Free Ion Spectroscopic Terms for dn Configurations Electronelectron


![Electronic Spectra of 3 d Transition Metal Complexes [M(H 2 O)6]2+ 7 Electronic Spectra of 3 d Transition Metal Complexes [M(H 2 O)6]2+ 7](https://slidetodoc.com/presentation_image_h/a5d799f1684912562c55a88bcd8edafe/image-7.jpg)
- Slides: 7

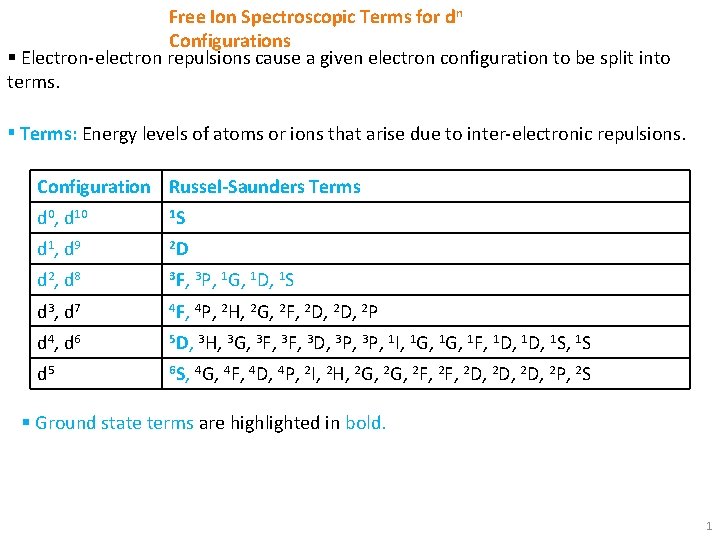
Free Ion Spectroscopic Terms for dn Configurations § Electron-electron repulsions cause a given electron configuration to be split into terms. ▪ Terms: Energy levels of atoms or ions that arise due to inter-electronic repulsions. Configuration Russel-Saunders Terms d 0, d 10 1 S d 1 , d 9 2 D d 2 , d 8 3 F, 3 P, 1 G, 1 D, 1 S d 3 , d 7 4 F, 4 P, 2 H, 2 G, 2 F, 2 D, 2 P d 4 , d 6 5 D, 3 H, 3 G, 3 F, 3 D, 3 P, 1 I, 1 G, 1 F, 1 D, 1 S d 5 6 S, 4 G, 4 F, 4 D, 4 P, 2 I, 2 H, 2 G, 2 F, 2 D, 2 D, 2 P, 2 S § Ground state terms are highlighted in bold. 1

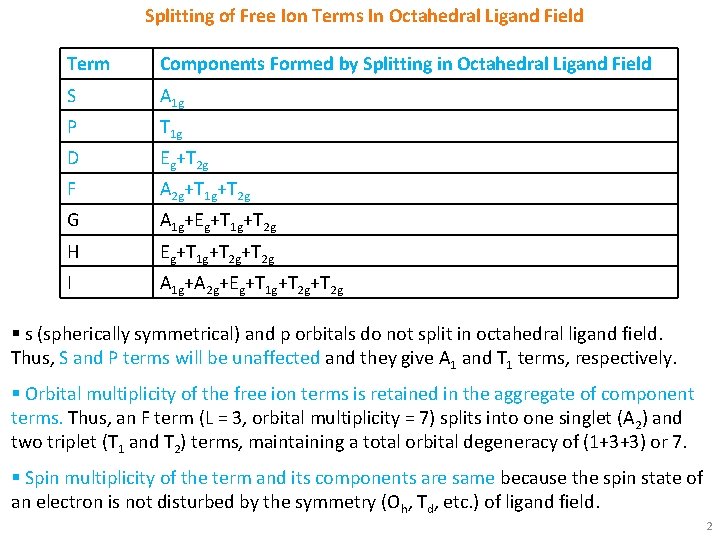
Splitting of Free Ion Terms In Octahedral Ligand Field Term Components Formed by Splitting in Octahedral Ligand Field S A 1 g P T 1 g D Eg+T 2 g F A 2 g+T 1 g+T 2 g G A 1 g+Eg+T 1 g+T 2 g H Eg+T 1 g+T 2 g I A 1 g+A 2 g+Eg+T 1 g+T 2 g § s (spherically symmetrical) and p orbitals do not split in octahedral ligand field. Thus, S and P terms will be unaffected and they give A 1 and T 1 terms, respectively. § Orbital multiplicity of the free ion terms is retained in the aggregate of component terms. Thus, an F term (L = 3, orbital multiplicity = 7) splits into one singlet (A 2) and two triplet (T 1 and T 2) terms, maintaining a total orbital degeneracy of (1+3+3) or 7. § Spin multiplicity of the term and its components are same because the spin state of an electron is not disturbed by the symmetry (Oh, Td, etc. ) of ligand field. 2

Splitting of D and F Terms in Tetrahedral and Octahedral Symmetries § Hole concept suggests an inversion of term splitting between dn and d 10 -n configurations. § For example, a d 9 metal ion has an electron vacancy or “hole” in its d level and thus can be regarded as the inverse of a d 1 arrangement. § An inversion of term splitting also occurs between tetrahedral and octahedral symmetries. Since ligand fields of these two symmetries produce inverse splitting patterns for d orbitals, any free ion term will also split into new terms by tetrahedral and octahedral fields, but the energy ordering will be opposite for two symmetries. 3

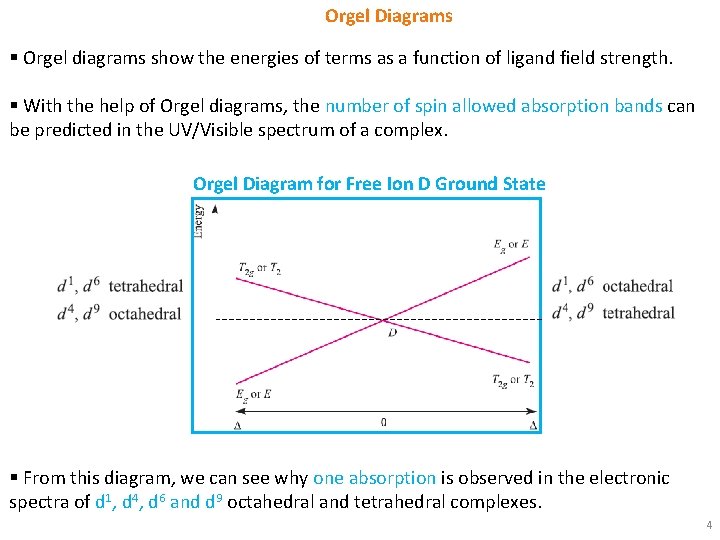
Orgel Diagrams § Orgel diagrams show the energies of terms as a function of ligand field strength. § With the help of Orgel diagrams, the number of spin allowed absorption bands can be predicted in the UV/Visible spectrum of a complex. Orgel Diagram for Free Ion D Ground State § From this diagram, we can see why one absorption is observed in the electronic spectra of d 1, d 4, d 6 and d 9 octahedral and tetrahedral complexes. 4

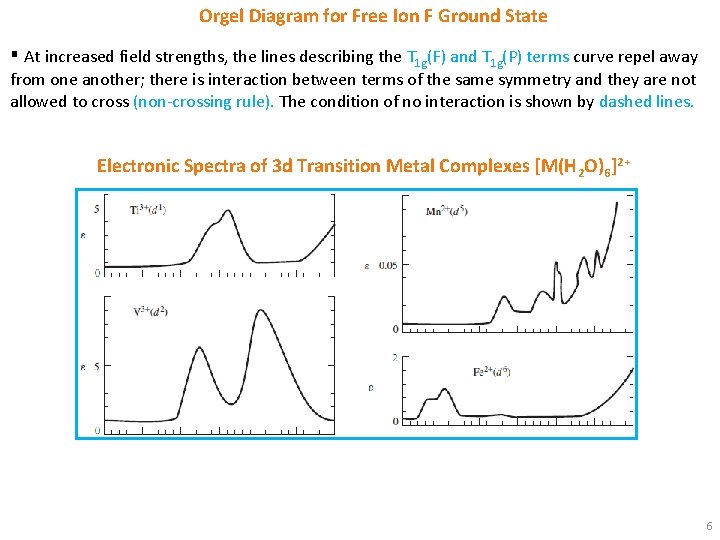
Orgel Diagram for Free Ion F Ground State 0 § From this diagram, we can see why three absorptions are observed in the electronic spectra of d 2, d 3, d 7 and d 8 octahedral and tetrahedral complexes. § Labels on Orgel diagrams do not include spin multiplicity designations. Spin multiplicities must be added when discussing specific transitions. 5

Orgel Diagram for Free Ion F Ground State ▪ At increased field strengths, the lines describing the T 1 g(F) and T 1 g(P) terms curve repel away from one another; there is interaction between terms of the same symmetry and they are not allowed to cross (non-crossing rule). The condition of no interaction is shown by dashed lines. Electronic Spectra of 3 d Transition Metal Complexes [M(H 2 O)6]2+ 6
![Electronic Spectra of 3 d Transition Metal Complexes MH 2 O62 7 Electronic Spectra of 3 d Transition Metal Complexes [M(H 2 O)6]2+ 7](https://slidetodoc.com/presentation_image_h/a5d799f1684912562c55a88bcd8edafe/image-7.jpg)
Electronic Spectra of 3 d Transition Metal Complexes [M(H 2 O)6]2+ 7