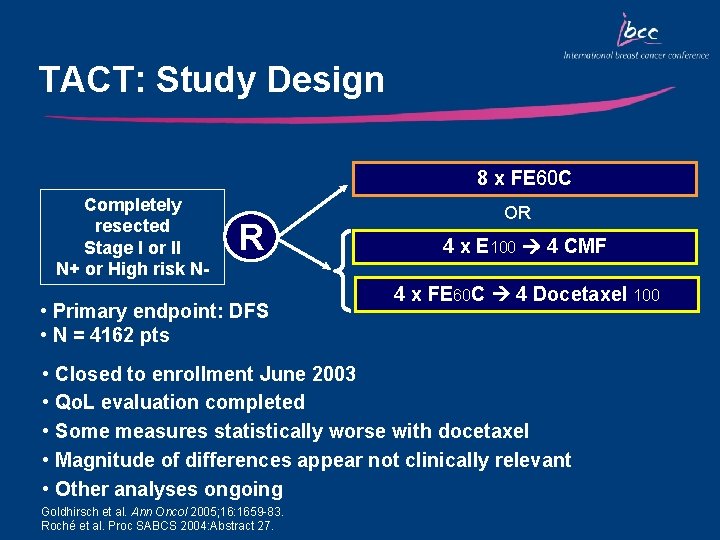
TACT: Study Design 8 x FE 60 C Completely resected Stage I or II N+ or High risk N- R • Primary endpoint: DFS • N = 4162 pts • • • OR 4 x E 100 4 CMF 4 x FE 60 C 4 Docetaxel 100 Closed to enrollment June 2003 Qo. L evaluation completed Some measures statistically worse with docetaxel Magnitude of differences appear not clinically relevant Other analyses ongoing Goldhirsch et al. Ann Oncol 2005; 16: 1659 -83. Roché et al. Proc SABCS 2004: Abstract 27.

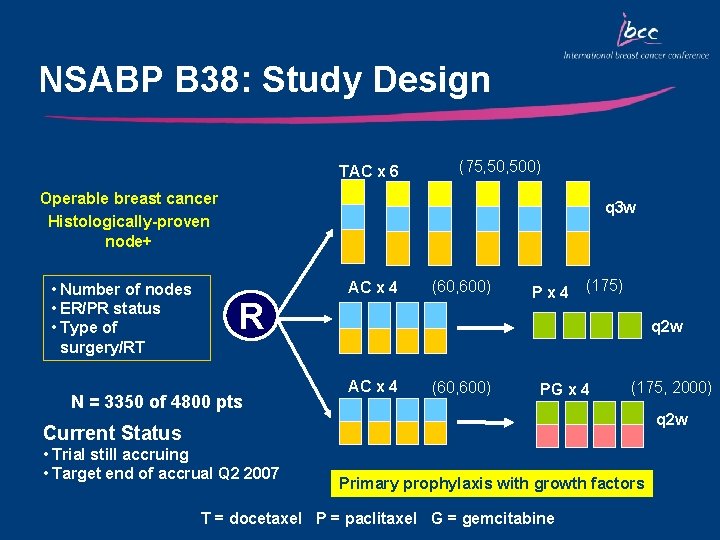
NSABP B 38: Study Design TAC x 6 (75, 500) Operable breast cancer Histologically-proven node+ • Number of nodes • ER/PR status • Type of surgery/RT q 3 w R N = 3350 of 4800 pts AC x 4 (60, 600) Px 4 (175) q 2 w AC x 4 (60, 600) PG x 4 (175, 2000) q 2 w Current Status • Trial still accruing • Target end of accrual Q 2 2007 Primary prophylaxis with growth factors T = docetaxel P = paclitaxel G = gemcitabine

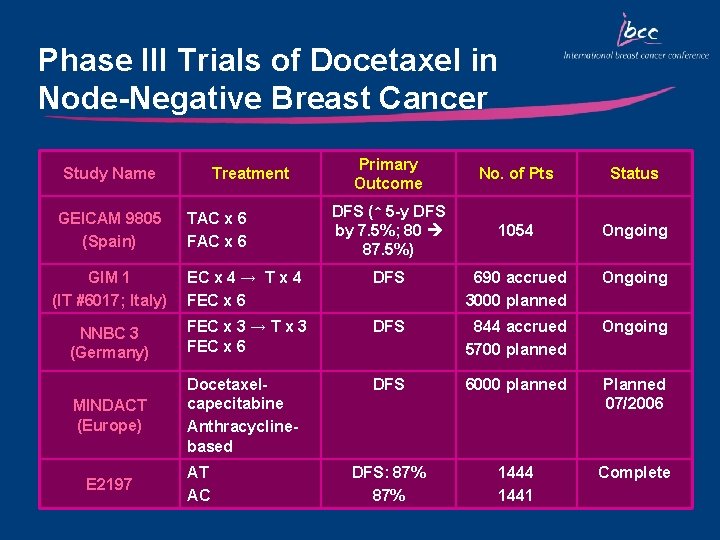
Phase III Trials of Docetaxel in Node-Negative Breast Cancer Study Name GEICAM 9805 (Spain) Treatment TAC x 6 FAC x 6 Primary Outcome No. of Pts Status DFS (↑ 5 -y DFS by 7. 5%; 80 87. 5%) 1054 Ongoing GIM 1 (IT #6017; Italy) EC x 4 → T x 4 FEC x 6 DFS 690 accrued 3000 planned Ongoing NNBC 3 (Germany) FEC x 3 → T x 3 FEC x 6 DFS 844 accrued 5700 planned Ongoing DFS 6000 planned MINDACT (Europe) Docetaxelcapecitabine Anthracyclinebased Planned 07/2006 DFS: 87% 1444 1441 Complete E 2197 AT AC

Management of Docetaxel. Induced Toxicities

Managing Docetaxel-Induced Toxicities: Guidelines for Colony-Stimulating Factors ASCO • “…Reduction in febrile neutropenia (FN) is an important clinical outcome that justifies use of CSFs, regardless of impact on other factors, when the risk of FN is ~ 20%” • Examples for appropriate use of CSF primary prophylaxis: – “…adjuvant treatment of early-stage breast cancer with more intensive regimens such as TAC or FEC 100 EORTC • “…When using a chemotherapy regimen associated with FN in > 20% of patients, prophylactic G-CSF is recommended. ” • “…If reductions in chemotherapy dose intensity or density are known to be associated with a poor prognosis, primary G-CSF prophylaxis may be used to maintain chemotherapy. ” Smith TJ et al. J Clin Oncol. 2006; 24: 3187– 3205. Aapro MS et al. Eur J Cancer. (E-pub, 5 Jun 2006; doi: 10. 1016/j. ejca. 2006)

NCCN Algorithm for G-CSF Primary Prophylaxis Risk of febrile neutropenia or of other neutropenic events compromising treatment High risk (>20%) Intermediate risk (10– 20%) Low risk (<10%) Lyman et al. J NCCN. 2005; 3: 557 -71. Curative adjuvant Prolong survival/ quality of life Symptom management/ quality of life CSF CSF Consider CSF No CSF

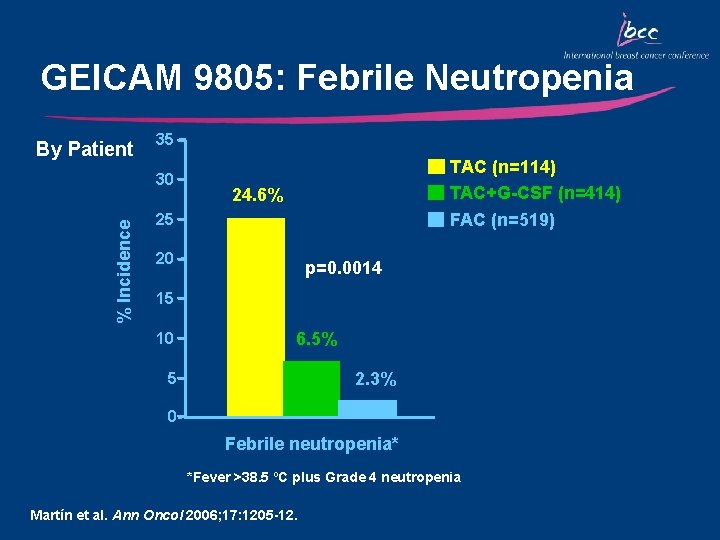
GEICAM 9805: Febrile Neutropenia By Patient 35 % Incidence 30 TAC (n=114) TAC+G-CSF (n=414) 24. 6% FAC (n=519) 25 20 p=0. 0014 15 10 6. 5% 2. 3% 5 0 Febrile neutropenia* *Fever >38. 5 ºC plus Grade 4 neutropenia Martín et al. Ann Oncol 2006; 17: 1205 -12.

Docetaxel in Early-Stage Breast Cancer • Docetaxel is one of the most, if not the most, active chemotherapy agents for breast cancer patients • Five large randomized studies established the benefit of docetaxel, given in combination or in sequence, in EBC (BCIRG 001, PACS 01, BIG 2 -98, USO 9735, Fin. HER) • Docetaxel–trastuzumab combination benefit demonstrated in adjuvant EBC (BCIRG 006) • Docetaxel toxicities in early stage disease can be managed by dose reduction, primary prophylaxis with G-CSF, or sequential versus concomitant administration – FN with TAC in BCIRG 001 = 24. 7% – FN in PACS 01 with FEC → docetaxel = 11. 2% Main Menu
 Future continuous and future perfect
Future continuous and future perfect Future perfect simple continuous
Future perfect simple continuous Current technologies of media and information
Current technologies of media and information A balanced delta connected load having an impedance 20-j15
A balanced delta connected load having an impedance 20-j15 Line vs phase voltage
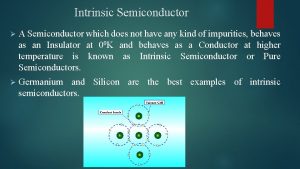
Line vs phase voltage Drift current and diffusion current in semiconductor
Drift current and diffusion current in semiconductor Ac systems lesson 4
Ac systems lesson 4 Drift current and diffusion current in semiconductor
Drift current and diffusion current in semiconductor Ceramic composition resistors
Ceramic composition resistors Line currents
Line currents Holding current and latching current
Holding current and latching current Diffusion current formula
Diffusion current formula Future directions of erp
Future directions of erp Current and future issues in corrections
Current and future issues in corrections Drain current in the constant-current region increases when
Drain current in the constant-current region increases when A splice in a welding cable should never be any closer than
A splice in a welding cable should never be any closer than Touch current vs leakage current
Touch current vs leakage current Non planar circuit
Non planar circuit Future continuous and future perfect
Future continuous and future perfect Tenses summary
Tenses summary Future plans and finished future actions
Future plans and finished future actions Future perfect x
Future perfect x Nulti i prvi kondicional
Nulti i prvi kondicional Current state vs future state diagram
Current state vs future state diagram Perfect future tense
Perfect future tense Have en futuro simple
Have en futuro simple Future nurse future midwife e learning
Future nurse future midwife e learning Past present continuous
Past present continuous How to use present continuous for future
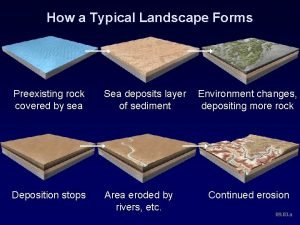
How to use present continuous for future Landscape development and environmental changes
Landscape development and environmental changes Positive and negative space landscape
Positive and negative space landscape Ore mines in india
Ore mines in india Landscape and the fall of icarus
Landscape and the fall of icarus Devegetation and defacing of landscape
Devegetation and defacing of landscape Elements and principles of landscape design
Elements and principles of landscape design Observe the rocks and landscape in this photograph
Observe the rocks and landscape in this photograph 3 planes of the body
3 planes of the body Medicine wheel examples
Medicine wheel examples Cardinal intermediate directions
Cardinal intermediate directions Prepositions of direction
Prepositions of direction Body planes definition
Body planes definition Anatomical regions of the body
Anatomical regions of the body Thoracic cavity and abdominal cavity
Thoracic cavity and abdominal cavity 7.2 body planes directions and cavities
7.2 body planes directions and cavities Listen to the dialogue and fill in the gaps
Listen to the dialogue and fill in the gaps Chapter 4 the muscular system labeling exercises
Chapter 4 the muscular system labeling exercises Body planes and directions
Body planes and directions Left lower quadrant organs female
Left lower quadrant organs female Terms of position and direction
Terms of position and direction 180 degrees longitude
180 degrees longitude Asking the way and giving directions
Asking the way and giving directions Vendor ppm
Vendor ppm Aerial perspective landscape painting
Aerial perspective landscape painting Scientific poster template 70x100
Scientific poster template 70x100 Semiotic landscape examples
Semiotic landscape examples Objectives of landscaping
Objectives of landscaping Robert arneson typewriter
Robert arneson typewriter Pop art landscape
Pop art landscape Panorama android
Panorama android Societal marketing concept
Societal marketing concept Louis comfort tiffany autumn landscape
Louis comfort tiffany autumn landscape Cultural landscape convergence
Cultural landscape convergence No bird soars too high essay
No bird soars too high essay Policy analysis problem statement example
Policy analysis problem statement example Impresionism
Impresionism Depositional landforms
Depositional landforms What is the meaning of cultural landscape
What is the meaning of cultural landscape Ap human geography the cultural landscape
Ap human geography the cultural landscape Sheeler american landscape
Sheeler american landscape Nomadic warrior hypothesis
Nomadic warrior hypothesis Artifact example ap human geography
Artifact example ap human geography Landscape with two poplars
Landscape with two poplars Limestone
Limestone Content marketing platforms forrester
Content marketing platforms forrester Arizona landscape contractors association
Arizona landscape contractors association Omellum
Omellum Martin biewenga
Martin biewenga Space analysis template
Space analysis template Uniform landscape definition
Uniform landscape definition Landscape face
Landscape face Digital twin landscape
Digital twin landscape Pharma 101
Pharma 101 Navigating the digital landscape
Navigating the digital landscape 11 surefire landscape photography tips
11 surefire landscape photography tips Salvador dali landscape near figueras
Salvador dali landscape near figueras Landscape mode photography
Landscape mode photography Landscape hotels world congress
Landscape hotels world congress Landscape art definition
Landscape art definition Rainier landscape design
Rainier landscape design Hanging valley glacier
Hanging valley glacier Charles sheeler, classic landscape, 1931.
Charles sheeler, classic landscape, 1931. Winter landscape with church friedrich
Winter landscape with church friedrich Charles sheeler american landscape
Charles sheeler american landscape Painting mastery test
Painting mastery test Landscape with the flight into egypt (carracci)
Landscape with the flight into egypt (carracci) Fallen angel alexandre cabanel wikipedia
Fallen angel alexandre cabanel wikipedia What makes landscapes distinctive
What makes landscapes distinctive Bear creek landscape
Bear creek landscape Map
Map Machine learning landscape
Machine learning landscape Site inventory architecture
Site inventory architecture Denominational switching
Denominational switching What is narrative stylistics
What is narrative stylistics Romantic era landscape painting
Romantic era landscape painting Landscape elements
Landscape elements 6-1 a changing landscape
6-1 a changing landscape Mountains and sea
Mountains and sea Landscape
Landscape Landscape drafting tools
Landscape drafting tools Objective of landscaping
Objective of landscaping