Disease Continuum in Prostate Cancer Death Docetaxel Castration

































- Slides: 54











Disease Continuum in Prostate Cancer Death Docetaxel* Castration Tumor volume Local Therapy PSA Local Disease Symptoms Asymptomatic Non-Metastatic Castration Sensitive Castration Resistant Death from Prostate Cancer Time 11






צ'רלס היגינס 1966 חתן פרס נובל • The first series of patients with prostatic cancer treated by orchiectomy 16 comprised 21 patients with far advanced metastases; only 4 of them survived for more than 12 years. Despite regressions of great magnitude, it is obvious that there were many failures of endocrine therapy to control the disease but on the whole, the life span had been extended by the novel treatments and there had been a decrease of man-pain hours. • Stilbestrol, which had been discovered in 1938 by E. C. Dodds et al. 15, was the first synthetic substance to control cancer; hence the study of the prostate cancers was the start of chemotherapy of malignant disease.



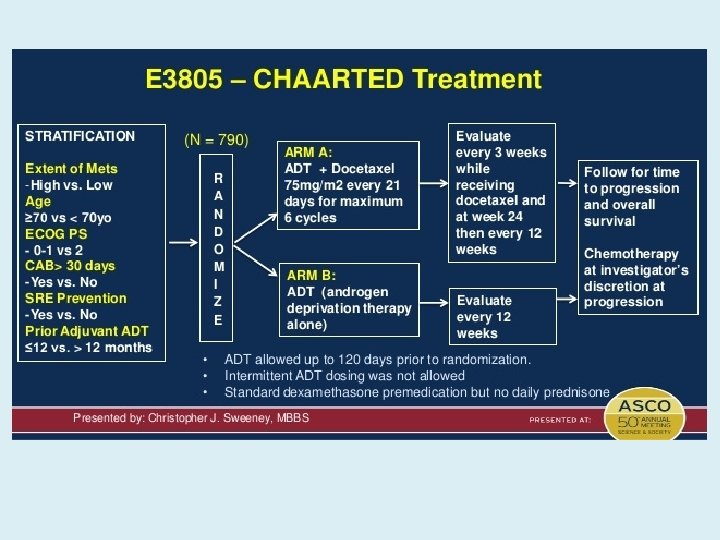
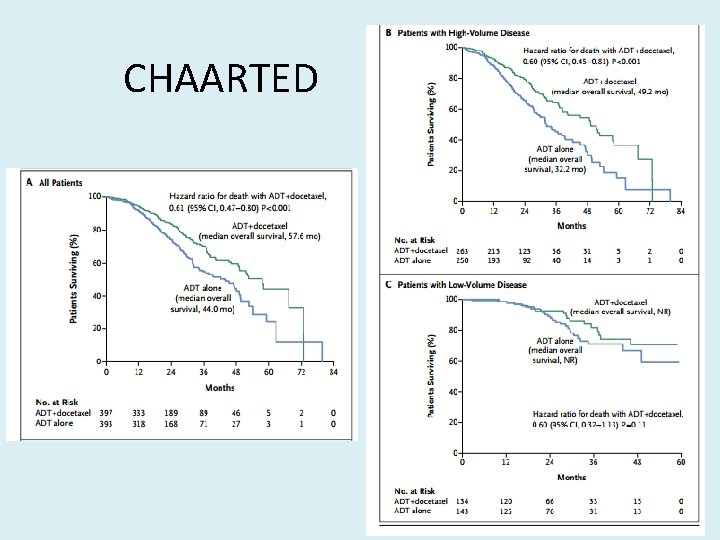
CHAARTED





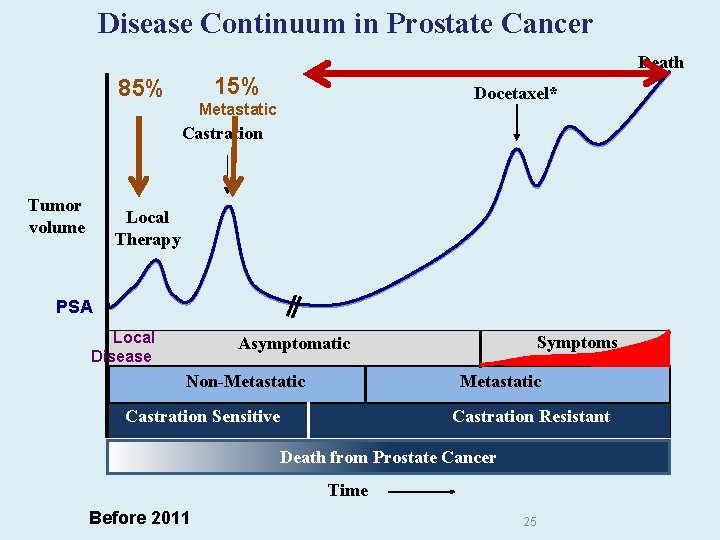
Disease Continuum in Prostate Cancer Death 15% 85% Docetaxel* Metastatic Castration Tumor volume Local Therapy PSA Local Disease Symptoms Asymptomatic Non-Metastatic Castration Sensitive Castration Resistant Death from Prostate Cancer Time Before 2011 25

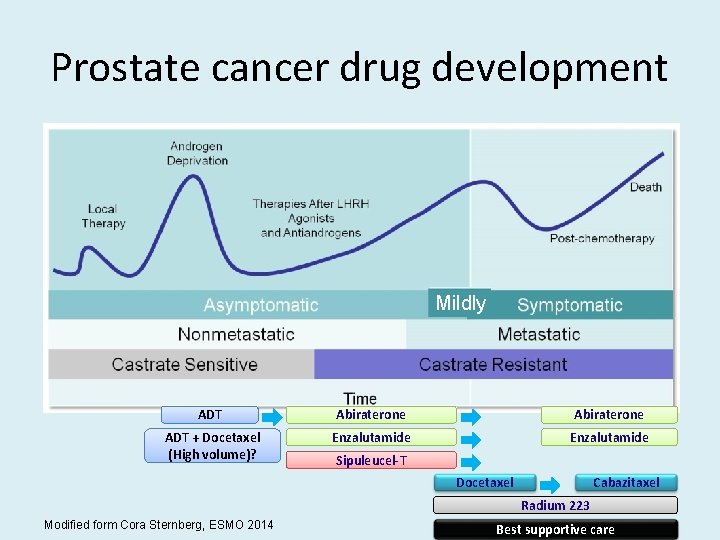
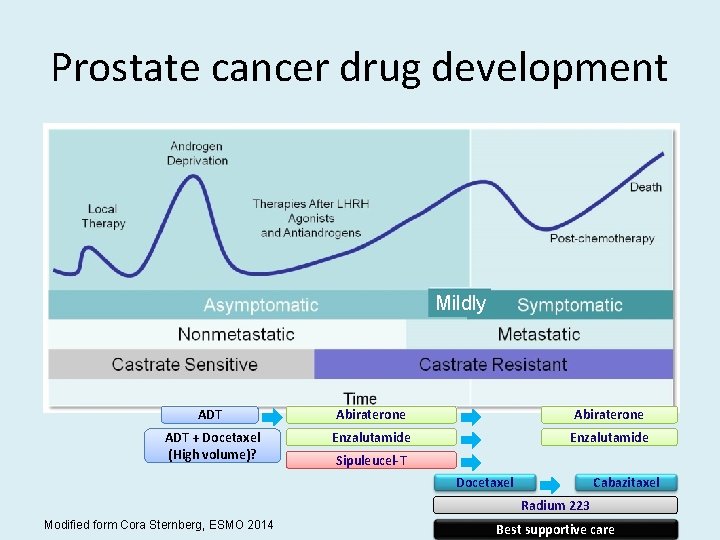
Prostate cancer drug development Mildly ADT Abiraterone ADT + Docetaxel (High volume)? Enzalutamide Sipuleucel-T Docetaxel Cabazitaxel Radium 223 Modified form Cora Sternberg, ESMO 2014 Best supportive care

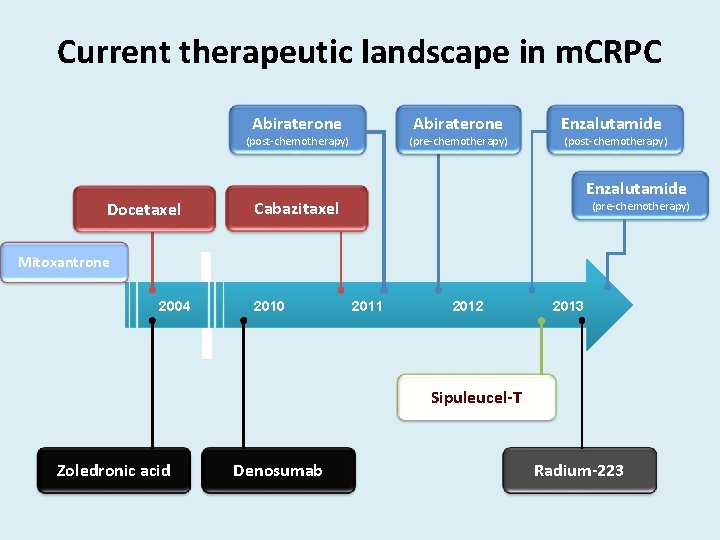
Current therapeutic landscape in m. CRPC Abiraterone (post-chemotherapy) Docetaxel (pre-chemotherapy) Enzalutamide (post-chemotherapy) Enzalutamide Cabazitaxel (pre-chemotherapy) Mitoxantrone 2004 2010 2011 2012 2013 Sipuleucel-T Zoledronic acid Denosumab Radium-223

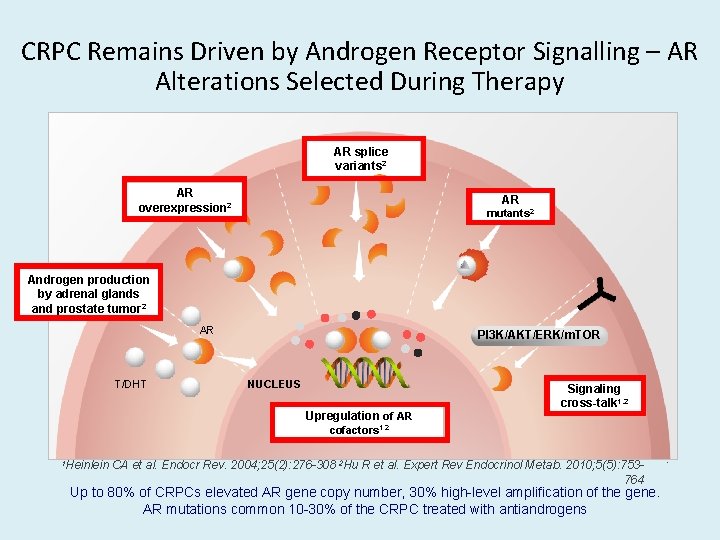
CRPC Remains Driven by Androgen Receptor Signalling – AR Alterations Selected During Therapy AR splice variants 2 AR overexpression 2 AR mutants 2 PI 3 K/AKT/ERK/m. TOR Androgen production by adrenal glands and prostate tumor 2 AR T/DHT PI 3 K/AKT/ERK/m. TOR NUCLEUS Upregulation of AR Signaling cross-talk 1, 2 cofactors 1, 2 1 Heinlein CA et al. Endocr Rev. 2004; 25(2): 276 -308 2 Hu R et al. Expert Rev Endocrinol Metab. 2010; 5(5): 753764 Up to 80% of CRPCs elevated AR gene copy number, 30% high-level amplification of the gene. AR mutations common 10 -30% of the CRPC treated with antiandrogens •

TAXOTERE




CABAZITAXEL

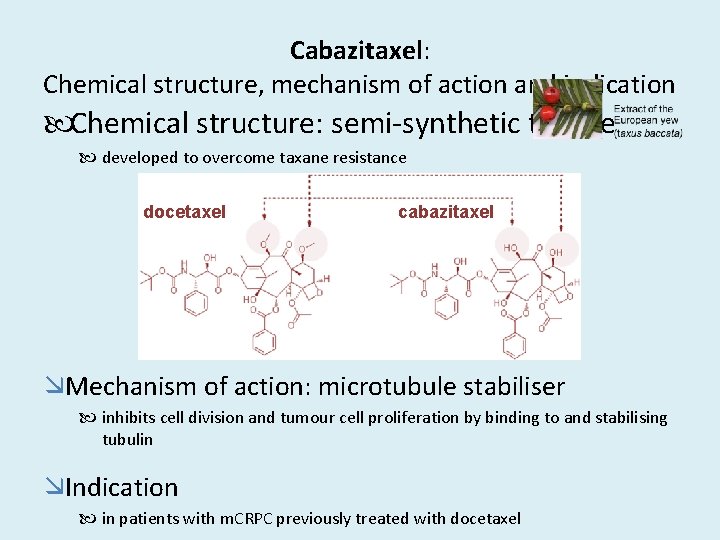
Cabazitaxel: Chemical structure, mechanism of action and indication Chemical structure: semi-synthetic taxane developed to overcome taxane resistance docetaxel cabazitaxel Mechanism of action: microtubule stabiliser inhibits cell division and tumour cell proliferation by binding to and stabilising tubulin Indication in patients with m. CRPC previously treated with docetaxel

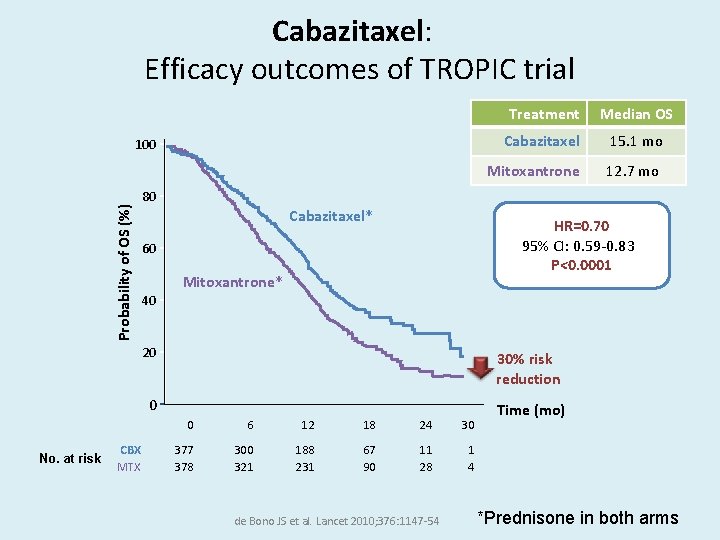
Cabazitaxel: Efficacy outcomes of TROPIC trial Probability of OS (%) 100 No. at risk CBX MTX Treatment Median OS Cabazitaxel 15. 1 mo Mitoxantrone 12. 7 mo 80 Cabazitaxel* HR=0. 70 95% CI: 0. 59 -0. 83 P<0. 0001 60 Mitoxantrone* 40 20 30% risk reduction 0 Time (mo) 0 6 12 18 24 30 377 378 300 321 188 231 67 90 11 28 1 4 de Bono JS et al. Lancet 2010; 376: 1147 -54 *Prednisone in both arms

ABIRATERONE

Abiraterone: Mechanism of action and indication Mechanism of action • inhibits testosterone production in testis, adrenal glands, and prostate – CYP 17 C 17, 20 lyase 17α-hydroxylase Pregnenolone Progesterone 17α-hydroxypregnenolone 17α-hydroxyprogesterone Abiraterone (steroidal) DHEA Androstenedione → Testosterone

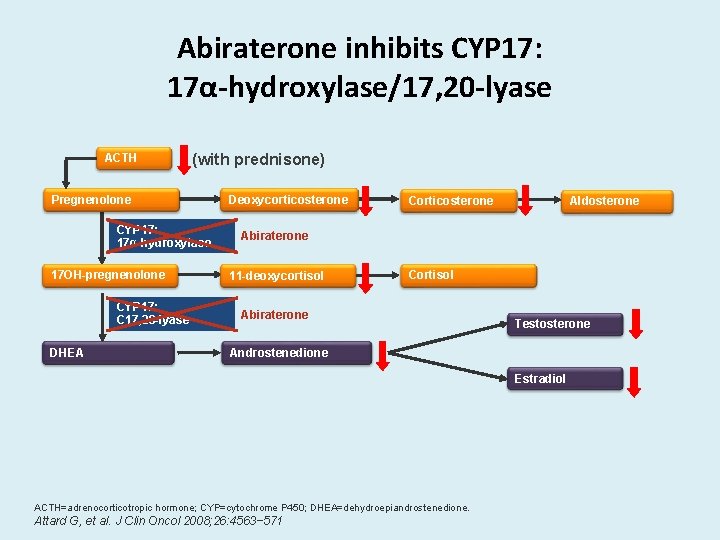
Abiraterone inhibits CYP 17: 17α-hydroxylase/17, 20 -lyase ACTH (with prednisone) Pregnenolone CYP 17: 17α-hydroxylase 17 OH-pregnenolone CYP 17: C 17, 20 -lyase DHEA Deoxycorticosterone Corticosterone Aldosterone Abiraterone 11 -deoxycortisol Cortisol Abiraterone Testosterone Androstenedione Estradiol ACTH=adrenocorticotropic hormone; CYP=cytochrome P 450; DHEA=dehydroepiandrostenedione. Attard G, et al. J Clin Oncol 2008; 26: 4563− 571

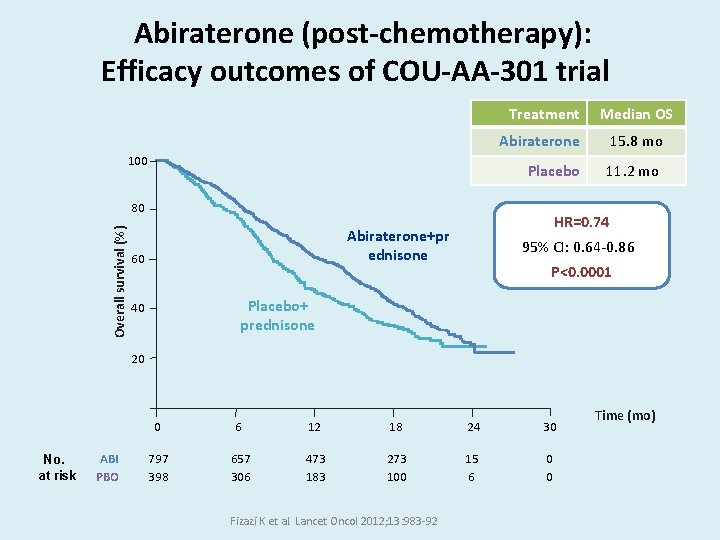
Abiraterone (post-chemotherapy): Efficacy outcomes of COU-AA-301 trial Treatment 100 Abiraterone 15. 8 mo Placebo 11. 2 mo Overall survival (%) 80 HR=0. 74 Abiraterone+pr ednisone 60 Median OS 95% CI: 0. 64 -0. 86 P<0. 0001 Placebo+ prednisone 40 20 No. at risk ABI PBO 0 6 12 18 24 30 797 398 657 306 473 183 273 100 15 6 0 0 Fizazi K et al. Lancet Oncol 2012; 13: 983 -92 Time (mo)

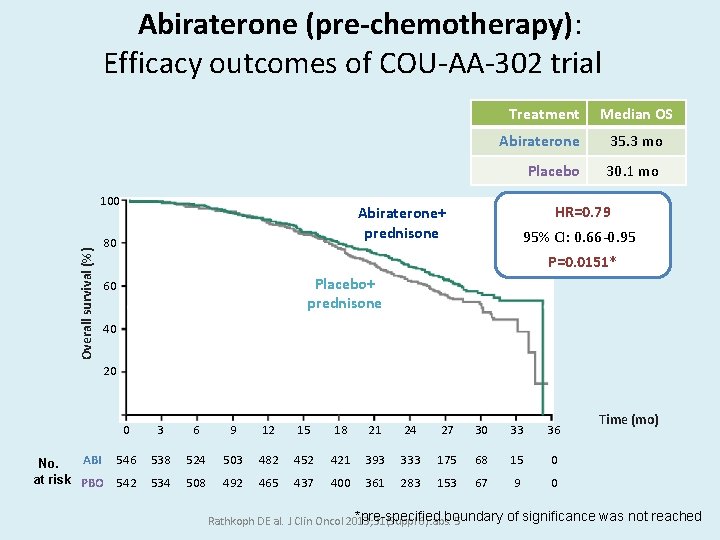
Abiraterone (pre-chemotherapy): Efficacy outcomes of COU-AA-302 trial Treatment Overall survival (%) 100 Abiraterone 35. 3 mo Placebo 30. 1 mo HR=0. 79 Abiraterone+ prednisone 80 Median OS 95% CI: 0. 66 -0. 95 P=0. 0151* Placebo+ prednisone 60 40 20 0 ABI 546 No. at risk PBO 542 3 6 9 12 15 18 21 24 27 30 33 36 538 524 503 482 452 421 393 333 175 68 15 0 534 508 492 465 437 400 361 283 153 67 9 0 Time (mo) *pre-specified boundary of significance was not reached Rathkoph DE al. J Clin Oncol 2013; 31(Suppl 6): abs. 5

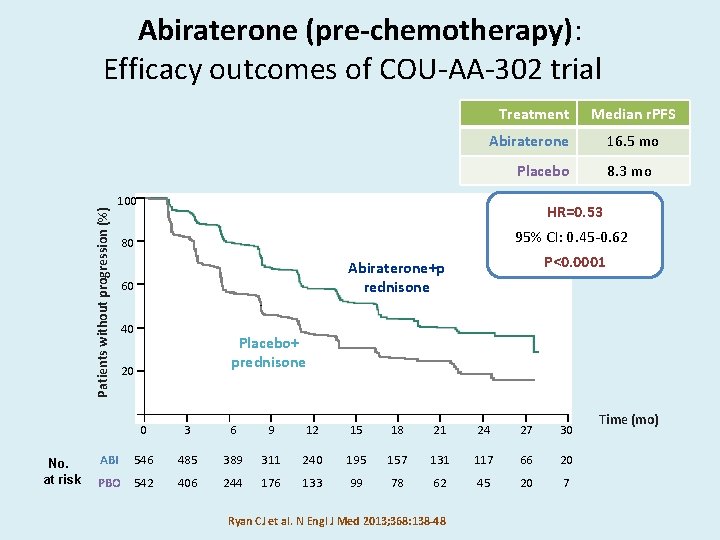
Abiraterone (pre-chemotherapy): Efficacy outcomes of COU-AA-302 trial Treatment Median r. PFS Abiraterone 16. 5 mo Patients without progression (%) Placebo No. at risk 100 HR=0. 53 95% CI: 0. 45 -0. 62 80 P<0. 0001 Abiraterone+p rednisone 60 40 Placebo+ prednisone 20 0 3 6 9 12 15 18 21 24 27 30 546 485 389 311 240 195 157 131 117 66 20 PBO 542 406 244 176 133 99 78 62 45 20 7 ABI 8. 3 mo Ryan CJ et al. N Engl J Med 2013; 368: 138 -48 Time (mo)

ENZALUTAMIDE

Enzalutamide: Mechanism of action and indication • Mechanism of action – androgen receptor (AR) signalling inhibitor Enzalutamide A Inhibits nuclear translocation of AR 2 A Enzalutamide 1 AR Inhibits binding of androgens to AR 3 AR Inhibits association of AR with DNA Cell nucleus Tumour death Scher HI et al. ASCO GU 2012. Poster LBA 1/A 2 Xtandi. Package insert and prescribing information. August 2012.

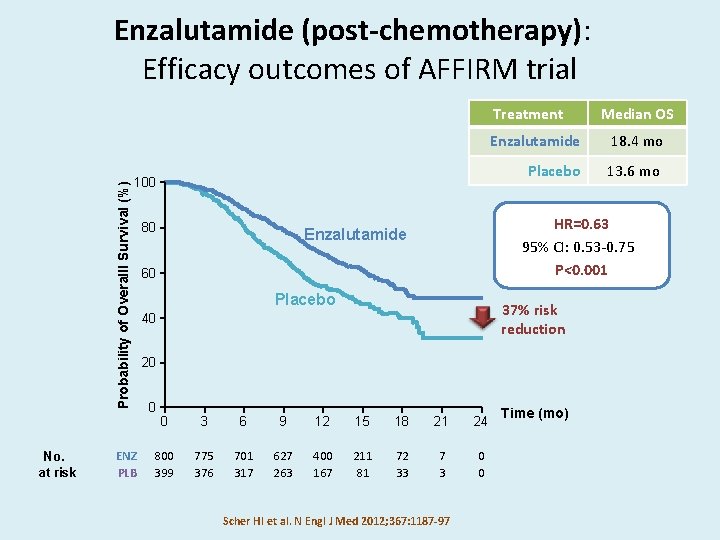
Enzalutamide (post-chemotherapy): Efficacy outcomes of AFFIRM trial Probability of Overalll Survival (%) Treatment No. at risk 100 ENZ PLB 80 Enzalutamide 18. 4 mo Placebo 13. 6 mo HR=0. 63 95% CI: 0. 53 -0. 75 P<0. 001 Enzalutamide 60 Placebo 37% risk reduction 40 20 0 Median OS 0 3 6 9 12 15 18 21 24 800 399 775 376 701 317 627 263 400 167 211 81 72 33 7 3 0 0 Scher HI et al. N Engl J Med 2012; 367: 1187 -97 Time (mo)

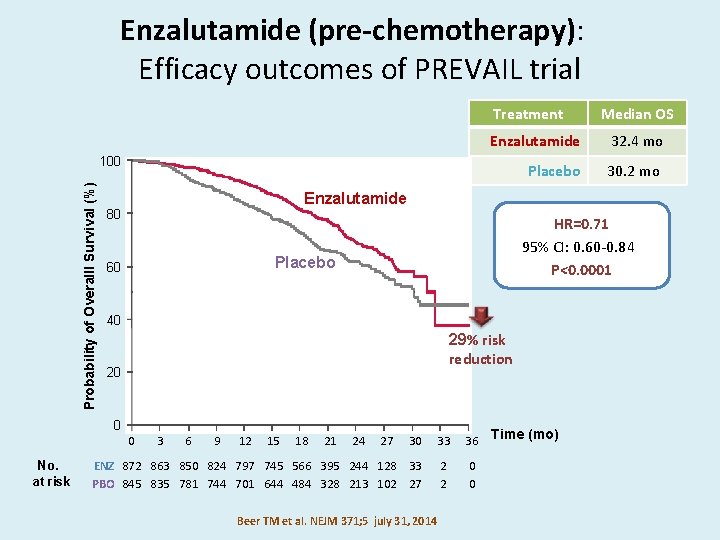
Enzalutamide (pre-chemotherapy): Efficacy outcomes of PREVAIL trial Treatment Enzalutamide 32. 4 mo Placebo 30. 2 mo Probability of Overalll Survival (%) 100 No. at risk Enzalutamide 80 HR=0. 71 95% CI: 0. 60 -0. 84 P<0. 0001 Placebo 60 40 29% risk reduction 20 0 Median OS 30 33 36 Time (mo) ENZ 872 863 850 824 797 745 566 395 244 128 33 PBO 845 835 781 744 701 644 484 328 213 102 27 2 2 0 0 0 3 6 9 12 15 18 21 24 27 Beer TM et al. NEJM 371; 5 july 31, 2014

RADIUM-223

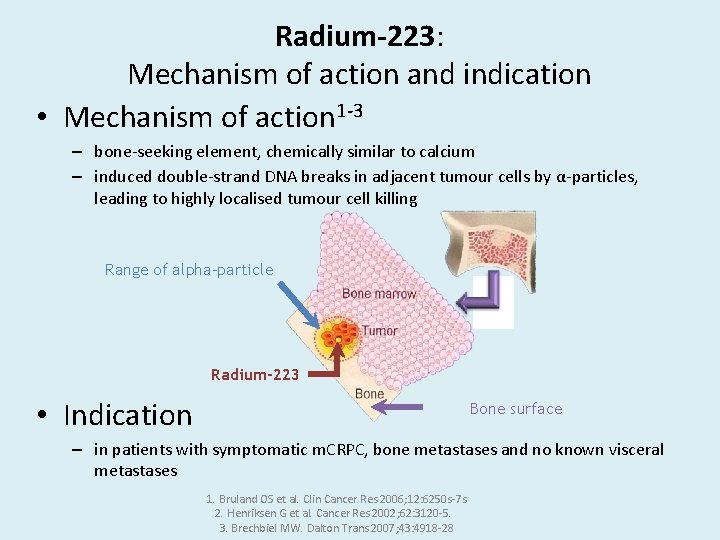
Radium-223: Mechanism of action and indication • Mechanism of action 1 -3 – bone-seeking element, chemically similar to calcium – induced double-strand DNA breaks in adjacent tumour cells by α-particles, leading to highly localised tumour cell killing Range of alpha-particle Radium-223 • Indication Bone surface – in patients with symptomatic m. CRPC, bone metastases and no known visceral metastases 1. Bruland OS et al. Clin Cancer Res 2006; 12: 6250 s-7 s 2. Henriksen G et al. Cancer Res 2002; 62: 3120 -5. 3. Brechbiel MW. Dalton Trans 2007; 43: 4918 -28

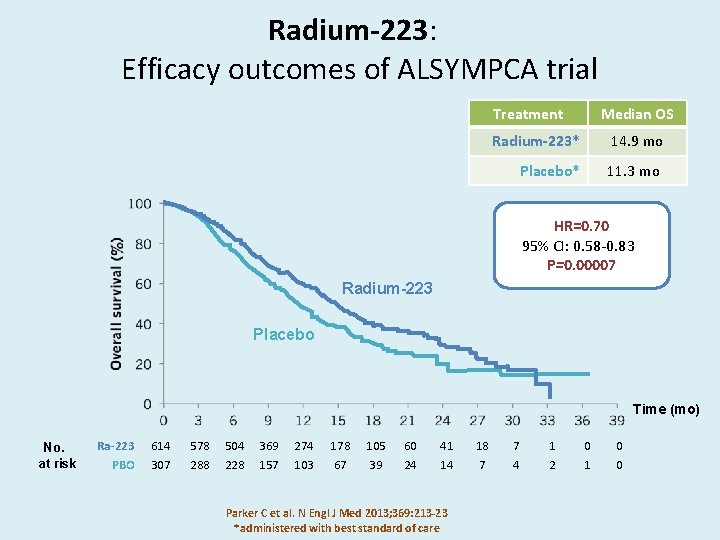
Radium-223: Efficacy outcomes of ALSYMPCA trial Treatment Median OS Radium-223* 14. 9 mo Placebo* 11. 3 mo HR=0. 70 95% CI: 0. 58 -0. 83 P=0. 00007 Radium-223 Placebo Time (mo) No. at risk Ra-223 PBO 614 307 578 288 504 228 369 157 274 103 178 67 105 39 60 24 41 14 Parker C et al. N Engl J Med 2013; 369: 213 -23 *administered with best standard of care 18 7 7 4 1 2 0 1 0 0

Prostate cancer drug development Mildly ADT Abiraterone ADT + Docetaxel (High volume)? Enzalutamide Sipuleucel-T Docetaxel Cabazitaxel Radium 223 Modified form Cora Sternberg, ESMO 2014 Best supportive care

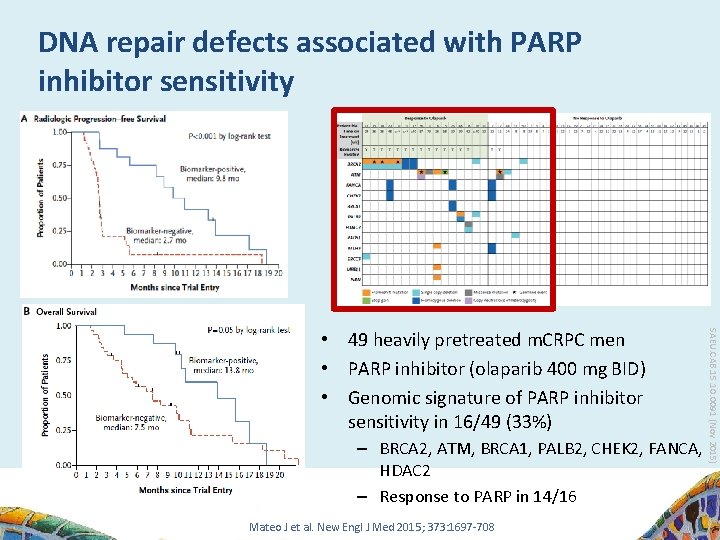
DNA repair defects associated with PARP inhibitor sensitivity – BRCA 2, ATM, BRCA 1, PALB 2, CHEK 2, FANCA, HDAC 2 – Response to PARP in 14/16 Mateo J et al. New Engl J Med 2015; 373: 1697 -708 SAEU. CAB. 15. 10. 0091 (Nov 2015) • 49 heavily pretreated m. CRPC men • PARP inhibitor (olaparib 400 mg BID) • Genomic signature of PARP inhibitor sensitivity in 16/49 (33%)




 Prostate cancer staging
Prostate cancer staging Prostate cancer tnm classification
Prostate cancer tnm classification Mdv3100 prostate cancer
Mdv3100 prostate cancer Quel taux de psa pour un cancer de la prostate ?
Quel taux de psa pour un cancer de la prostate ? Prostate cancer survival rates
Prostate cancer survival rates Laprp
Laprp Cancer burden of disease
Cancer burden of disease Cancer death poem
Cancer death poem Tools used for castration
Tools used for castration Is a veterinarian
Is a veterinarian Defence mechanism sigmund freud
Defence mechanism sigmund freud Henderson castration tool
Henderson castration tool Neuroendocrine syndrome in gynecology
Neuroendocrine syndrome in gynecology Castration
Castration Sissy castration
Sissy castration Castration anxiety
Castration anxiety Grooming objectives
Grooming objectives Communicable disease and non communicable disease
Communicable disease and non communicable disease Red death poem
Red death poem Somatic death
Somatic death Istilah sebutan pembentukan sperma adalah
Istilah sebutan pembentukan sperma adalah Prostate pathology
Prostate pathology Prostate
Prostate Dartos muscle
Dartos muscle Irm de prostate
Irm de prostate Tuip prostate
Tuip prostate Function of the ductus deferens
Function of the ductus deferens Irm prostate
Irm prostate Pi-rads v
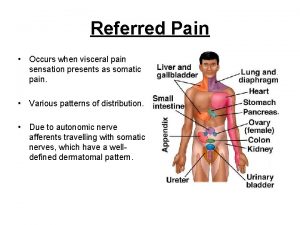
Pi-rads v Viscerosomatic reflex
Viscerosomatic reflex Function of prostate gland
Function of prostate gland Prostate pathology
Prostate pathology Silodasin
Silodasin Prostate referred pain
Prostate referred pain Celine duperron
Celine duperron Prostate gland
Prostate gland Prostatakreft symptomer
Prostatakreft symptomer Score de gleason
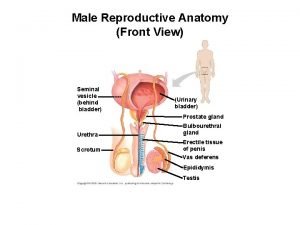
Score de gleason Primary sex organ of the male reproductive system? *
Primary sex organ of the male reproductive system? * Prostate histology
Prostate histology Normal weight of prostate
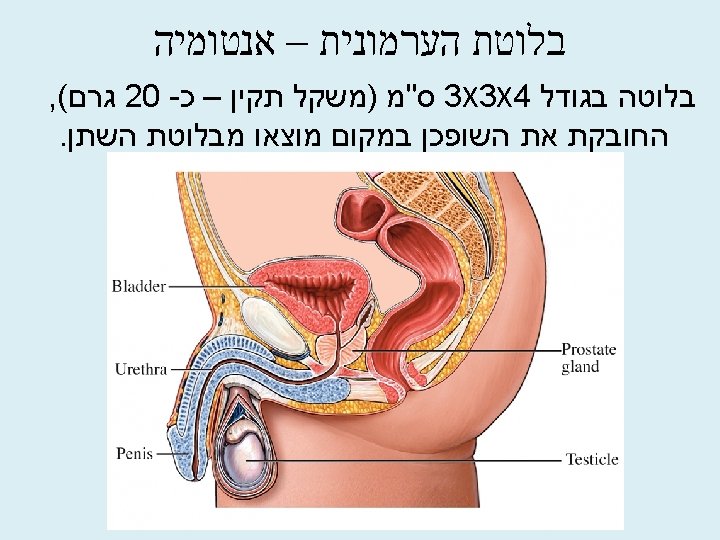
Normal weight of prostate Prostate
Prostate Lipoma
Lipoma Goods services continuum diagram
Goods services continuum diagram Use of force continuum chart
Use of force continuum chart What is risk continuum
What is risk continuum Maturity continuum
Maturity continuum Continuum care greensboro nc
Continuum care greensboro nc Phonemic awareness continuum
Phonemic awareness continuum Pa career ready skills continuum
Pa career ready skills continuum 3 reasons to use supportive stance
3 reasons to use supportive stance Compassion continuum
Compassion continuum Turkey economic continuum
Turkey economic continuum Cpi verbal escalation continuum
Cpi verbal escalation continuum Ethical interpersonal communication
Ethical interpersonal communication