Conformation and Diastereomeric Specific Spectroscopic Investigation of peptides


- Slides: 23

Conformation and Diastereomeric Specific Spectroscopic Investigation of α/β-peptides Ac-Phe-β 3 -h. Ala-NHMe and Ac-β 3 -h. Ala-Phe-NHMe MG 09 William H. James III, Esteban E. Baquero, and Timothy S. Zwier Purdue University, West Lafayette IN 47907 Soo Hyuk Choi and Samuel H. Gellman University of Wisconsin-Madison, Madison WI 53706 June 16, 2008

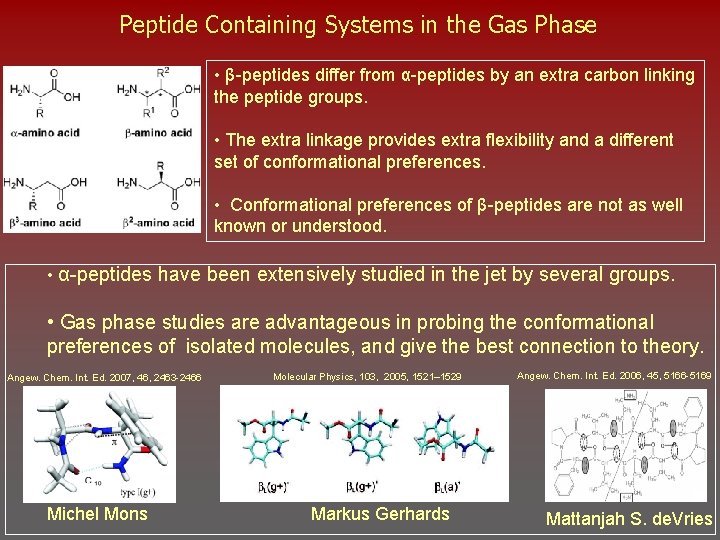
Peptide Containing Systems in the Gas Phase • β-peptides differ from α-peptides by an extra carbon linking the peptide groups. • The extra linkage provides extra flexibility and a different set of conformational preferences. • Conformational preferences of β-peptides are not as well known or understood. • α-peptides have been extensively studied in the jet by several groups. • Gas phase studies are advantageous in probing the conformational preferences of isolated molecules, and give the best connection to theory. Angew. Chem. Int. Ed. 2007, 46, 2463 -2466 Michel Mons Molecular Physics, 103, 2005, 1521– 1529 Markus Gerhards Angew. Chem. Int. Ed. 2006, 45, 5166 -5169 Mattanjah S. de. Vries

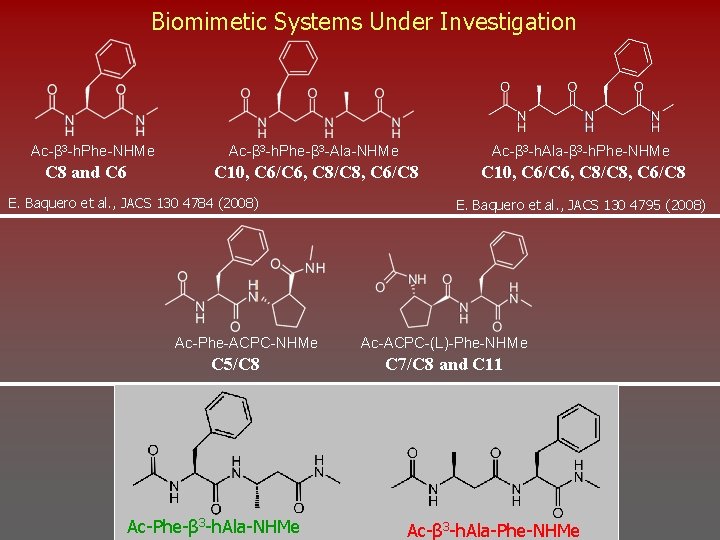
Biomimetic Systems Under Investigation Ac-β 3 -h. Phe-NHMe C 8 and C 6 Ac-β 3 -h. Phe-β 3 -Ala-NHMe Ac-β 3 -h. Ala-β 3 -h. Phe-NHMe C 10, C 6/C 6, C 8/C 8, C 6/C 8 E. Baquero et al. , JACS 130 4784 (2008) Ac-Phe-ACPC-NHMe C 5/C 8 Ac-Phe-β 3 -h. Ala-NHMe E. Baquero et al. , JACS 130 4795 (2008) Ac-ACPC-(L)-Phe-NHMe C 7/C 8 and C 11 Ac-β 3 -h. Ala-Phe-NHMe

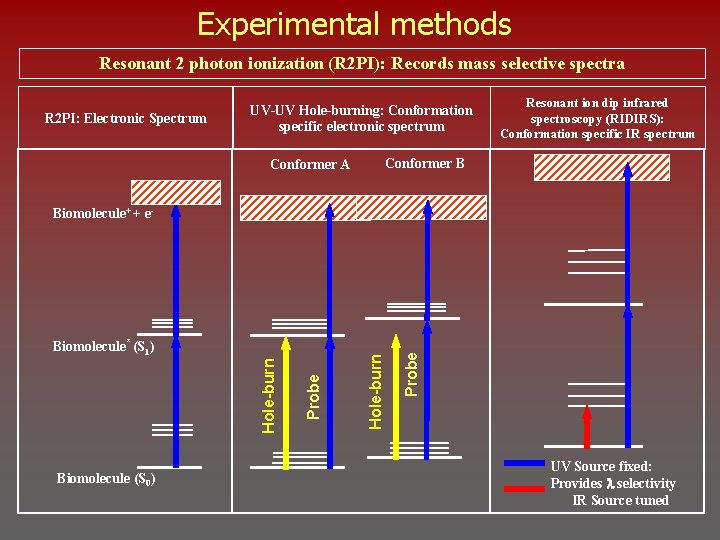
Experimental methods Resonant 2 photon ionization (R 2 PI): Records mass selective spectra R 2 PI: Electronic Spectrum UV-UV Hole-burning: Conformation specific electronic spectrum Resonant ion dip infrared spectroscopy (RIDIRS): Conformation specific IR spectrum Conformer B Conformer A Biomolecule (S 0) Probe Hole-burn Biomolecule* (S 1) Hole-burn Biomolecule+ + e- UV Source fixed: Provides l selectivity IR Source tuned

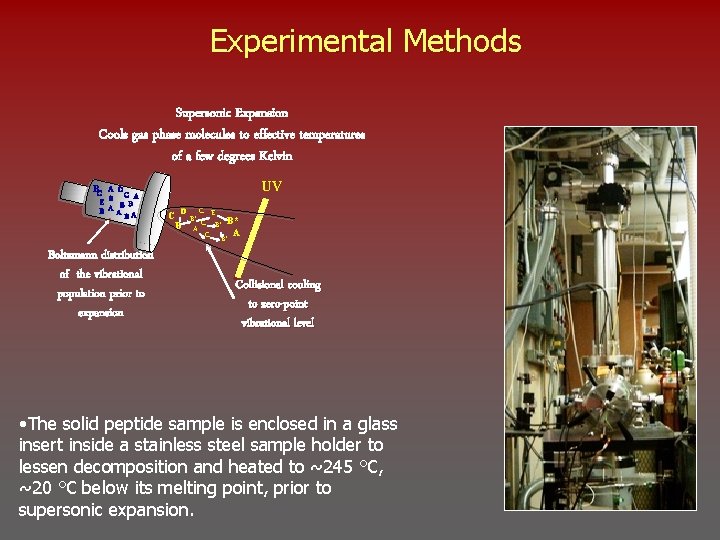
Experimental Methods Supersonic Expansion Cools gas phase molecules to effective temperatures of a few degrees Kelvin BC A D C A E B B B A A B Boltzmann distribution of the vibrational population prior to expansion UV D C C B* E B* B A C B* A Collisional cooling to zero-point vibrational level • The solid peptide sample is enclosed in a glass insert inside a stainless steel sample holder to lessen decomposition and heated to ~245 °C, ~20 °C below its melting point, prior to supersonic expansion.

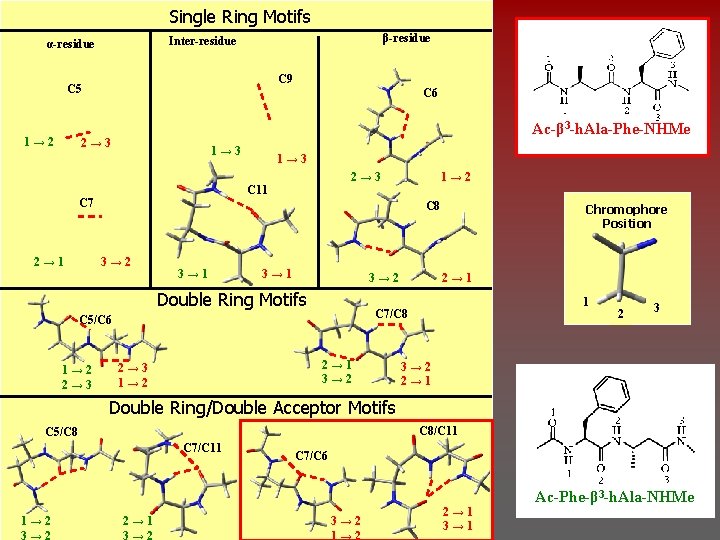
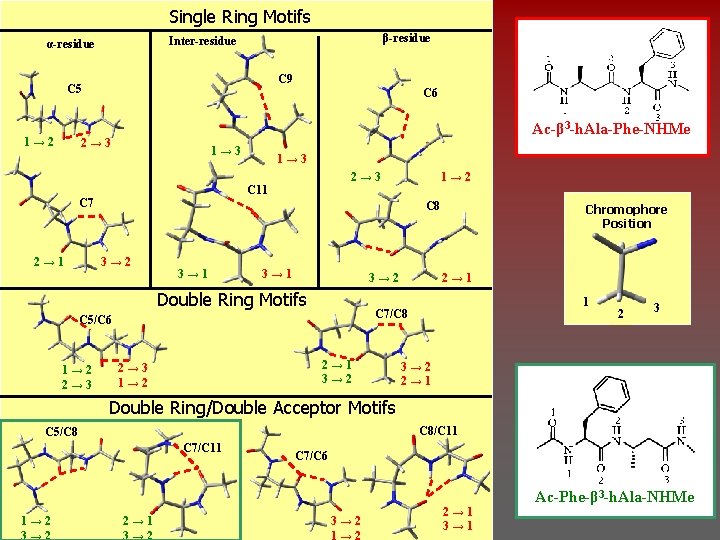
Single Ring Motifs β-residue Inter-residue α-residue C 9 C 5 1→ 2 C 6 Ac-β 3 -h. Ala-Phe-NHMe 2→ 3 1→ 3 2→ 3 C 11 C 7 2→ 1 1→ 3 C 8 3→ 2 3→ 1 3→ 2 Double Ring Motifs 2→ 1 3→ 2 2→ 3 1→ 2 Chromophore Position 2→ 1 1 C 7/C 8 C 5/C 6 1→ 2 2→ 3 1→ 2 2 3 3→ 2 2→ 1 Double Ring/Double Acceptor Motifs C 8/C 11 C 5/C 8 C 7/C 11 1→ 2 3→ 2 2→ 1 3→ 2 C 7/C 6 3→ 2 1→ 2 2→ 1 3→ 1 Ac-Phe-β 3 -h. Ala-NHMe

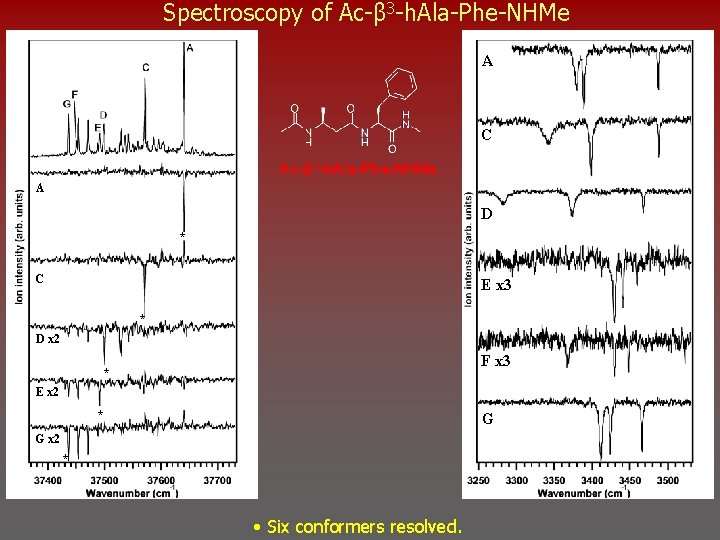
Spectroscopy of Ac-β 3 -h. Ala-Phe-NHMe A C Ac-β 3 -h. Ala-Phe-NHMe A D * C E x 3 * D x 2 F x 3 * E x 2 * G G x 2 * • Six conformers resolved.

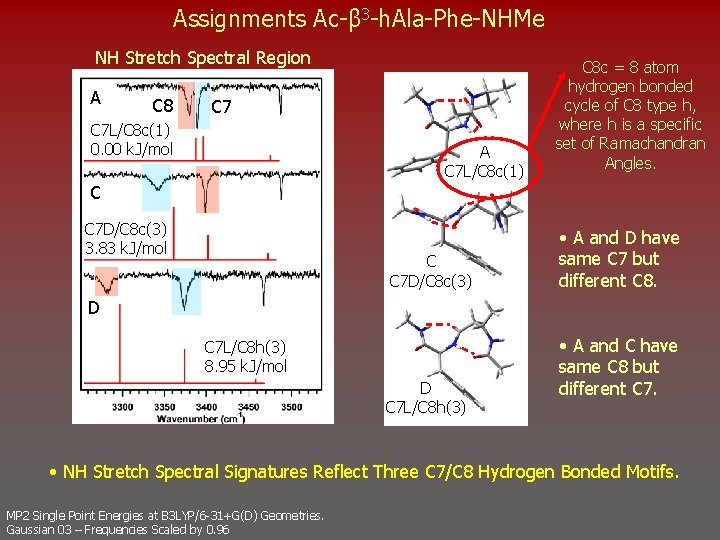
Assignments Ac-β 3 -h. Ala-Phe-NHMe NH Stretch Spectral Region A C 8 C 7 L/C 8 c(1) 0. 00 k. J/mol A C 7 L/C 8 c(1) C 8 c = 8 atom hydrogen bonded cycle of C 8 type h, where h is a specific set of Ramachandran Angles. C C 7 D/C 8 c(3) 3. 83 k. J/mol C C 7 D/C 8 c(3) • A and D have same C 7 but different C 8. D C 7 L/C 8 h(3) 8. 95 k. J/mol D C 7 L/C 8 h(3) • A and C have same C 8 but different C 7. • NH Stretch Spectral Signatures Reflect Three C 7/C 8 Hydrogen Bonded Motifs. MP 2 Single Point Energies at B 3 LYP/6 -31+G(D) Geometries. Gaussian 03 – Frequencies Scaled by 0. 96

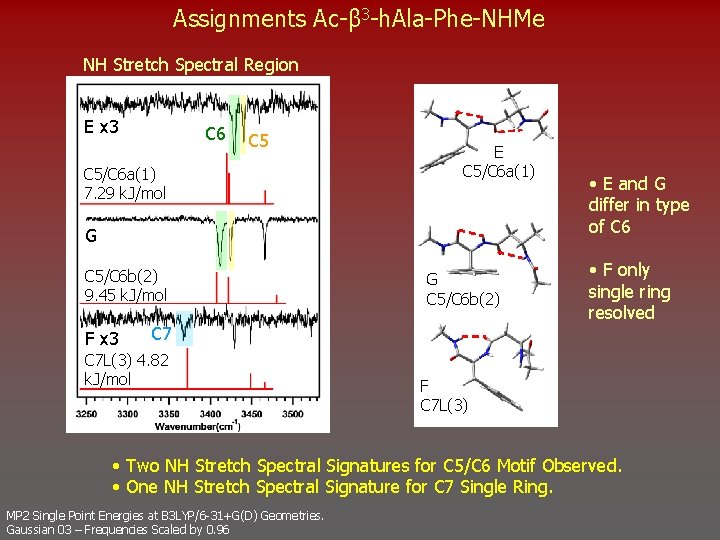
Assignments Ac-β 3 -h. Ala-Phe-NHMe NH Stretch Spectral Region E x 3 C 6 C 5/C 6 a(1) 7. 29 k. J/mol E C 5/C 6 a(1) G C 5/C 6 b(2) 9. 45 k. J/mol F x 3 G C 5/C 6 b(2) C 7 L(3) 4. 82 k. J/mol • E and G differ in type of C 6 • F only single ring resolved F C 7 L(3) • Two NH Stretch Spectral Signatures for C 5/C 6 Motif Observed. • One NH Stretch Spectral Signature for C 7 Single Ring. MP 2 Single Point Energies at B 3 LYP/6 -31+G(D) Geometries. Gaussian 03 – Frequencies Scaled by 0. 96

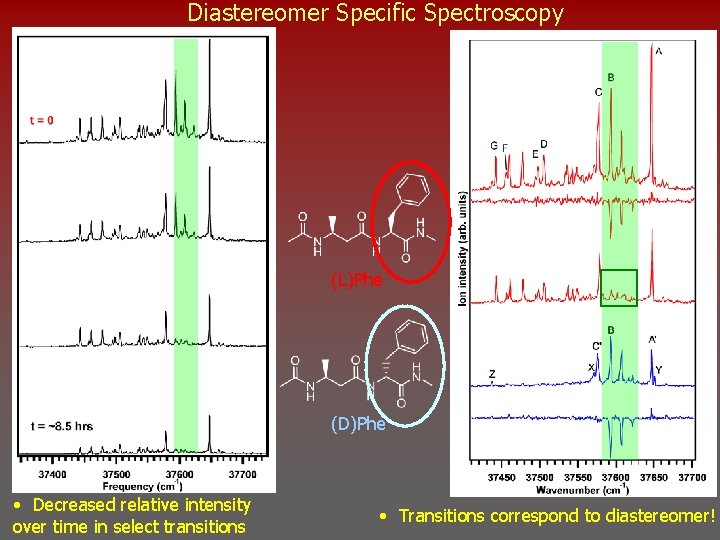
Diastereomer Specific Spectroscopy (L)Phe (D)Phe • Decreased relative intensity over time in select transitions • Transitions correspond to diastereomer!

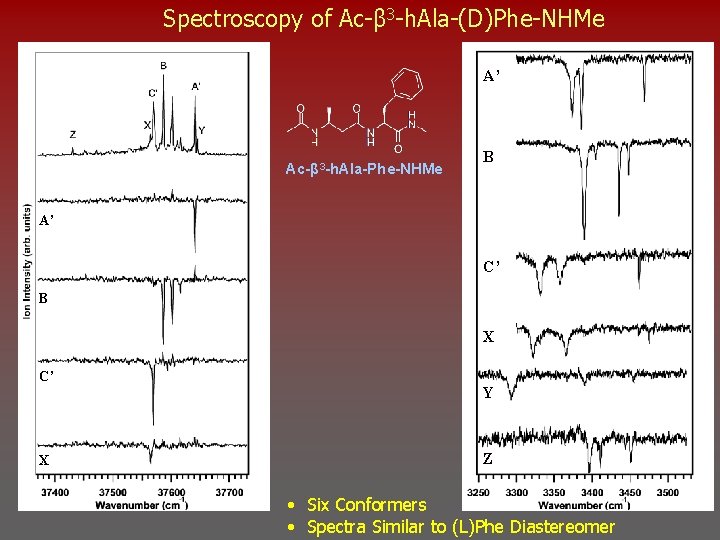
Spectroscopy of Ac-β 3 -h. Ala-(D)Phe-NHMe A’ Ac-β 3 -h. Ala-Phe-NHMe B A’ C’ B X C’ X Y Z • Six Conformers • Spectra Similar to (L)Phe Diastereomer

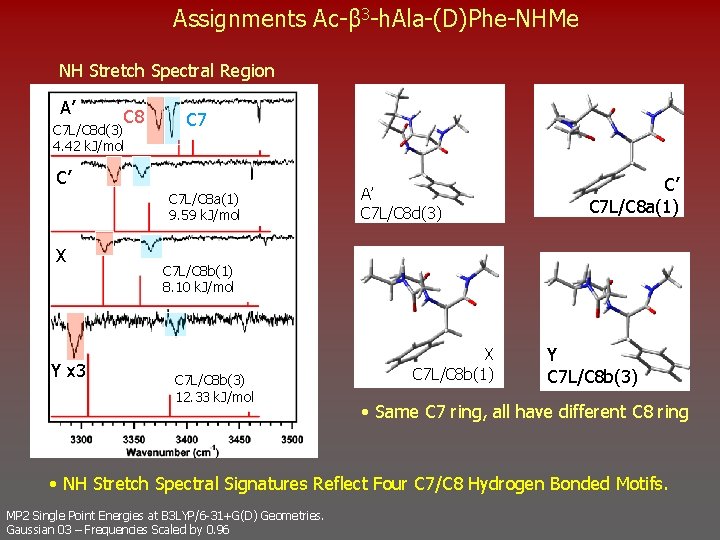
Assignments Ac-β 3 -h. Ala-(D)Phe-NHMe NH Stretch Spectral Region A’ C 8 C 7 L/C 8 d(3) 4. 42 k. J/mol C 7 C’ C 7 L/C 8 a(1) 9. 59 k. J/mol X Y x 3 A’ C 7 L/C 8 d(3) C’ C 7 L/C 8 a(1) C 7 L/C 8 b(1) 8. 10 k. J/mol C 7 L/C 8 b(3) 12. 33 k. J/mol X C 7 L/C 8 b(1) Y C 7 L/C 8 b(3) • Same C 7 ring, all have different C 8 ring • NH Stretch Spectral Signatures Reflect Four C 7/C 8 Hydrogen Bonded Motifs. MP 2 Single Point Energies at B 3 LYP/6 -31+G(D) Geometries. Gaussian 03 – Frequencies Scaled by 0. 96

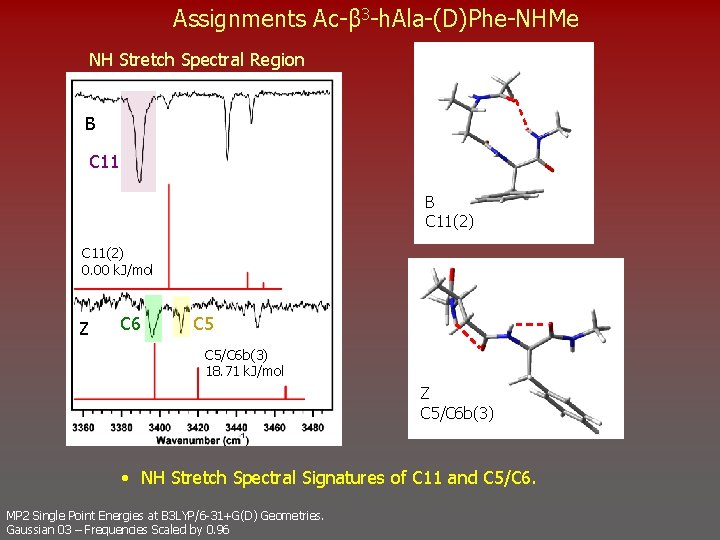
Assignments Ac-β 3 -h. Ala-(D)Phe-NHMe NH Stretch Spectral Region B C 11(2) 0. 00 k. J/mol Z C 6 C 5/C 6 b(3) 18. 71 k. J/mol Z C 5/C 6 b(3) • NH Stretch Spectral Signatures of C 11 and C 5/C 6. MP 2 Single Point Energies at B 3 LYP/6 -31+G(D) Geometries. Gaussian 03 – Frequencies Scaled by 0. 96

Single Ring Motifs β-residue Inter-residue α-residue C 9 C 5 1→ 2 C 6 Ac-β 3 -h. Ala-Phe-NHMe 2→ 3 1→ 3 2→ 3 C 11 C 7 2→ 1 1→ 3 C 8 3→ 2 3→ 1 3→ 2 Double Ring Motifs 2→ 1 3→ 2 2→ 3 1→ 2 Chromophore Position 2→ 1 1 C 7/C 8 C 5/C 6 1→ 2 2→ 3 1→ 2 2 3 3→ 2 2→ 1 Double Ring/Double Acceptor Motifs C 8/C 11 C 5/C 8 C 7/C 11 1→ 2 3→ 2 2→ 1 3→ 2 C 7/C 6 3→ 2 1→ 2 2→ 1 3→ 1 Ac-Phe-β 3 -h. Ala-NHMe

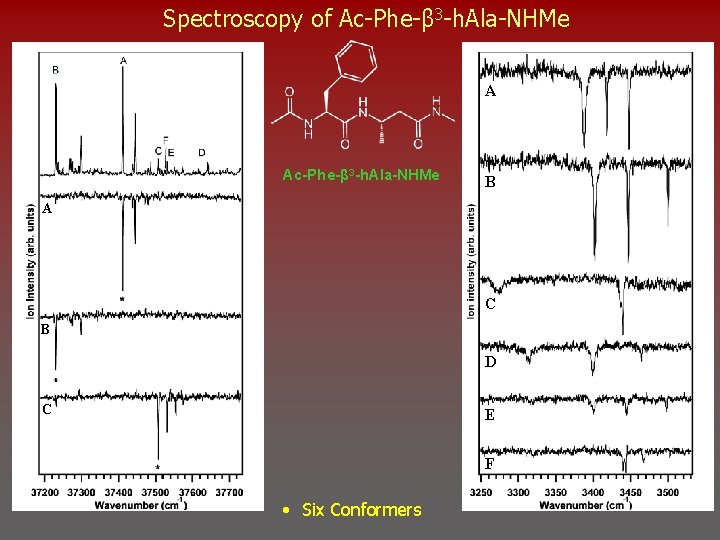
Spectroscopy of Ac-Phe-β 3 -h. Ala-NHMe A Ac-Phe-β 3 -h. Ala-NHMe B A C B D C E F • Six Conformers

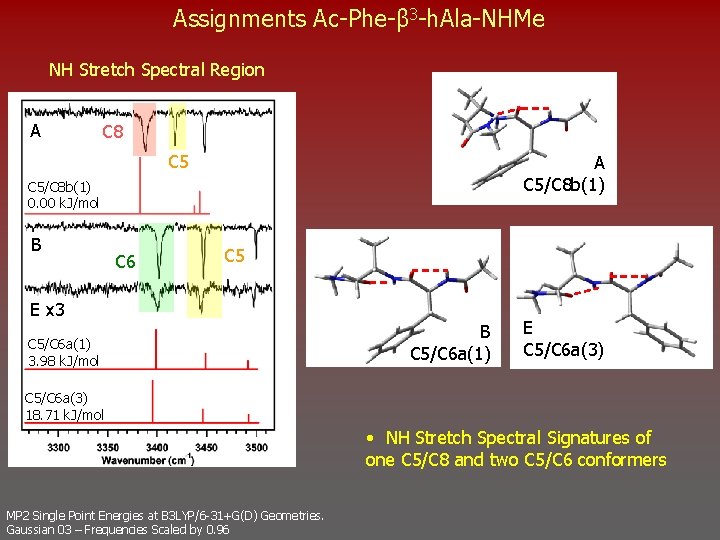
Assignments Ac-Phe-β 3 -h. Ala-NHMe NH Stretch Spectral Region A C 8 C 5 A C 5/C 8 b(1) 0. 00 k. J/mol B C 6 C 5 E x 3 C 5/C 6 a(1) 3. 98 k. J/mol B C 5/C 6 a(1) E C 5/C 6 a(3) 18. 71 k. J/mol • NH Stretch Spectral Signatures of one C 5/C 8 and two C 5/C 6 conformers MP 2 Single Point Energies at B 3 LYP/6 -31+G(D) Geometries. Gaussian 03 – Frequencies Scaled by 0. 96

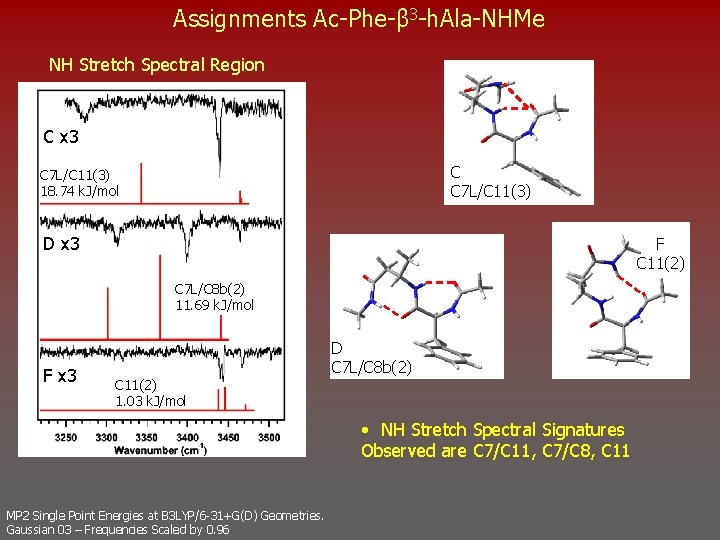
Assignments Ac-Phe-β 3 -h. Ala-NHMe NH Stretch Spectral Region C x 3 C C 7 L/C 11(3) 18. 74 k. J/mol D x 3 F C 11(2) C 7 L/C 8 b(2) 11. 69 k. J/mol F x 3 C 11(2) 1. 03 k. J/mol D C 7 L/C 8 b(2) • NH Stretch Spectral Signatures Observed are C 7/C 11, C 7/C 8, C 11 MP 2 Single Point Energies at B 3 LYP/6 -31+G(D) Geometries. Gaussian 03 – Frequencies Scaled by 0. 96

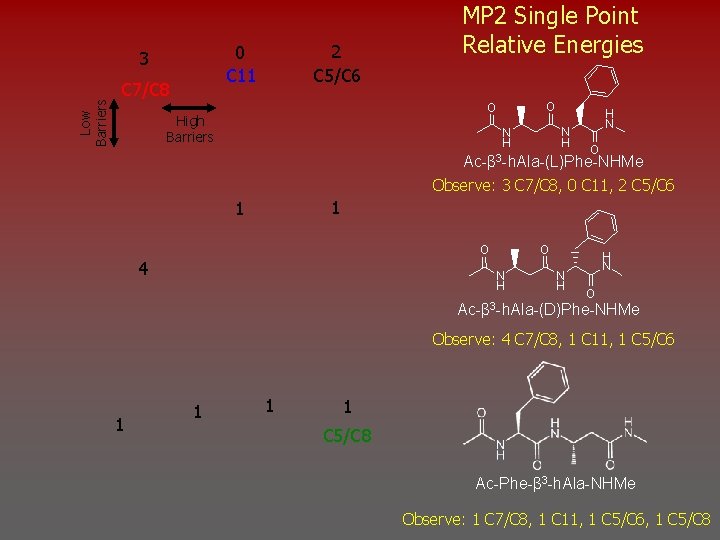
Low Barriers 2 C 5/C 6 0 C 11 3 C 7/C 8 MP 2 Single Point Relative Energies O O High Barriers N H H N O Ac-β 3 -h. Ala-(L)Phe-NHMe Observe: 3 C 7/C 8, 0 C 11, 2 C 5/C 6 1 1 O 4 O N H H N O Ac-β 3 -h. Ala-(D)Phe-NHMe Observe: 4 C 7/C 8, 1 C 11, 1 C 5/C 6 1 1 C 5/C 8 Ac-Phe-β 3 -h. Ala-NHMe Observe: 1 C 7/C 8, 1 C 11, 1 C 5/C 6, 1 C 5/C 8

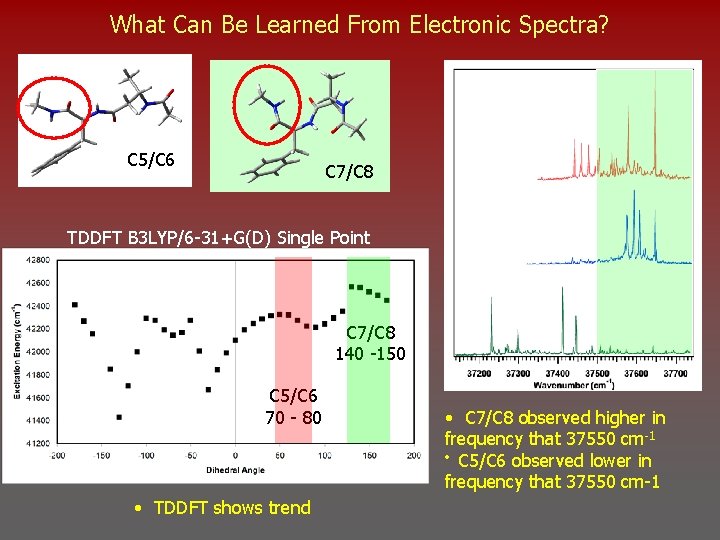
What Can Be Learned From Electronic Spectra? C 5/C 6 C 7/C 8 TDDFT B 3 LYP/6 -31+G(D) Single Point C 7/C 8 140 -150 C 5/C 6 70 - 80 • TDDFT shows trend • C 7/C 8 observed higher in frequency that 37550 cm-1 • C 5/C 6 observed lower in frequency that 37550 cm-1

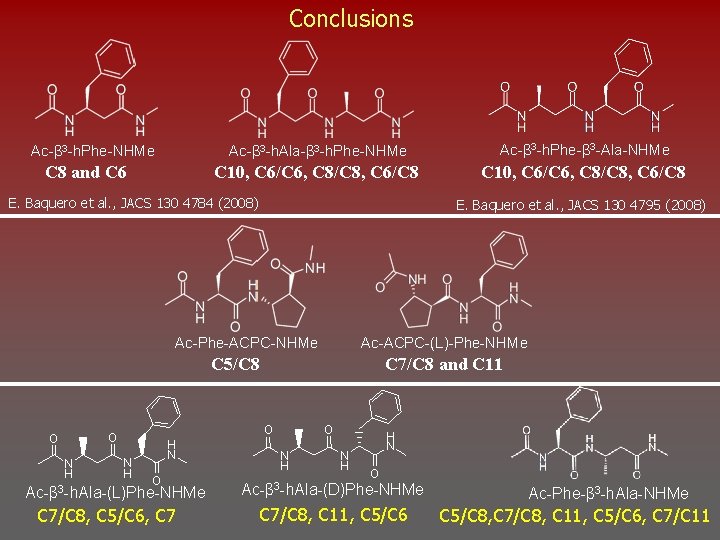
Conclusions Ac-β 3 -h. Phe-NHMe C 8 and C 6 Ac-β 3 -h. Ala-β 3 -h. Phe-NHMe Ac-β 3 -h. Phe-β 3 -Ala-NHMe C 10, C 6/C 6, C 8/C 8, C 6/C 8 E. Baquero et al. , JACS 130 4784 (2008) E. Baquero et al. , JACS 130 4795 (2008) Ac-Phe-ACPC-NHMe Ac-ACPC-(L)-Phe-NHMe C 5/C 8 O O O N H C 7/C 8 and C 11 N H H N O Ac-β 3 -h. Ala-(L)Phe-NHMe C 7/C 8, C 5/C 6, C 7 O N H H N O Ac-β 3 -h. Ala-(D)Phe-NHMe C 7/C 8, C 11, C 5/C 6 Ac-Phe-β 3 -h. Ala-NHMe C 5/C 8, C 7/C 8, C 11, C 5/C 6, C 7/C 11

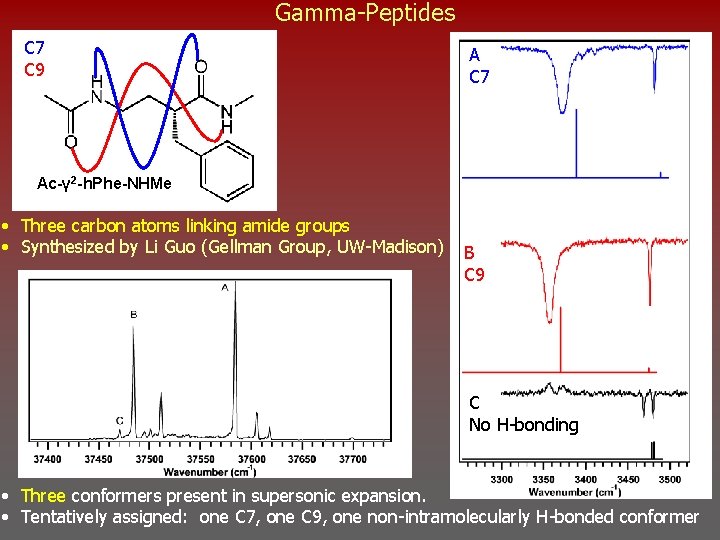
Gamma-Peptides C 7 C 9 A C 7 Ac-γ 2 -h. Phe-NHMe • Three carbon atoms linking amide groups • Synthesized by Li Guo (Gellman Group, UW-Madison) B C 9 C No H-bonding • Three conformers present in supersonic expansion. • Tentatively assigned: one C 7, one C 9, one non-intramolecularly H-bonded conformer

Acknowledgements Professor Samuel H. Gellman Soo Hyuk Choi Li Guo Professor Timothy S. Zwier Current Group Members: Dr. Christian Müller Tracy Le. Greve Nathan Pillsbury Joshua Newby Chirantha Rodrigo Joshua Sebree Evan Buchanan Former Group Members: Dr. Jaime Stearns Dr. Talitha Selby Dr. Jasper Clarkson Dr. Ching-Ping Liu Dr. Esteban Baquero Dr. V. Alvin Shubert FUNDING Computational Resources

 Peptides and proteins
Peptides and proteins Mobile phase in affinity chromatography
Mobile phase in affinity chromatography Ppgbqa
Ppgbqa Will amylase build keratin out of peptides
Will amylase build keratin out of peptides Local hormones
Local hormones Stability of chair and boat conformation
Stability of chair and boat conformation Alkyne condensed formula
Alkyne condensed formula Spectroscopic notation examples
Spectroscopic notation examples Spectroscopic data in chemistry
Spectroscopic data in chemistry Free ion configuration
Free ion configuration What is the mulliken symbols for 'f' spectroscopic term in
What is the mulliken symbols for 'f' spectroscopic term in Slope
Slope Tetrahydropyran chair conformation
Tetrahydropyran chair conformation Site:slidetodoc.com
Site:slidetodoc.com What does this image show
What does this image show Oxidation of carbohydrates
Oxidation of carbohydrates Isomérie optique
Isomérie optique How to calculate specific gravity
How to calculate specific gravity Pharmaceutical application of specific gravity
Pharmaceutical application of specific gravity System planning and initial investigation
System planning and initial investigation Fire and arson investigation ppt
Fire and arson investigation ppt Connected mathematics stretching and shrinking
Connected mathematics stretching and shrinking Phet charges and charged objects investigation
Phet charges and charged objects investigation Technological design and scientific investigation
Technological design and scientific investigation