CRT 2016 Protection Devices for Stroke Prevention Does









- Slides: 27

CRT 2016 Protection Devices for Stroke Prevention: Does the Data Support Routine Use? Eberhard Grube, MD, FACC, FSCAI University Hospital, Dept of Medicine II, Bonn, Germany Stanford University, Palo Alto, California, USA

Eberhard Grube, MD Physician Name Company/Relationship Eberhard Grube, MD Medtronic, Core. Valve: C, SB, AB, OF Liva. Nova: C, SB, AB Mitralign: AB, SB, E Boston Scientific: C, SB, AB Millipede: SB, C, AB Kona: AB, E Abbott Vascular: AB In. Seal Medical: AB, E, Valtech: E, SB, Claret: SB Keystone: AB Shockwave: E, AB Key G – Grant and or Research Support E – Equity Interests S – Salary, AB – Advisory Board C – Consulting fees, Honoraria R – Royalty Income I – Intellectual Property Rights SB – Speaker’s Bureau O – Ownership OF – Other Financial Benefits‘

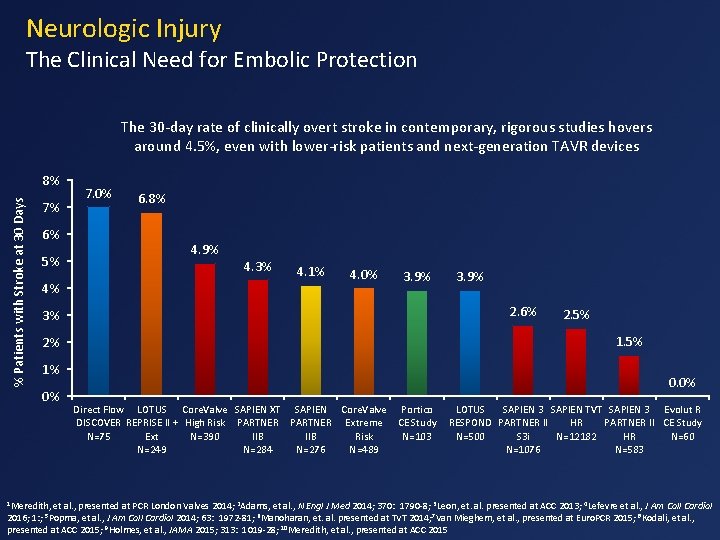
Neurologic Injury The Clinical Need for Embolic Protection The 30 -day rate of clinically overt stroke in contemporary, rigorous studies hovers around 4. 5%, even with lower-risk patients and next-generation TAVR devices % Patients with Stroke at 30 Days 8% 7. 0% 7% 6% 6. 8% 4. 9% 5% 4% 4. 3% 4. 1% 4. 0% 3. 9% 2. 6% 3% 1% 1 Meredith, 2. 5% 1. 5% 2% 0% 3. 9% 0. 0% Direct Flow LOTUS Core. Valve SAPIEN XT SAPIEN Core. Valve Portico DISCOVER REPRISE II + High Risk PARTNER Extreme CE Study N=75 Ext N=390 IIB Risk N=103 N=249 N=284 N=276 N=489 LOTUS SAPIEN 3 SAPIEN TVT SAPIEN 3 Evolut R RESPOND PARTNER II HR PARTNER II CE Study N=500 S 3 i N=12182 HR N=60 N=1076 N=583 et al. , presented at PCR London Valves 2014; 2 Adams, et al. , N Engl J Med 2014; 370: 1790 -8; 3 Leon, et. al. presented at ACC 2013; 4 Lefevre et al. , J Am Coll Cardiol 2016; 1: ; et al. , J Am Coll Cardiol 2014; 63: 1972 -81; 6 Manoharan, et. al. presented at TVT 2014; 7 Van Mieghem, et al. , presented at Euro. PCR 2015; 8 Kodali, et al. , presented at ACC 2015; 9 Holmes, et al. , JAMA 2015; 313: 1019 -28; 10 Meredith, et al. , presented at ACC 2015 5 Popma,

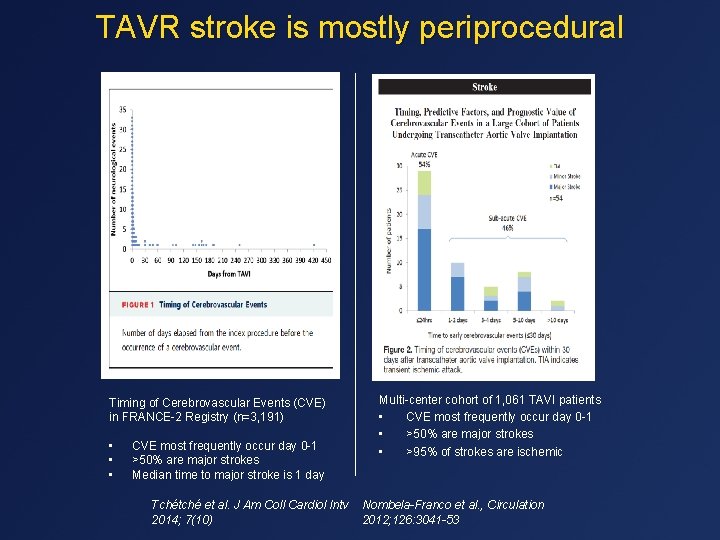
TAVR stroke is mostly periprocedural Timing of Cerebrovascular Events (CVE) in FRANCE-2 Registry (n=3, 191) • • • CVE most frequently occur day 0 -1 >50% are major strokes Median time to major stroke is 1 day Tchétché et al. J Am Coll Cardiol Intv 2014; 7(10) Multi-center cohort of 1, 061 TAVI patients • CVE most frequently occur day 0 -1 • >50% are major strokes • >95% of strokes are ischemic Nombela-Franco et al. , Circulation 2012; 126: 3041 -53

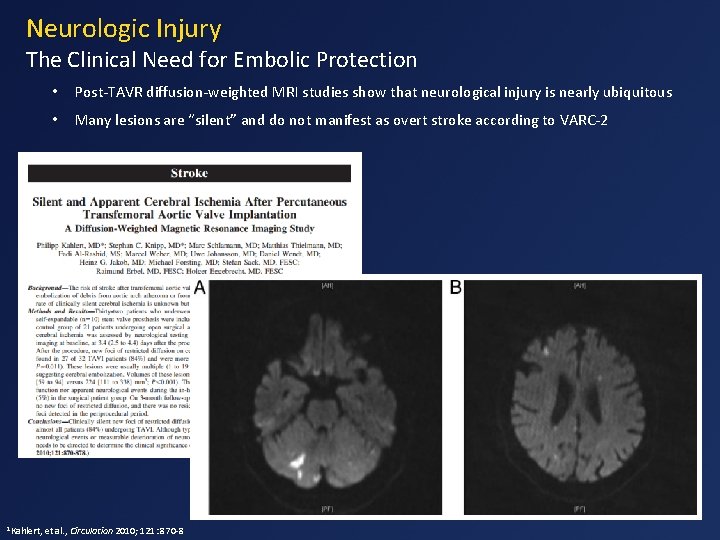
Neurologic Injury The Clinical Need for Embolic Protection 1 Kahlert, • Post-TAVR diffusion-weighted MRI studies show that neurological injury is nearly ubiquitous • Many lesions are “silent” and do not manifest as overt stroke according to VARC-2 et al. , Circulation 2010; 121: 870 -8

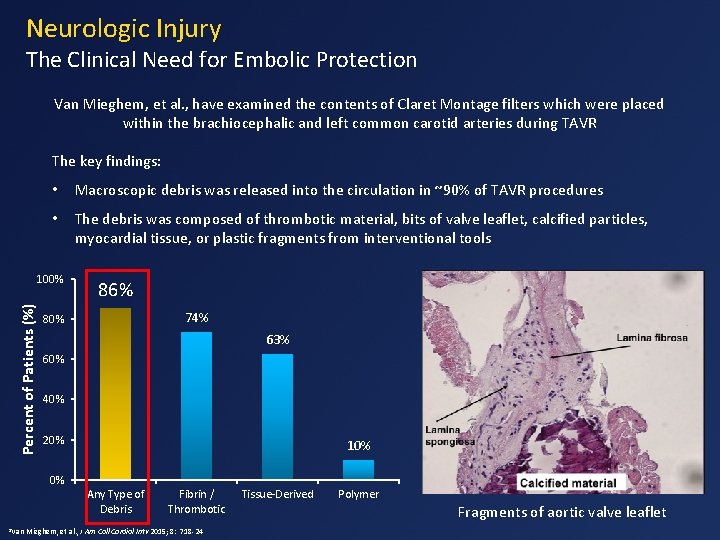
Neurologic Injury The Clinical Need for Embolic Protection Van Mieghem, et al. , have examined the contents of Claret Montage filters which were placed within the brachiocephalic and left common carotid arteries during TAVR The key findings: • Macroscopic debris was released into the circulation in ~90% of TAVR procedures • The debris was composed of thrombotic material, bits of valve leaflet, calcified particles, myocardial tissue, or plastic fragments from interventional tools Percent of Patients (%) 100% 86% 74% 80% 63% 60% 40% 20% 10% 0% Any Type of Debris 1 Van Fibrin / Thrombotic Mieghem, et al. , J Am Coll Cardiol Intv 2015; 8: 718 -24 Tissue-Derived Polymer Fragments of aortic valve leaflet

Neurologic Injury The clinical need for neuro-protective strategies in TAVR is established: • Next-generation devices and vast clinical experience have not effectively reduced the rate of stroke associated with TAVR. • One mechanism for neurologic injury is the release of embolic debris into the cerebral circulation during procedural manipulation of the aortic valve. • Imaging studies show that even patients without clinically overt stroke sustain neurologic injury. How much of this injury is clinically relevant? Is there an acceptable level that is not harmful to patients? • We know that silent infarcts have potential to cause neurocognitive deficits or predispose patients to neurodegenerative disease, so (much!) further study is (very!) necessary.

Embolic Protection Devices | The Evidence Base

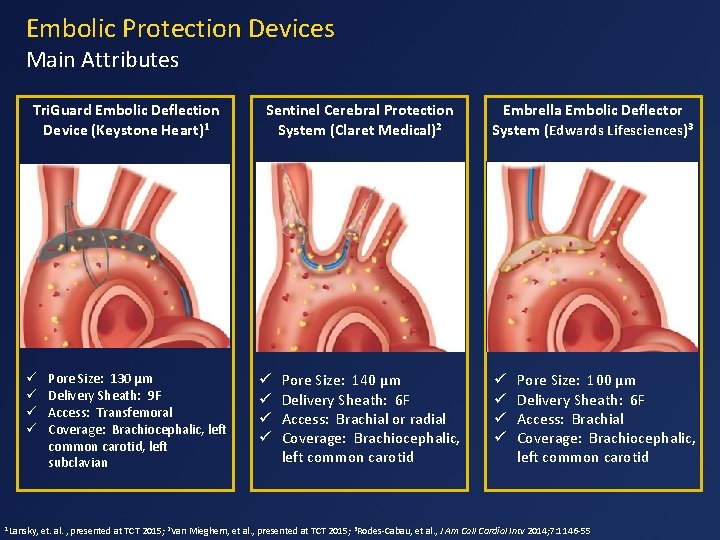
Embolic Protection Devices Main Attributes Tri. Guard Embolic Deflection Device (Keystone Heart)1 ü ü 1 Lansky, Pore Size: 130 µm Delivery Sheath: 9 F Access: Transfemoral Coverage: Brachiocephalic, left common carotid, left subclavian Sentinel Cerebral Protection System (Claret Medical)2 ü ü Pore Size: 140 µm Delivery Sheath: 6 F Access: Brachial or radial Coverage: Brachiocephalic, left common carotid Embrella Embolic Deflector System (Edwards Lifesciences)3 ü ü Pore Size: 100 µm Delivery Sheath: 6 F Access: Brachial Coverage: Brachiocephalic, left common carotid et. al. , presented at TCT 2015; 2 Van Mieghem, et al. , presented at TCT 2015; 3 Rodes-Cabau, et al. , J Am Coll Cardiol Intv 2014; 7: 1146 -55

Montage and Sentinel Dual Filters

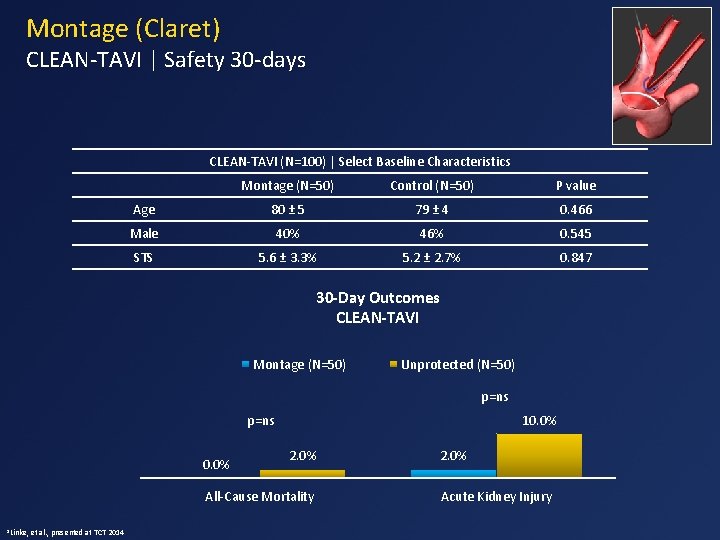
Montage (Claret) CLEAN-TAVI | Safety 30 -days CLEAN-TAVI (N=100) | Select Baseline Characteristics Montage (N=50) Control (N=50) P value Age 80 ± 5 79 ± 4 0. 466 Male 40% 46% 0. 545 STS 5. 6 ± 3. 3% 5. 2 ± 2. 7% 0. 847 30 -Day Outcomes CLEAN-TAVI Montage (N=50) Unprotected (N=50) p=ns 10. 0% p=ns 0. 0% 2. 0% All-Cause Mortality 1 Linke, et al. , presented at TCT 2014 2. 0% Acute Kidney Injury

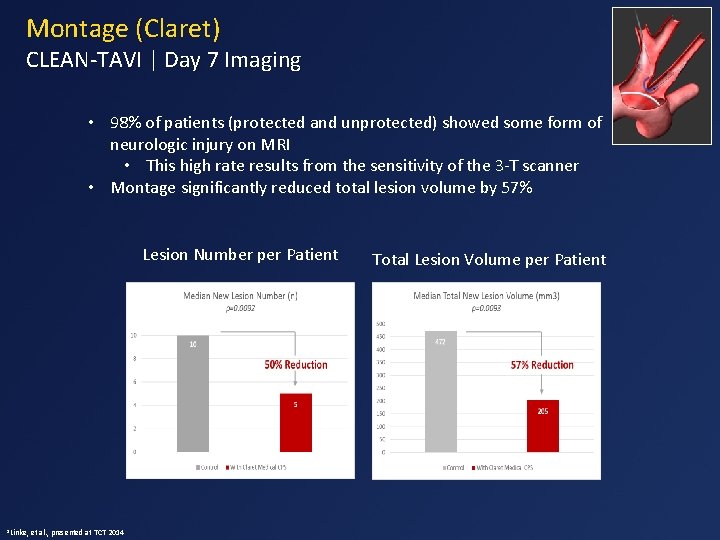
Montage (Claret) CLEAN-TAVI | Day 7 Imaging • 98% of patients (protected and unprotected) showed some form of neurologic injury on MRI • This high rate results from the sensitivity of the 3 -T scanner • Montage significantly reduced total lesion volume by 57% Lesion Number per Patient 1 Linke, et al. , presented at TCT 2014 Total Lesion Volume per Patient

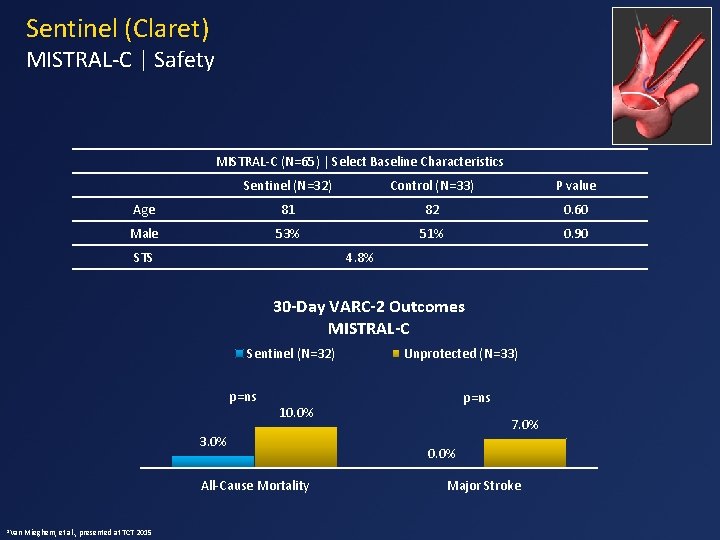
Sentinel (Claret) MISTRAL-C | Safety MISTRAL-C (N=65) | Select Baseline Characteristics Sentinel (N=32) Control (N=33) P value Age 81 82 0. 60 Male 53% 51% 0. 90 STS 4. 8% 30 -Day VARC-2 Outcomes MISTRAL-C Sentinel (N=32) p=ns All-Cause Mortality Mieghem, et al. , presented at TCT 2015 p=ns 10. 0% 3. 0% 1 Van Unprotected (N=33) 7. 0% 0. 0% Major Stroke

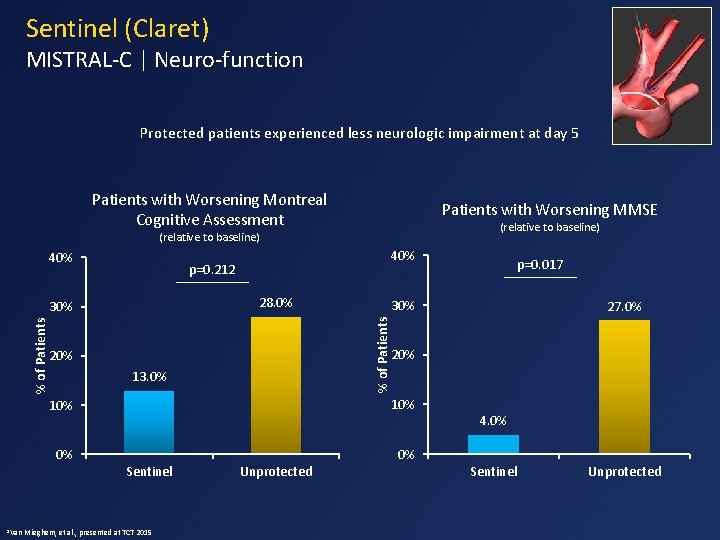
Sentinel (Claret) MISTRAL-C | Neuro-function Protected patients experienced less neurologic impairment at day 5 Patients with Worsening Montreal Cognitive Assessment Patients with Worsening MMSE (relative to baseline) 40% p=0. 212 28. 0% 20% 13. 0% 10% 0% 0% Mieghem, et al. , presented at TCT 2015 Unprotected 27. 0% 20% 10% Sentinel p=0. 017 30% % of Patients 30% 1 Van 40% 4. 0% Sentinel Unprotected

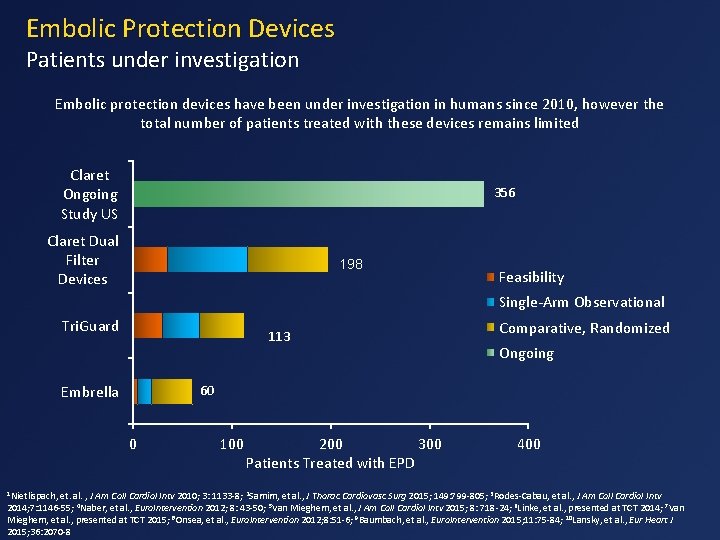
Embolic Protection Devices Patients under investigation Embolic protection devices have been under investigation in humans since 2010, however the total number of patients treated with these devices remains limited Claret Ongoing Study US 356 Claret Dual Filter Devices 198 Feasibility Single-Arm Observational Tri. Guard 113 Ongoing 60 Embrella 0 1 Nietlispach, Comparative, Randomized 100 200 300 Patients Treated with EPD 400 et. al. , J Am Coll Cardiol Intv 2010; 3: 1133 -8; 2 Samim, et al. , J Thorac Cardiovasc Surg 2015; 149: 799 -805; 3 Rodes-Cabau, et al. , J Am Coll Cardiol Intv 2014; 7: 1146 -55; 4 Naber, et al. , Euro. Intervention 2012; 8: 43 -50; 5 Van Mieghem, et al. , J Am Coll Cardiol Intv 2015; 8: 718 -24; 6 Linke, et al. , presented at TCT 2014; 7 Van Mieghem, et al. , presented at TCT 2015; 8 Onsea, et al. , Euro. Intervention 2012; 8: 51 -6; 9 Baumbach, et al. , Euro. Intervention 2015; 11: 75 -84; 10 Lansky, et al. , Eur Heart J 2015; 36: 2070 -8

Tri. Guard

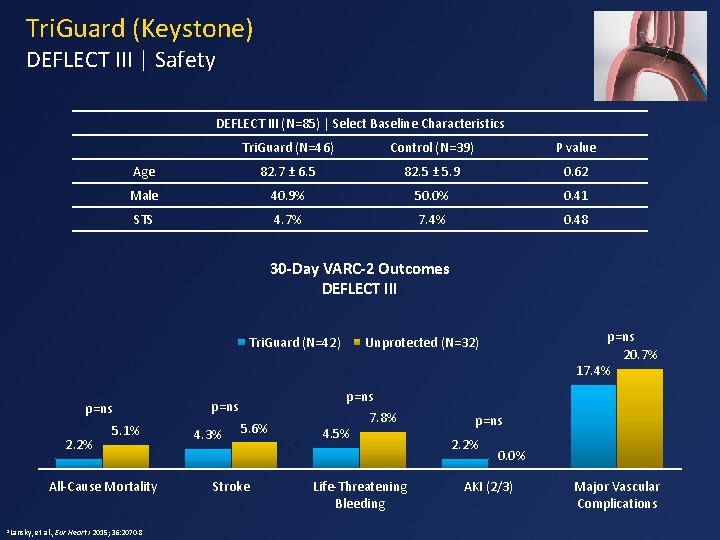
Tri. Guard (Keystone) DEFLECT III | Safety DEFLECT III (N=85) | Select Baseline Characteristics Tri. Guard (N=46) Control (N=39) P value Age 82. 7 ± 6. 5 82. 5 ± 5. 9 0. 62 Male 40. 9% 50. 0% 0. 41 STS 4. 7% 7. 4% 0. 48 30 -Day VARC-2 Outcomes DEFLECT III Tri. Guard (N=42) p=ns 2. 2% 5. 1% All-Cause Mortality 1 Lansky, et al. , Eur Heart J 2015; 36: 2070 -8 p=ns 4. 3% p=ns 20. 7% 17. 4% Unprotected (N=32) 5. 6% Stroke 4. 5% 7. 8% Life-Threatening Bleeding p=ns 2. 2% 0. 0% AKI (2/3) Major Vascular Complications

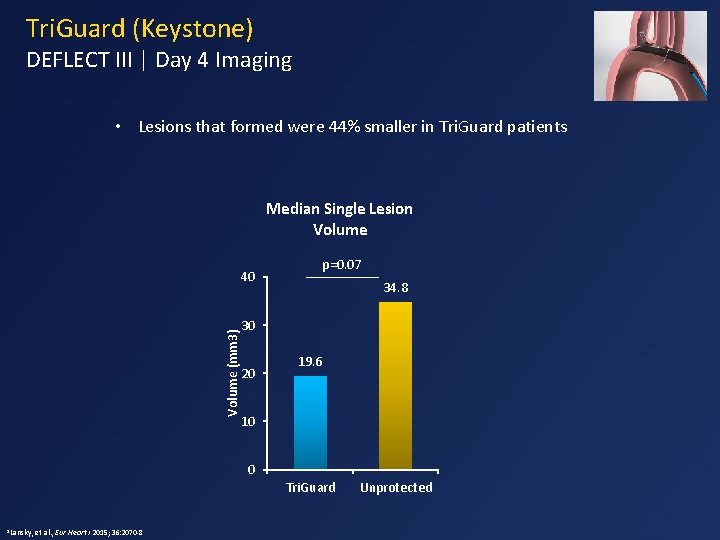
Tri. Guard (Keystone) DEFLECT III | Day 4 Imaging • Lesions that formed were 44% smaller in Tri. Guard patients Median Single Lesion Volume (mm 3) 40 p=0. 07 34. 8 30 20 19. 6 10 0 Tri. Guard 1 Lansky, et al. , Eur Heart J 2015; 36: 2070 -8 Unprotected

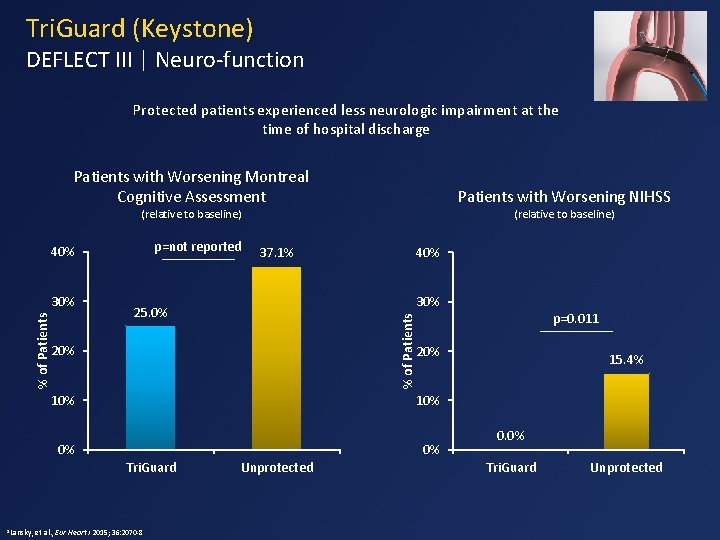
Tri. Guard (Keystone) DEFLECT III | Neuro-function Protected patients experienced less neurologic impairment at the time of hospital discharge Patients with Worsening Montreal Cognitive Assessment Patients with Worsening NIHSS (relative to baseline) p=not reported 40% 30% 25. 0% 20% 10% 0% 0% et al. , Eur Heart J 2015; 36: 2070 -8 Unprotected p=0. 011 20% 10% Tri. Guard 1 Lansky, 40% % of Patients 30% 37. 1% 15. 4% 0. 0% Tri. Guard Unprotected

Embrella

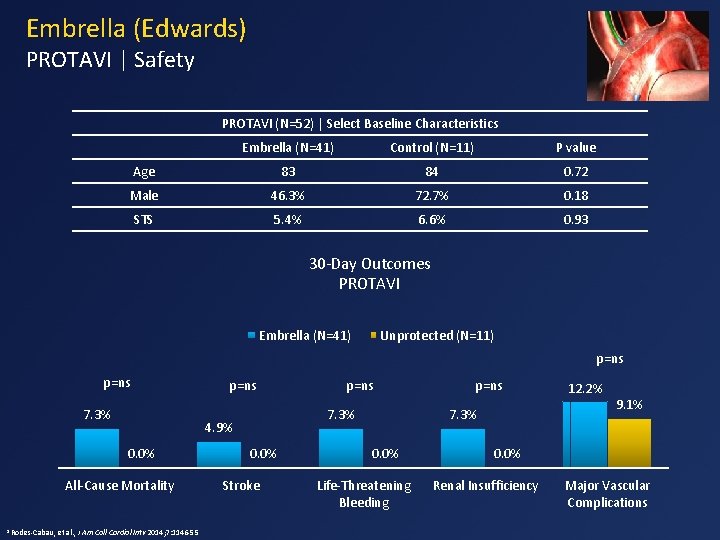
Embrella (Edwards) PROTAVI | Safety PROTAVI (N=52) | Select Baseline Characteristics Embrella (N=41) Control (N=11) P value Age 83 84 0. 72 Male 46. 3% 72. 7% 0. 18 STS 5. 4% 6. 6% 0. 93 30 -Day Outcomes PROTAVI Embrella (N=41) Unprotected (N=11) p=ns 7. 3% All-Cause Mortality et al. , J Am Coll Cardiol Intv 2014; 7: 1146 -55 p=ns 7. 3% 4. 9% 0. 0% 1 Rodes-Cabau, p=ns 0. 0% Stroke p=ns 7. 3% 0. 0% Life-Threatening Bleeding 12. 2% 9. 1% 0. 0% Renal Insufficiency Major Vascular Complications

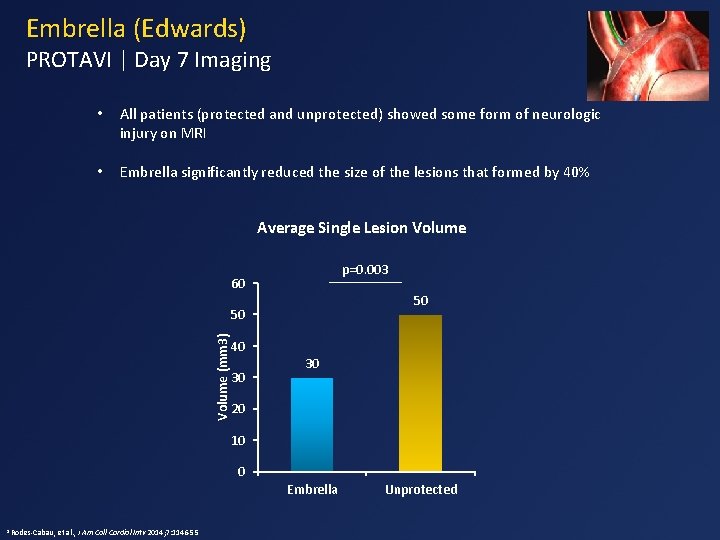
Embrella (Edwards) PROTAVI | Day 7 Imaging • All patients (protected and unprotected) showed some form of neurologic injury on MRI • Embrella significantly reduced the size of the lesions that formed by 40% Average Single Lesion Volume p=0. 003 60 50 Volume (mm 3) 50 40 30 30 20 10 0 Embrella 1 Rodes-Cabau, et al. , J Am Coll Cardiol Intv 2014; 7: 1146 -55 Unprotected

In Summary…

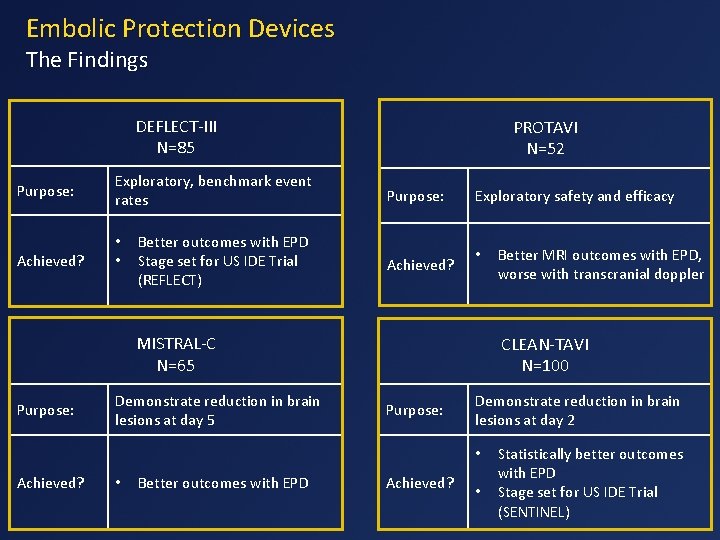
Embolic Protection Devices The Findings DEFLECT-III N=85 Purpose: Achieved? Exploratory, benchmark event rates • • Better outcomes with EPD Stage set for US IDE Trial (REFLECT) PROTAVI N=52 Purpose: Achieved? Exploratory safety and efficacy • MISTRAL-C N=65 Purpose: Demonstrate reduction in brain lesions at day 5 CLEAN-TAVI N=100 Purpose: Demonstrate reduction in brain lesions at day 2 • Achieved? • Better outcomes with EPD Better MRI outcomes with EPD, worse with transcranial doppler Achieved? • Statistically better outcomes with EPD Stage set for US IDE Trial (SENTINEL)

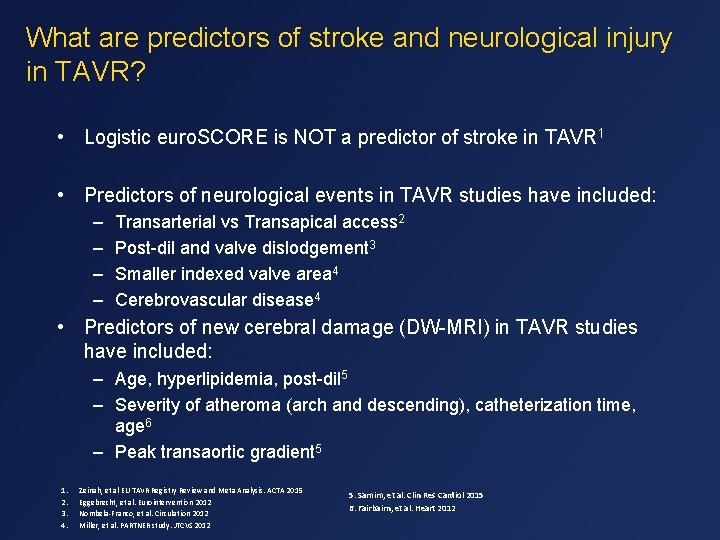
What are predictors of stroke and neurological injury in TAVR? • Logistic euro. SCORE is NOT a predictor of stroke in TAVR 1 • Predictors of neurological events in TAVR studies have included: – – Transarterial vs Transapical access 2 Post-dil and valve dislodgement 3 Smaller indexed valve area 4 Cerebrovascular disease 4 • Predictors of new cerebral damage (DW-MRI) in TAVR studies have included: – Age, hyperlipidemia, post-dil 5 – Severity of atheroma (arch and descending), catheterization time, age 6 – Peak transaortic gradient 5 1. 2. 3. 4. Zeinah, et al EU TAVR Registry Review and Meta Analysis. ACTA 2015 Eggebrecht, et al. Eurointervention 2012 Nombela-Franco, et al. Circulation 2012 Miller, et al. PARTNER study. JTCVS 2012 5. Samim, et al. Clin Res Cardiol 2015 6. Fairbairn, et al. Heart 2012

Final Thoughts • These studies have fulfilled their intended purpose: • They validate the notion that reduced embolic debris in the cerebral circulation results in fewer signals on MRI, and this translates clinically into better neurocognitive function. • They provide information on sample size and assessment tools needed to a show statistically significant benefit of embolic protection in larger studies. • With the evolution to younger, lower and Intermediate risk patients, can any level of neurologic injury be tolerated? • CLEAN TAVI, MISTRAL and DEFLECT series have set the stage for future US pivotal trials.

Thank you for your kind attention!