Chapter 3 Conformational Steric and Stereo Electronic Effects
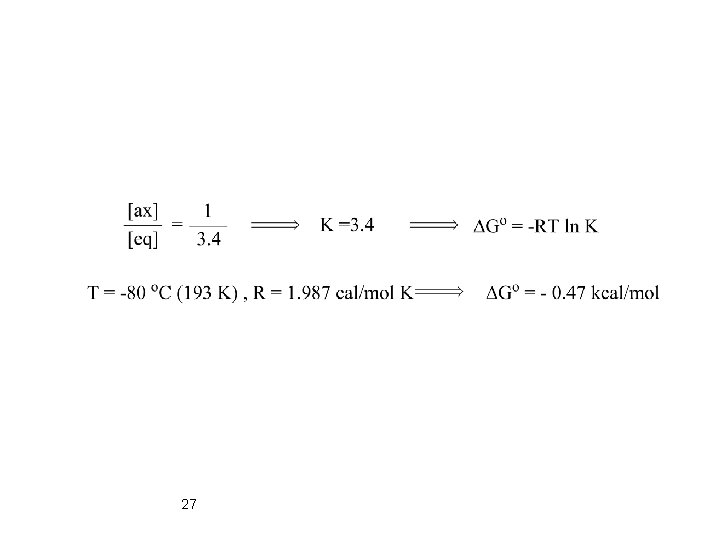

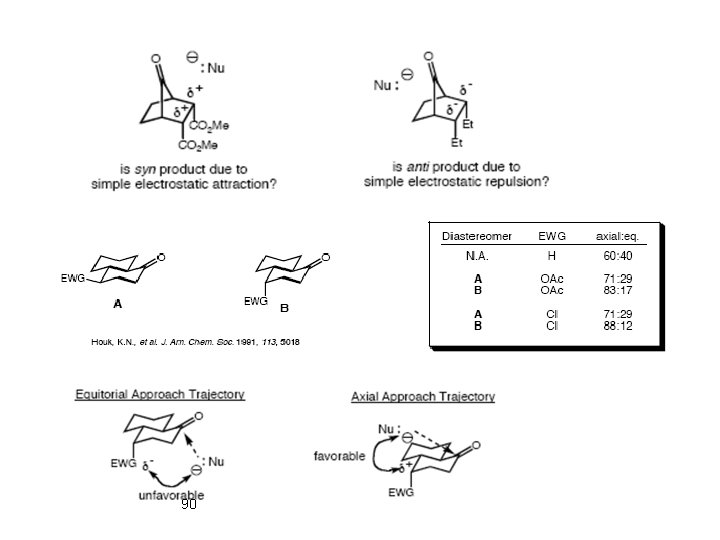
- Slides: 90

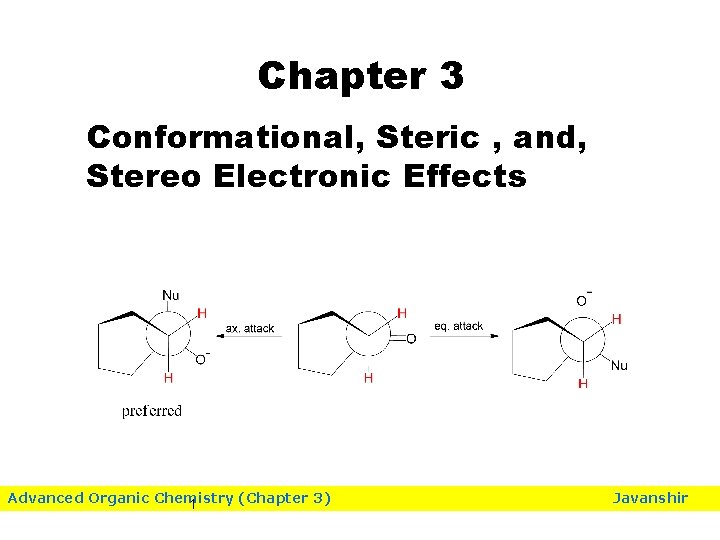
Chapter 3 Conformational, Steric , and, Stereo Electronic Effects Advanced Organic Chemistry (Chapter 3) 1 Javanshir

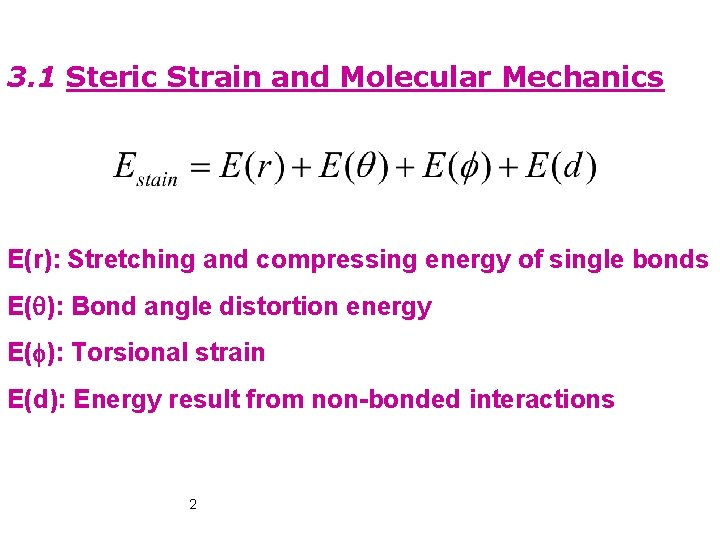
3. 1 Steric Strain and Molecular Mechanics E(r): Stretching and compressing energy of single bonds E(q): Bond angle distortion energy E(f): Torsional strain E(d): Energy result from non-bonded interactions 2

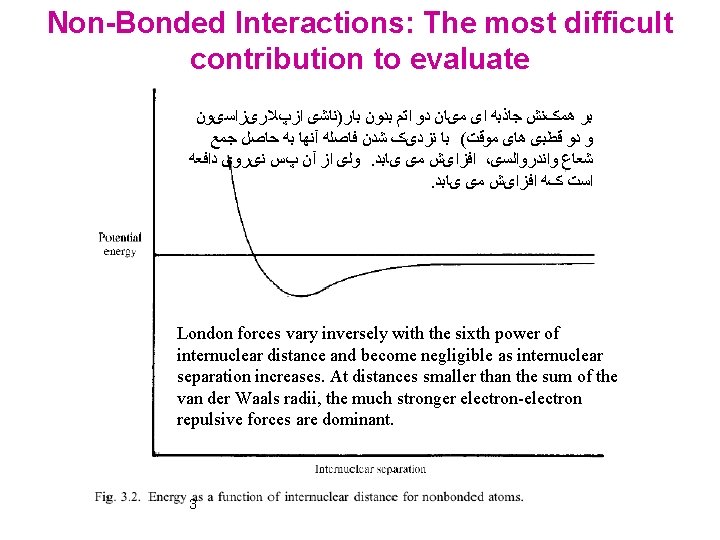
Non-Bonded Interactions: The most difficult contribution to evaluate ﺑﺮ ﻫﻤکﻨﺶ ﺟﺎﺫﺑﻪ ﺍی ﻣیﺎﻥ ﺩﻭ ﺍﺗﻢ ﺑﺪﻭﻥ ﺑﺎﺭ)ﻧﺎﺷی ﺍﺯپﻼﺭیﺰﺍﺳیﻮﻥ ﻭ ﺩﻭ ﻗﻄﺒی ﻫﺎی ﻣﻮﻗﺖ( ﺑﺎ ﻧﺰﺩیک ﺷﺪﻥ ﻓﺎﺻﻠﻪ آﻨﻬﺎ ﺑﻪ ﺣﺎﺻﻞ ﺟﻤﻊ ﻭﻟی ﺍﺯ آﻦ پﺲ ﻧیﺮﻭی ﺩﺍﻓﻌﻪ. ﺍﻓﺰﺍیﺶ ﻣی یﺎﺑﺪ ، ﺷﻌﺎﻉ ﻭﺍﻧﺪﺭﻭﺍﻟﺴی . ﺍﺳﺖ کﻪ ﺍﻓﺰﺍیﺶ ﻣی یﺎﺑﺪ London forces vary inversely with the sixth power of internuclear distance and become negligible as internuclear separation increases. At distances smaller than the sum of the van der Waals radii, the much stronger electron-electron repulsive forces are dominant. 3

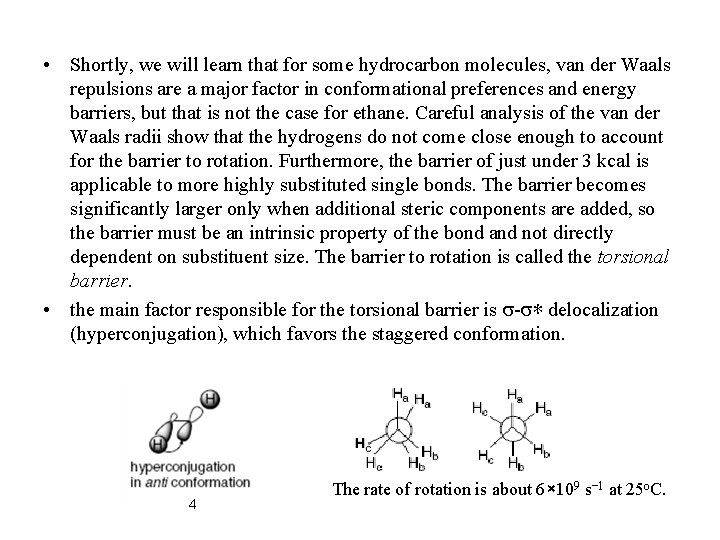
• Shortly, we will learn that for some hydrocarbon molecules, van der Waals repulsions are a major factor in conformational preferences and energy barriers, but that is not the case for ethane. Careful analysis of the van der Waals radii show that the hydrogens do not come close enough to account for the barrier to rotation. Furthermore, the barrier of just under 3 kcal is applicable to more highly substituted single bonds. The barrier becomes significantly larger only when additional steric components are added, so the barrier must be an intrinsic property of the bond and not directly dependent on substituent size. The barrier to rotation is called the torsional barrier. • the main factor responsible for the torsional barrier is s-s∗ delocalization (hyperconjugation), which favors the staggered conformation. 4 The rate of rotation is about 6× 109 s− 1 at 25 o. C.

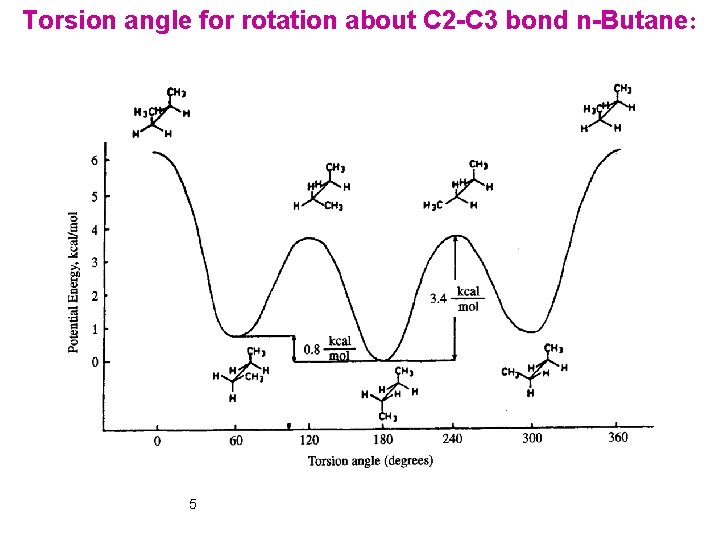
Torsion angle for rotation about C 2 -C 3 bond n-Butane: 5

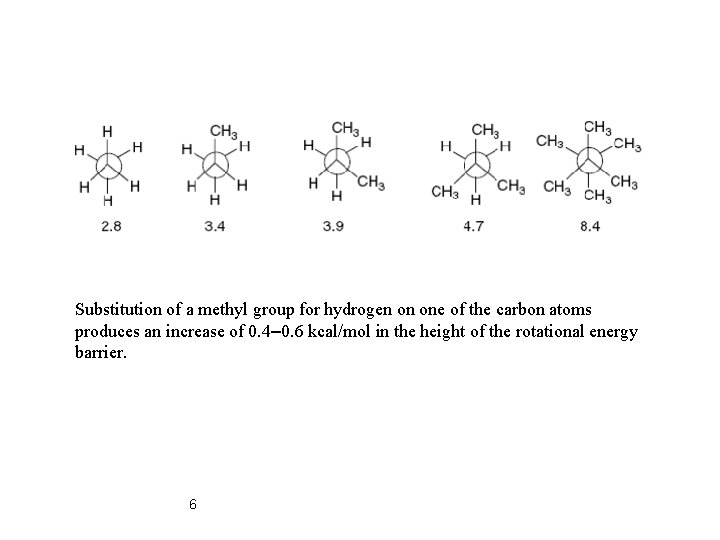
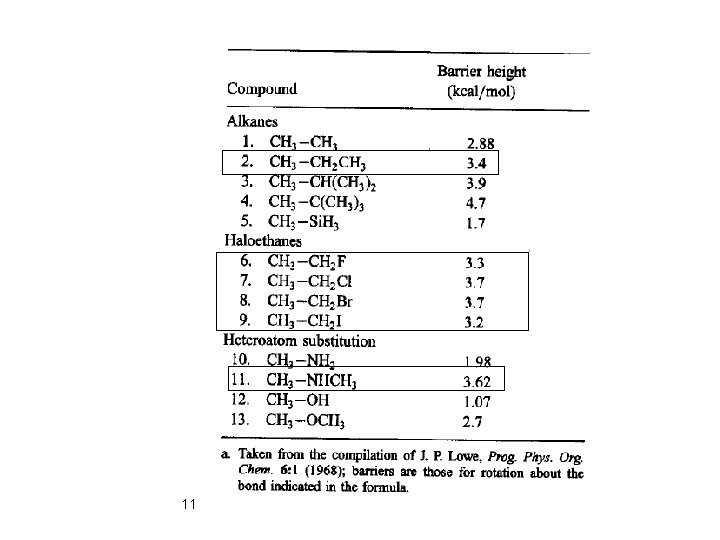
Substitution of a methyl group for hydrogen on one of the carbon atoms produces an increase of 0. 4– 0. 6 kcal/mol in the height of the rotational energy barrier. 6


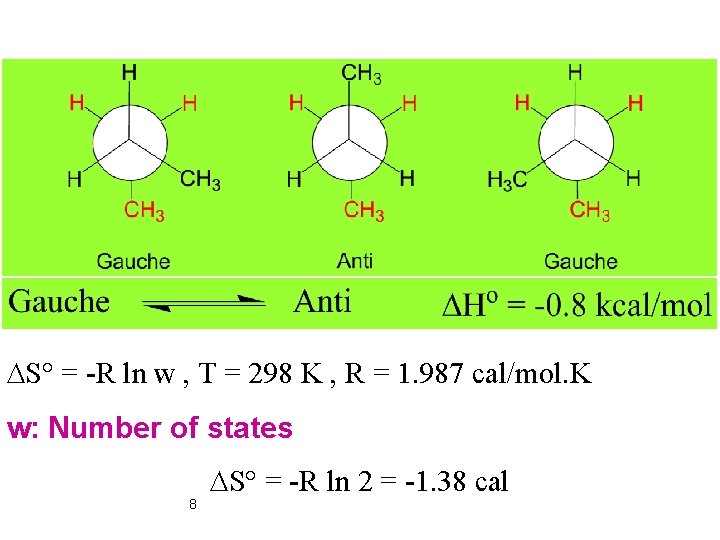
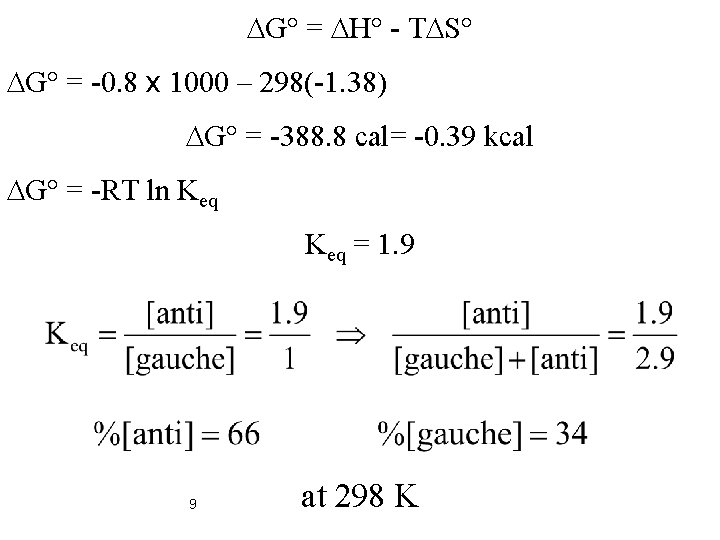
DS° = -R ln w , T = 298 K , R = 1. 987 cal/mol. K w: Number of states 8 DS° = -R ln 2 = -1. 38 cal

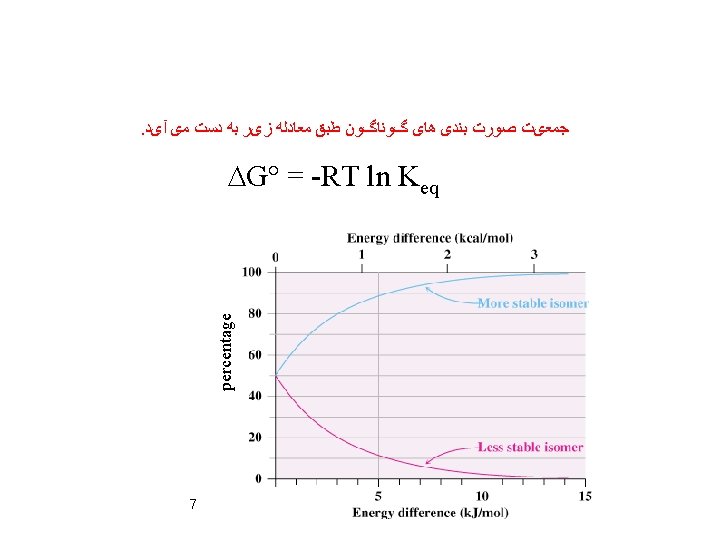
DG° = DH° - TDS° DG° = -0. 8 x 1000 – 298(-1. 38) DG° = -388. 8 cal= -0. 39 kcal DG° = -RT ln Keq = 1. 9 9 at 298 K

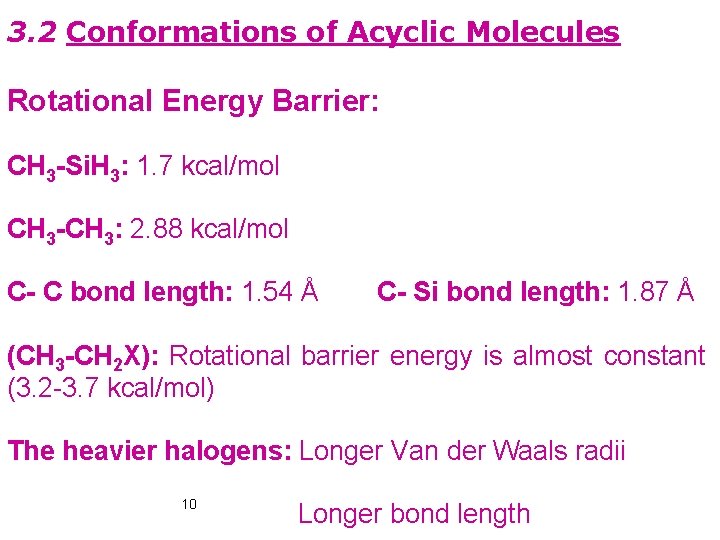
3. 2 Conformations of Acyclic Molecules Rotational Energy Barrier: CH 3 -Si. H 3: 1. 7 kcal/mol CH 3 -CH 3: 2. 88 kcal/mol C- C bond length: 1. 54 Å C- Si bond length: 1. 87 Å (CH 3 -CH 2 X): Rotational barrier energy is almost constant (3. 2 -3. 7 kcal/mol) The heavier halogens: Longer Van der Waals radii 10 Longer bond length

11

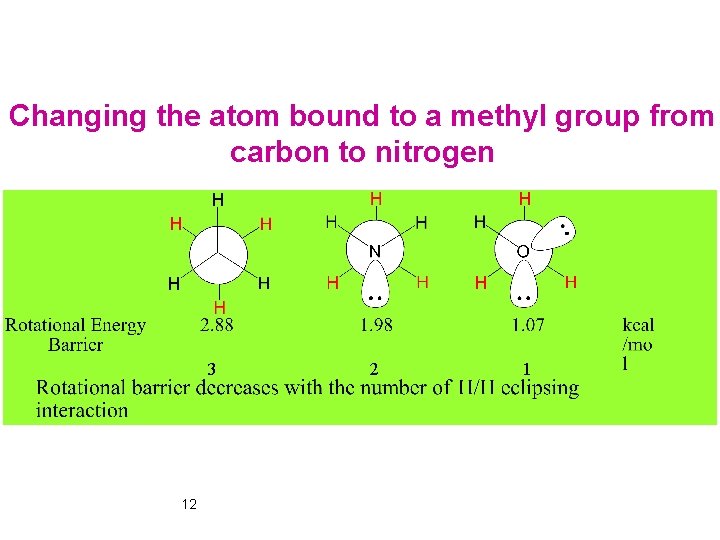
Changing the atom bound to a methyl group from carbon to nitrogen 3 12 2 1

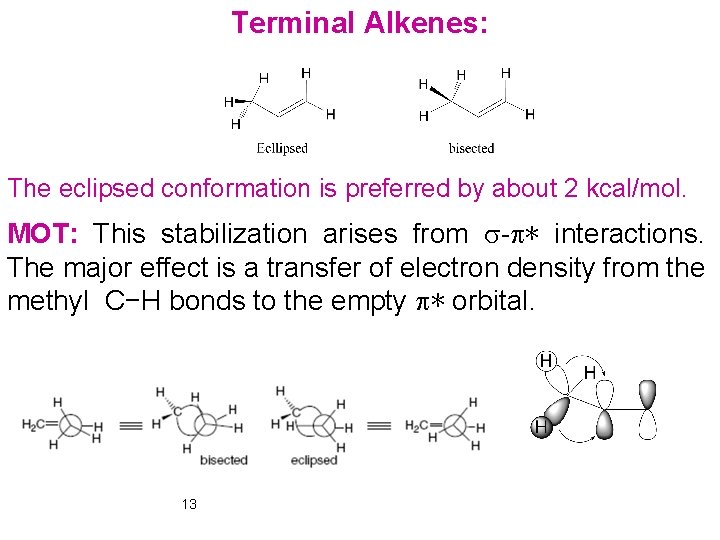
Terminal Alkenes: The eclipsed conformation is preferred by about 2 kcal/mol. MOT: This stabilization arises from s-p∗ interactions. The major effect is a transfer of electron density from the methyl C−H bonds to the empty p∗ orbital. 13

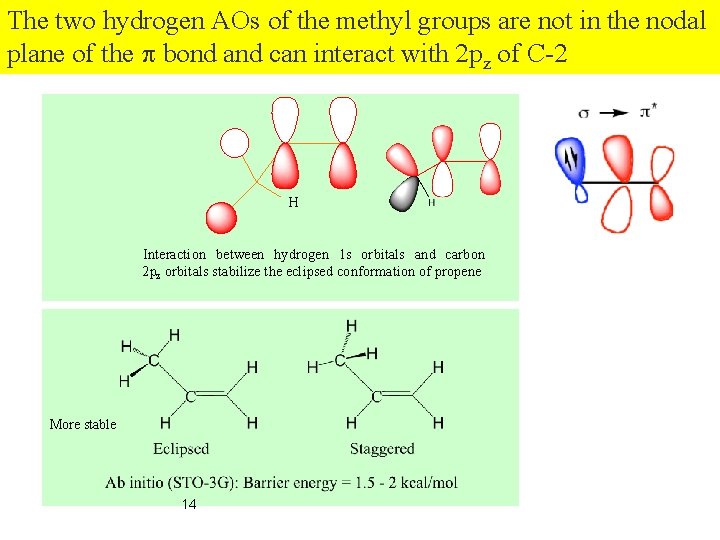
The two hydrogen AOs of the methyl groups are not in the nodal plane of the p bond and can interact with 2 pz of C-2 H Interaction between hydrogen 1 s orbitals and carbon 2 pz orbitals stabilize the eclipsed conformation of propene. More stable 14

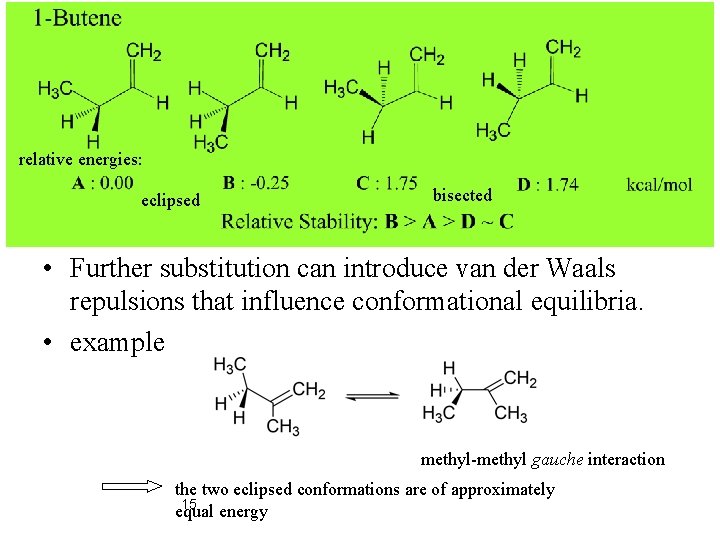
relative energies: eclipsed bisected • Further substitution can introduce van der Waals repulsions that influence conformational equilibria. • example methyl-methyl gauche interaction the two eclipsed conformations are of approximately 15 equal energy

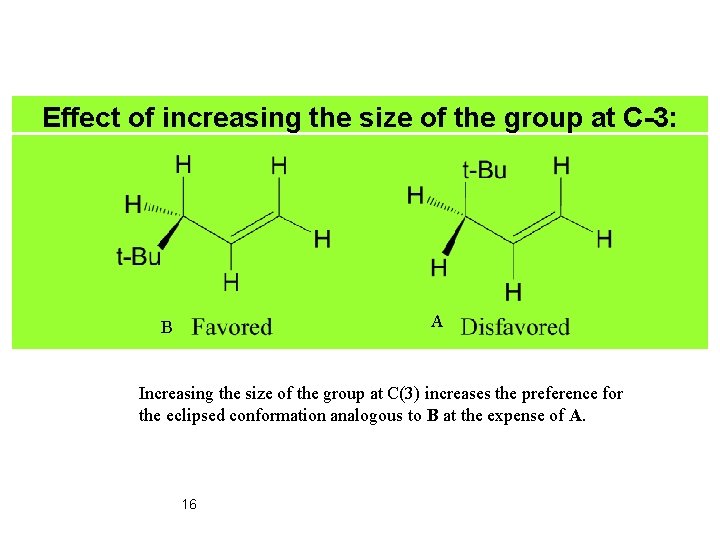
Effect of increasing the size of the group at C-3: A B Increasing the size of the group at C(3) increases the preference for the eclipsed conformation analogous to B at the expense of A. 16

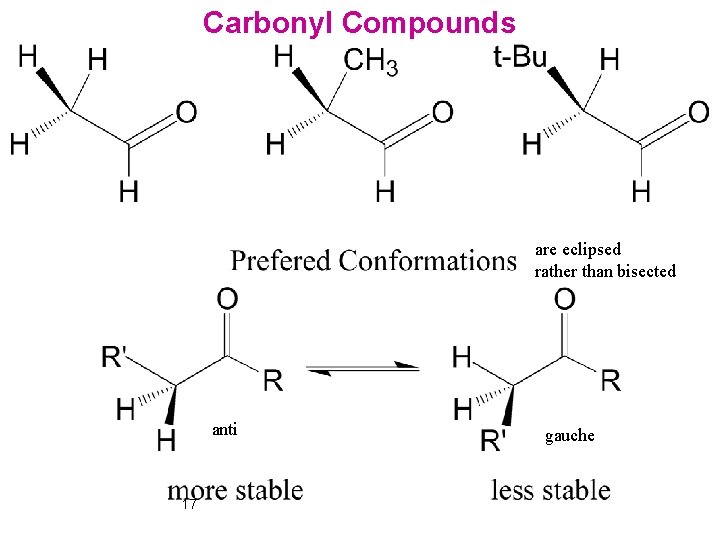
Carbonyl Compounds are eclipsed rather than bisected anti 17 gauche


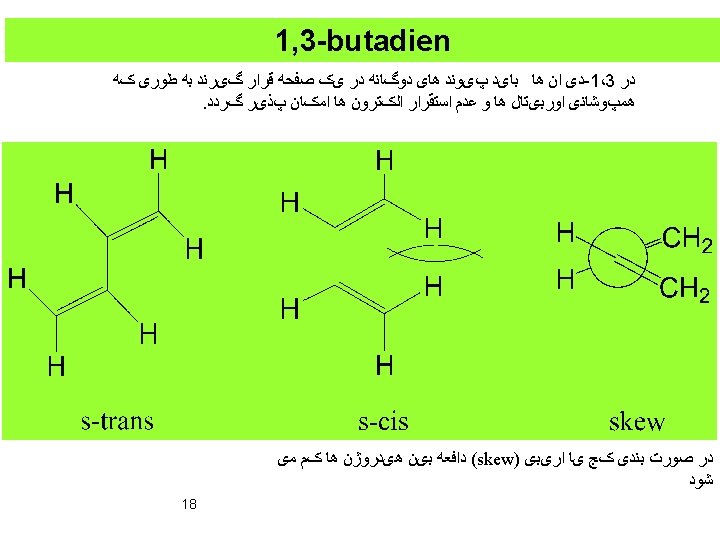
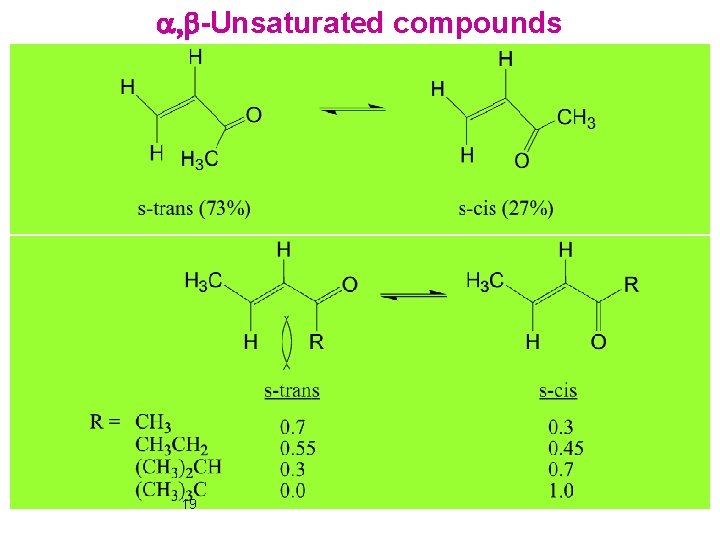
a, b-Unsaturated compounds 19

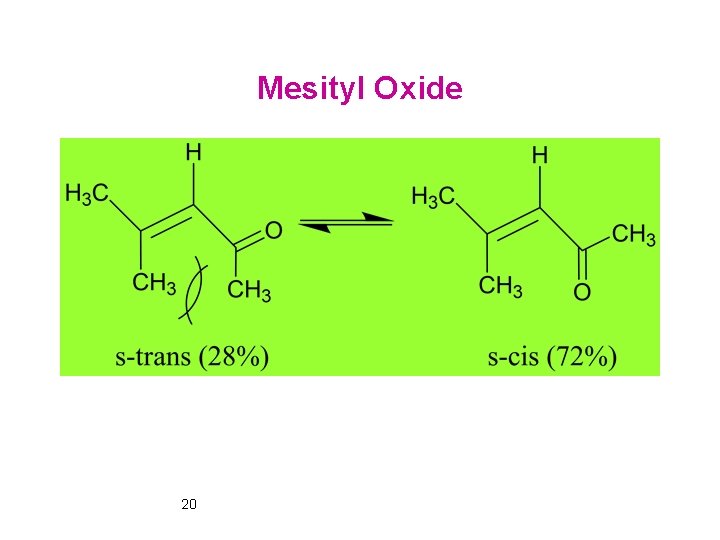
Mesityl Oxide 20




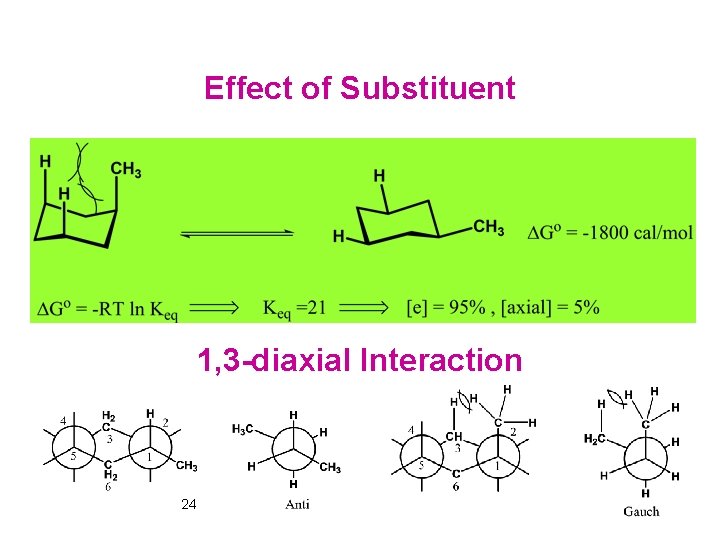
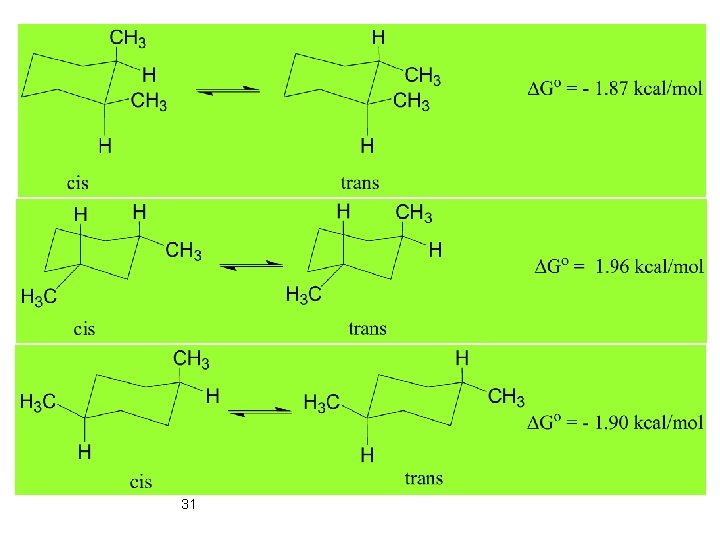
Effect of Substituent 1, 3 -diaxial Interaction 24

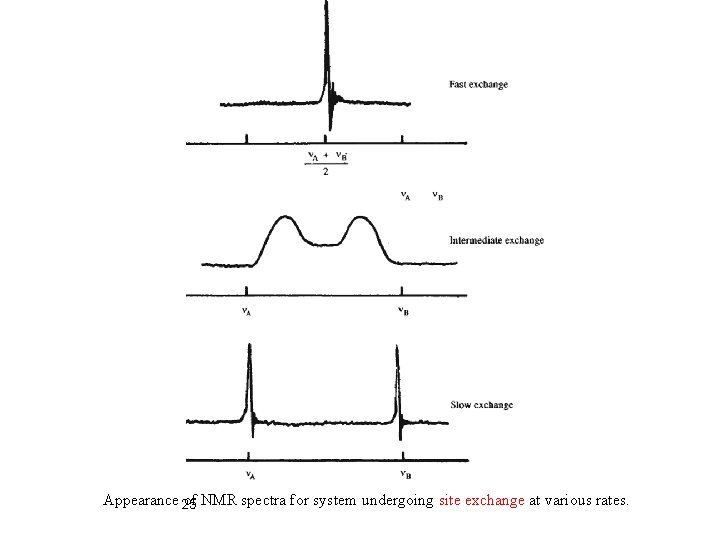
Appearance 25 of NMR spectra for system undergoing site exchange at various rates.

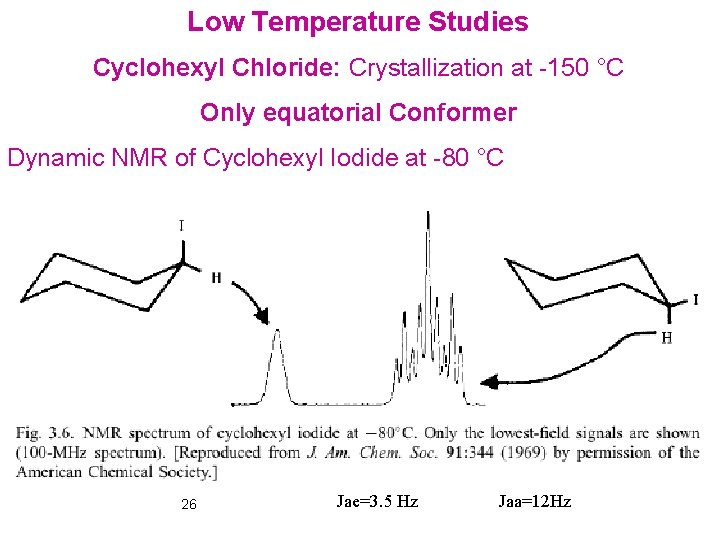
Low Temperature Studies Cyclohexyl Chloride: Crystallization at -150 °C Only equatorial Conformer Dynamic NMR of Cyclohexyl Iodide at -80 °C 26 Jae=3. 5 Hz Jaa=12 Hz
27

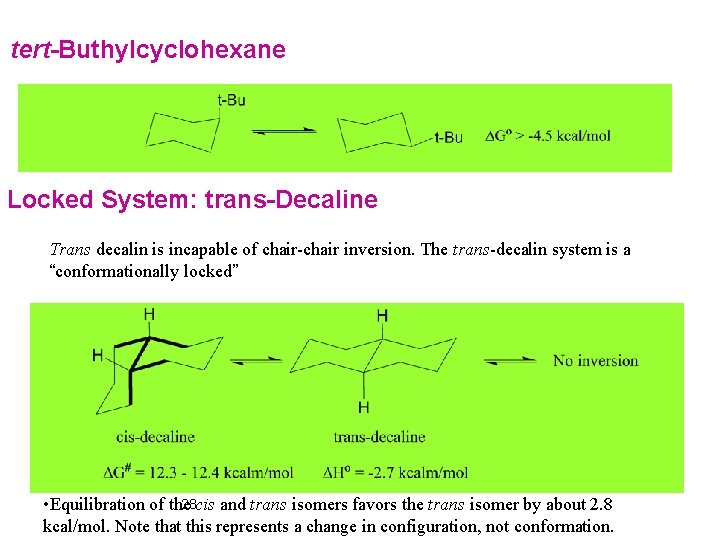
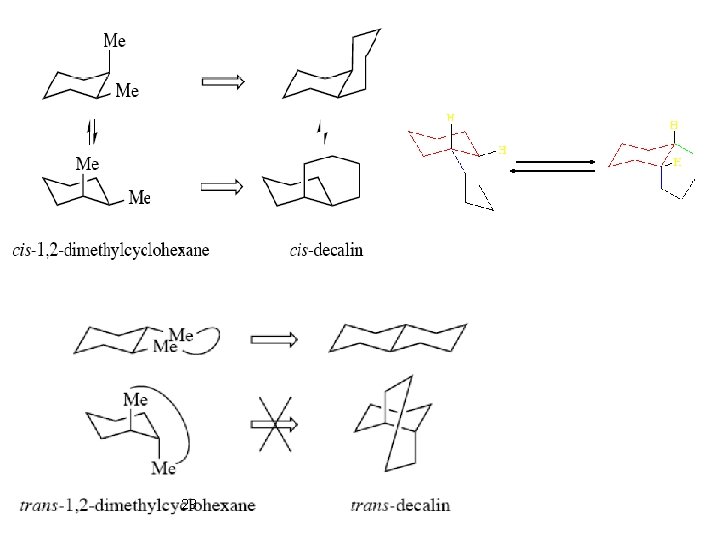
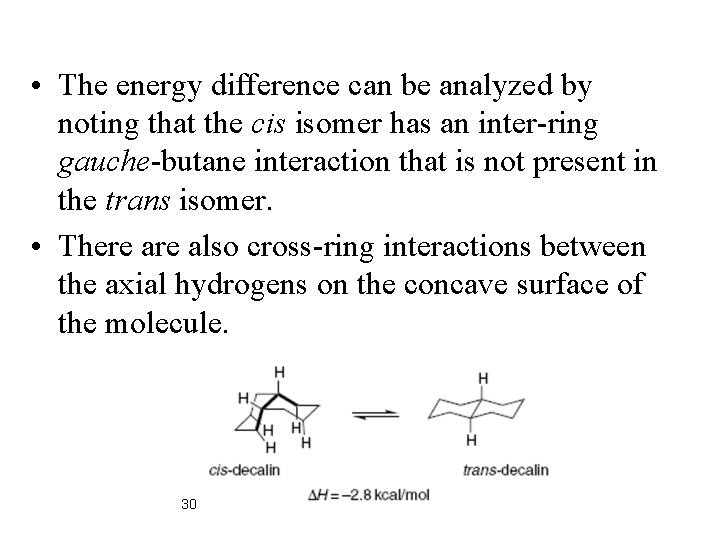
tert-Buthylcyclohexane Locked System: trans-Decaline Trans decalin is incapable of chair-chair inversion. The trans-decalin system is a “conformationally locked” 28 cis and trans isomers favors the trans isomer by about 2. 8 • Equilibration of the kcal/mol. Note that this represents a change in configuration, not conformation.

29

• The energy difference can be analyzed by noting that the cis isomer has an inter-ring gauche-butane interaction that is not present in the trans isomer. • There also cross-ring interactions between the axial hydrogens on the concave surface of the molecule. 30

31

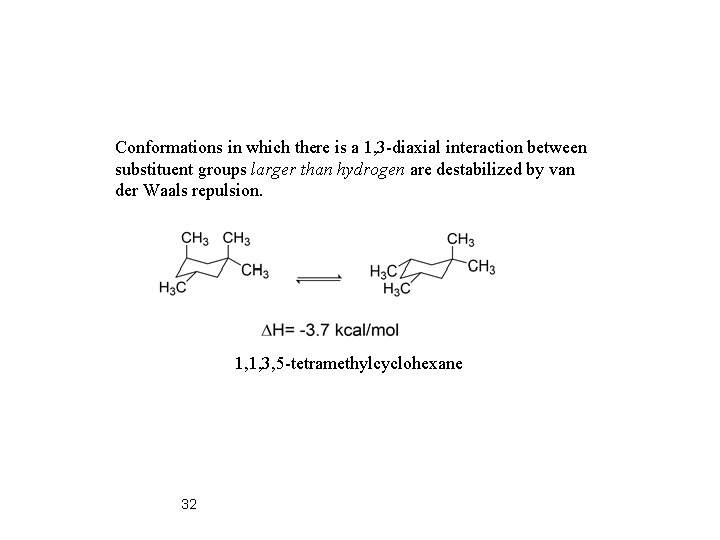
Conformations in which there is a 1, 3 -diaxial interaction between substituent groups larger than hydrogen are destabilized by van der Waals repulsion. 1, 1, 3, 5 -tetramethylcyclohexane 32

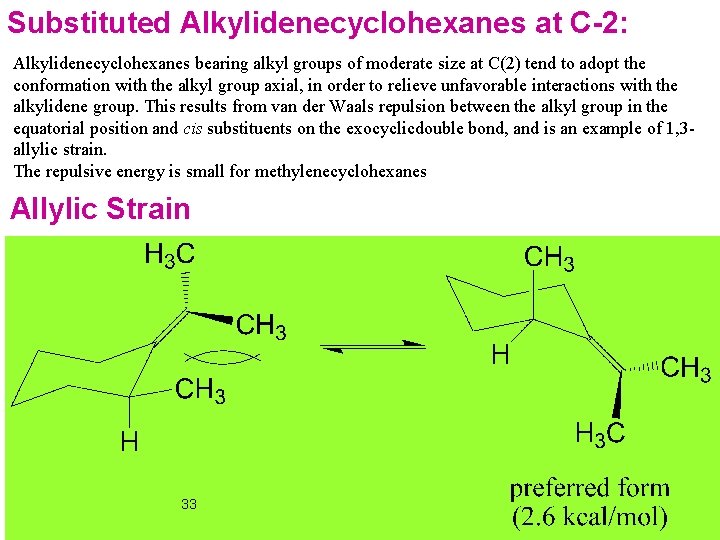
Substituted Alkylidenecyclohexanes at C-2: Alkylidenecyclohexanes bearing alkyl groups of moderate size at C(2) tend to adopt the conformation with the alkyl group axial, in order to relieve unfavorable interactions with the alkylidene group. This results from van der Waals repulsion between the alkyl group in the equatorial position and cis substituents on the exocyclicdouble bond, and is an example of 1, 3 allylic strain. The repulsive energy is small for methylenecyclohexanes Allylic Strain 33

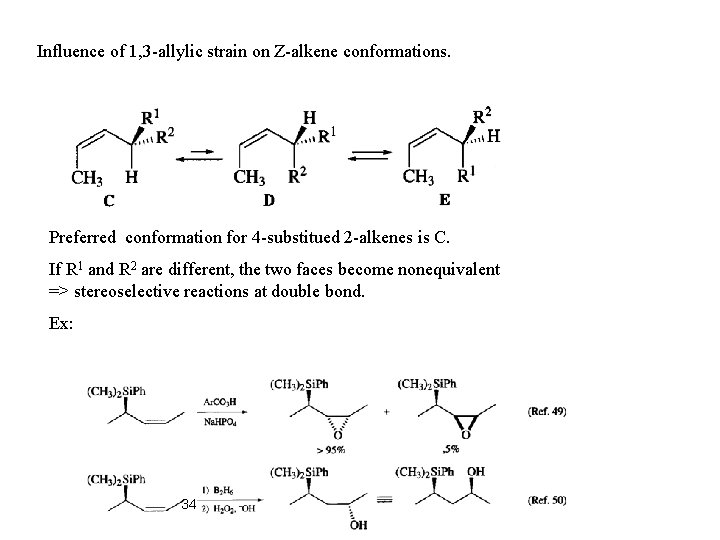
Influence of 1, 3 -allylic strain on Z-alkene conformations. Preferred conformation for 4 -substitued 2 -alkenes is C. If R 1 and R 2 are different, the two faces become nonequivalent => stereoselective reactions at double bond. Ex: 34

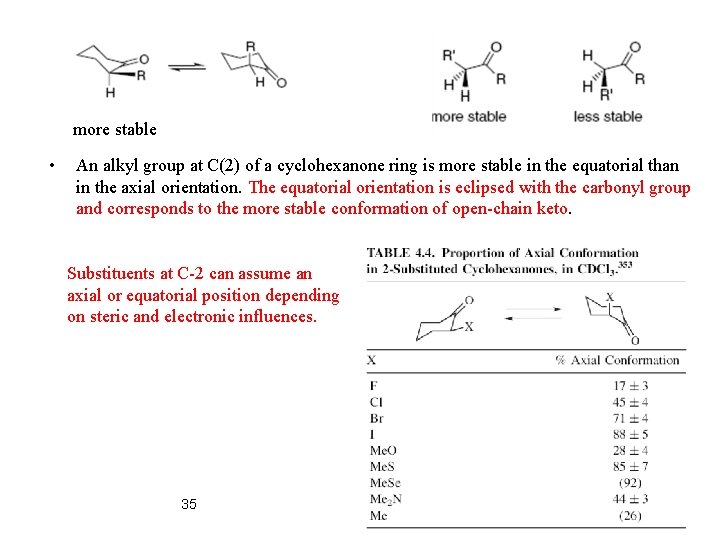
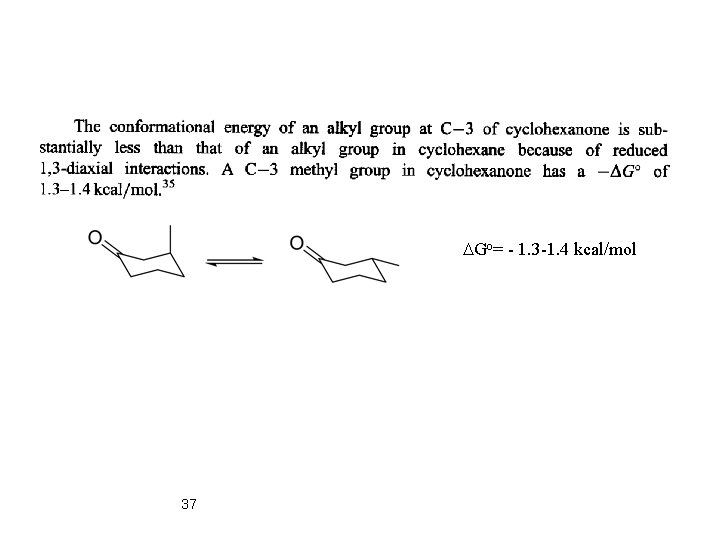
more stable • An alkyl group at C(2) of a cyclohexanone ring is more stable in the equatorial than in the axial orientation. The equatorial orientation is eclipsed with the carbonyl group and corresponds to the more stable conformation of open-chain keto. Substituents at C-2 can assume an axial or equatorial position depending on steric and electronic influences. 35

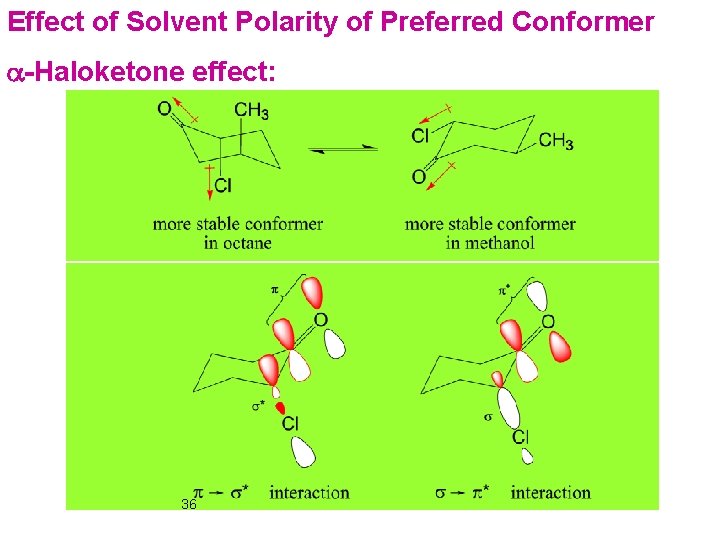
Effect of Solvent Polarity of Preferred Conformer a-Haloketone effect: 36

DGo= - 1. 3 -1. 4 kcal/mol 37

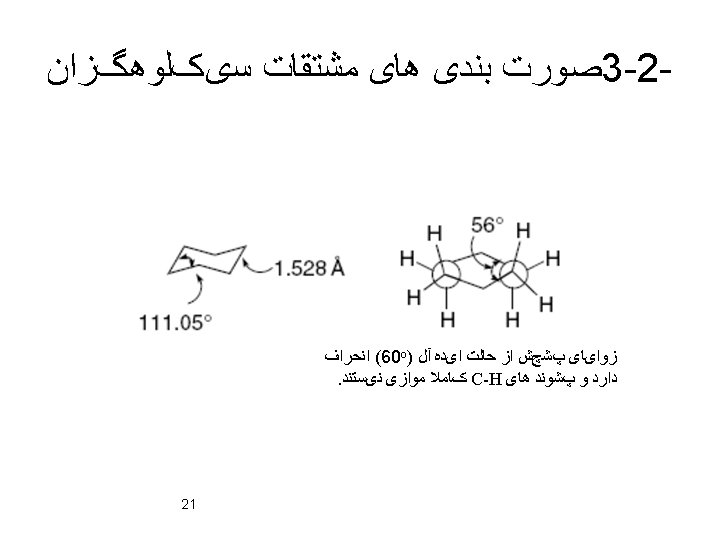
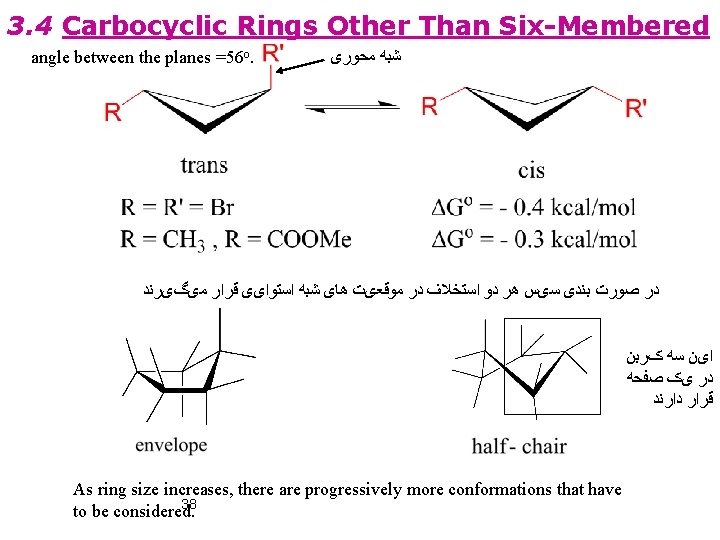
3. 4 Carbocyclic Rings Other Than Six-Membered angle between the planes =56 o. ﺷﺒﻪ ﻣﺤﻮﺭی ﺩﺭ ﺻﻮﺭﺕ ﺑﻨﺪی ﺳیﺲ ﻫﺮ ﺩﻭ ﺍﺳﺘﺨﻼﻑ ﺩﺭ ﻣﻮﻗﻌیﺖ ﻫﺎی ﺷﺒﻪ ﺍﺳﺘﻮﺍیی ﻗﺮﺍﺭ ﻣیگیﺮﻧﺪ ﺍیﻦ ﺳﻪ کﺮﺑﻦ ﺩﺭ یک ﺻﻔﺤﻪ ﻗﺮﺍﺭ ﺩﺍﺭﻧﺪ As ring size increases, there are progressively more conformations that have 38 to be considered.

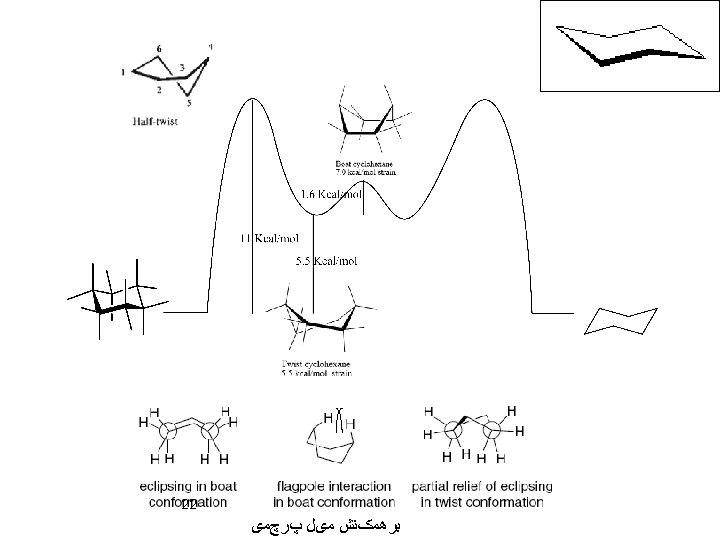
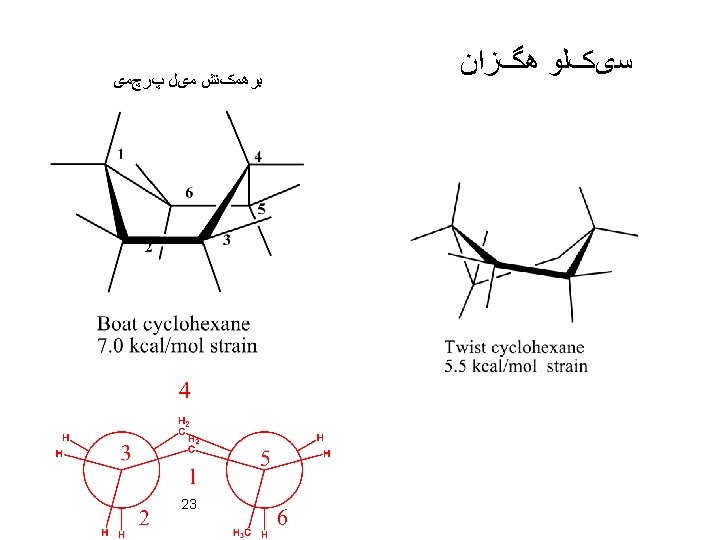
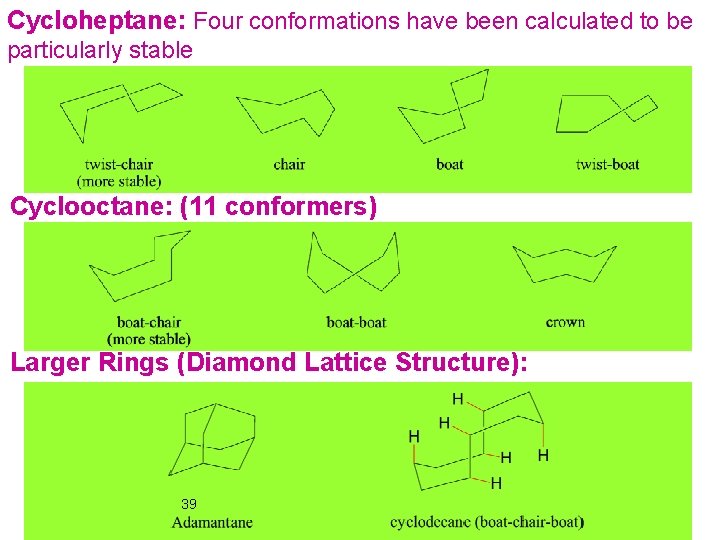
Cycloheptane: Four conformations have been calculated to be particularly stable Cyclooctane: (11 conformers) Larger Rings (Diamond Lattice Structure): 39

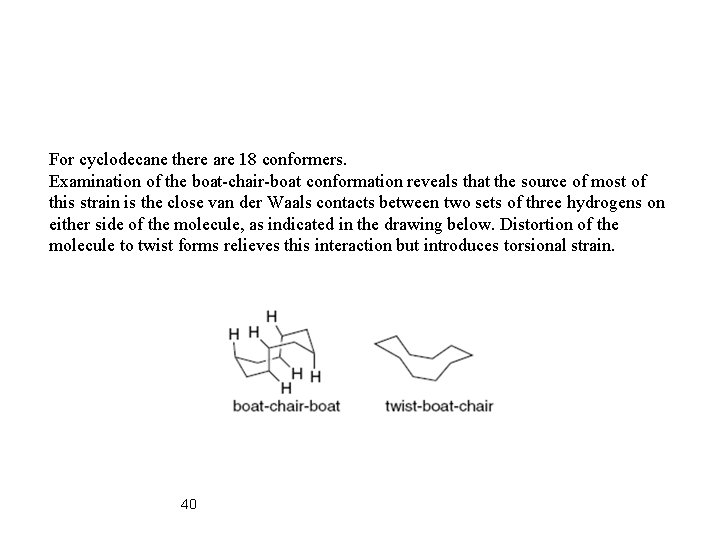
For cyclodecane there are 18 conformers. Examination of the boat-chair-boat conformation reveals that the source of most of this strain is the close van der Waals contacts between two sets of three hydrogens on either side of the molecule, as indicated in the drawing below. Distortion of the molecule to twist forms relieves this interaction but introduces torsional strain. 40

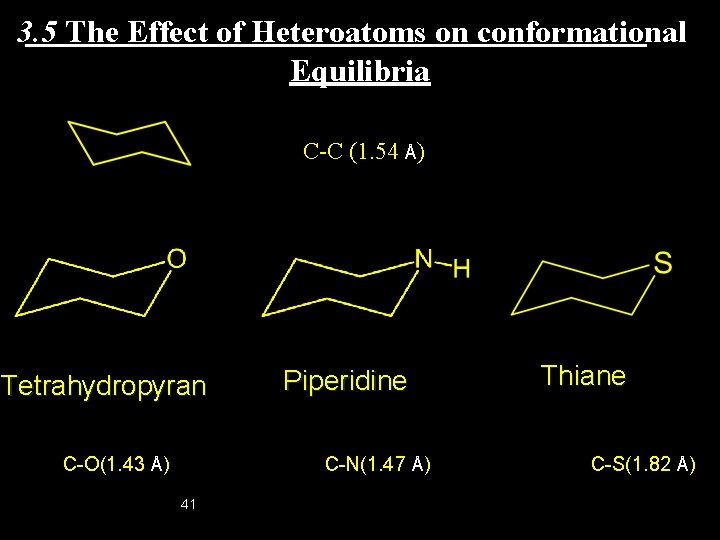
3. 5 The Effect of Heteroatoms on conformational Equilibria C-C (1. 54 Å) Tetrahydropyran C-O(1. 43 Å) Piperidine C-N(1. 47 Å) 41 Thiane C-S(1. 82 Å)

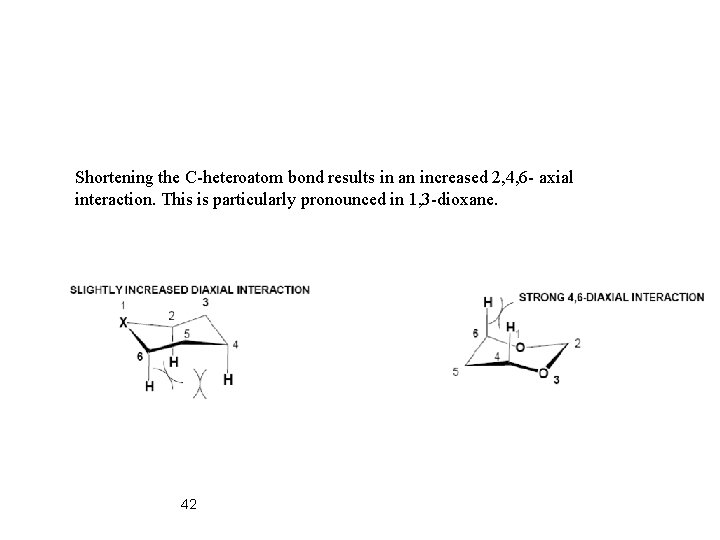
Shortening the C-heteroatom bond results in an increased 2, 4, 6 - axial interaction. This is particularly pronounced in 1, 3 -dioxane. 42

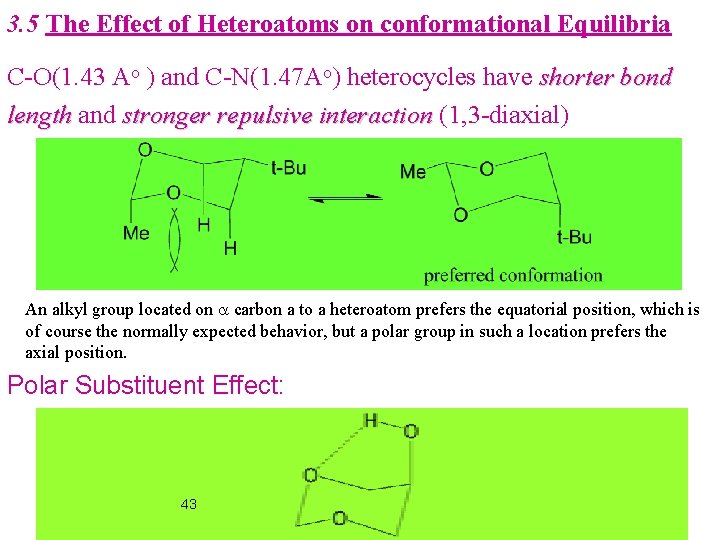
3. 5 The Effect of Heteroatoms on conformational Equilibria C-O(1. 43 Ao ) and C-N(1. 47 Ao) heterocycles have shorter bond length and stronger repulsive interaction (1, 3 -diaxial) An alkyl group located on a carbon a to a heteroatom prefers the equatorial position, which is of course the normally expected behavior, but a polar group in such a location prefers the axial position. Polar Substituent Effect: 43

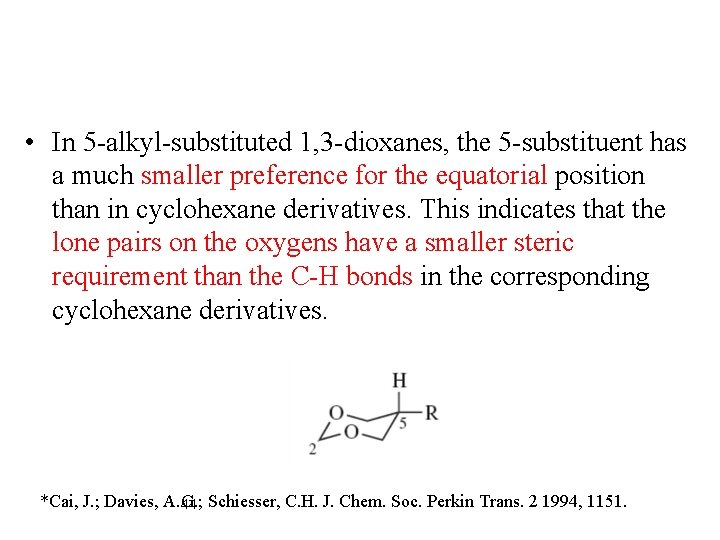
• In 5 -alkyl-substituted 1, 3 -dioxanes, the 5 -substituent has a much smaller preference for the equatorial position than in cyclohexane derivatives. This indicates that the lone pairs on the oxygens have a smaller steric requirement than the C-H bonds in the corresponding cyclohexane derivatives. *Cai, J. ; Davies, A. G. ; 44 Schiesser, C. H. J. Chem. Soc. Perkin Trans. 2 1994, 1151.

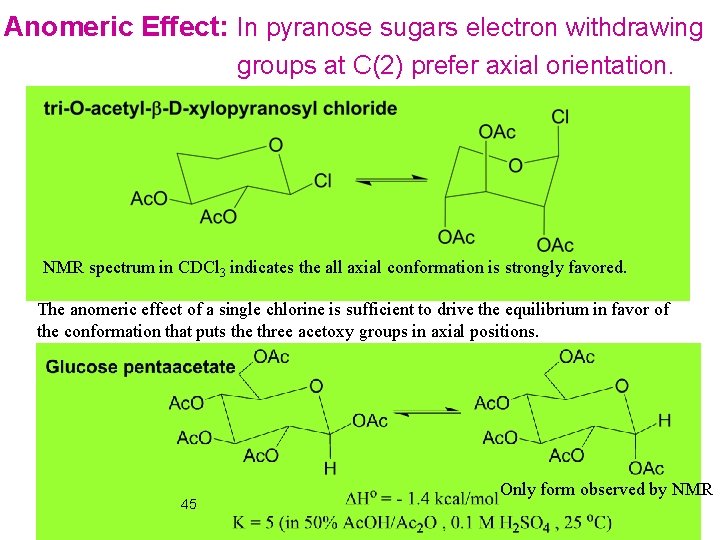
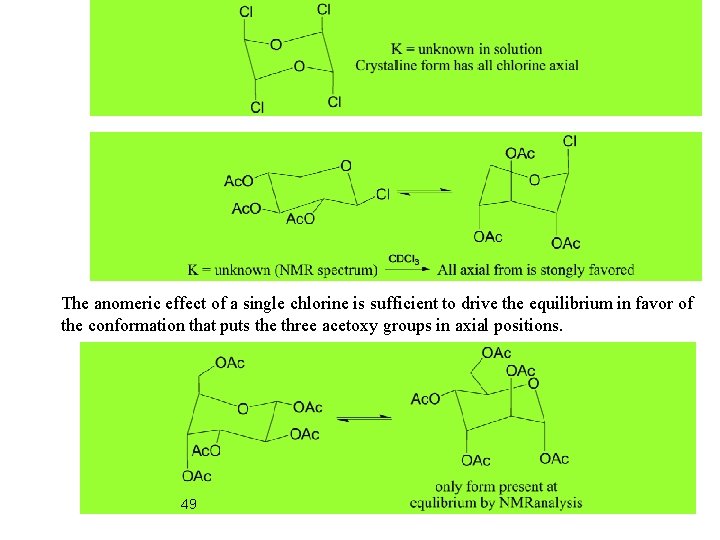
Anomeric Effect: In pyranose sugars electron withdrawing groups at C(2) prefer axial orientation. NMR spectrum in CDCl 3 indicates the all axial conformation is strongly favored. The anomeric effect of a single chlorine is sufficient to drive the equilibrium in favor of the conformation that puts the three acetoxy groups in axial positions. 45 Only form observed by NMR

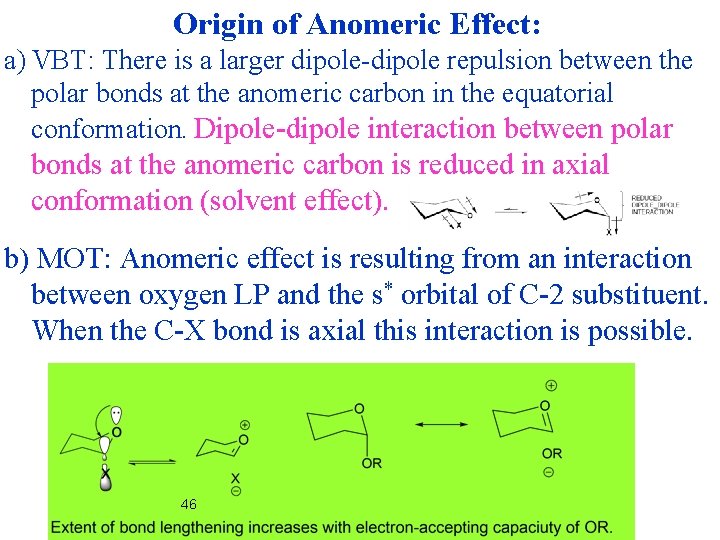
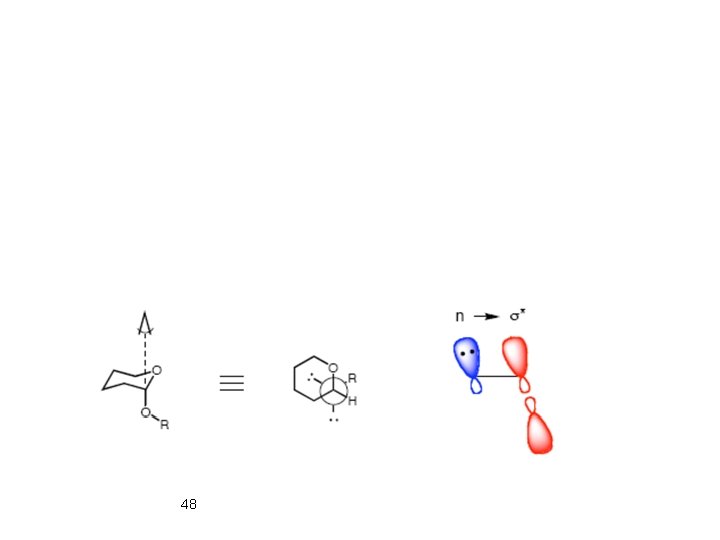
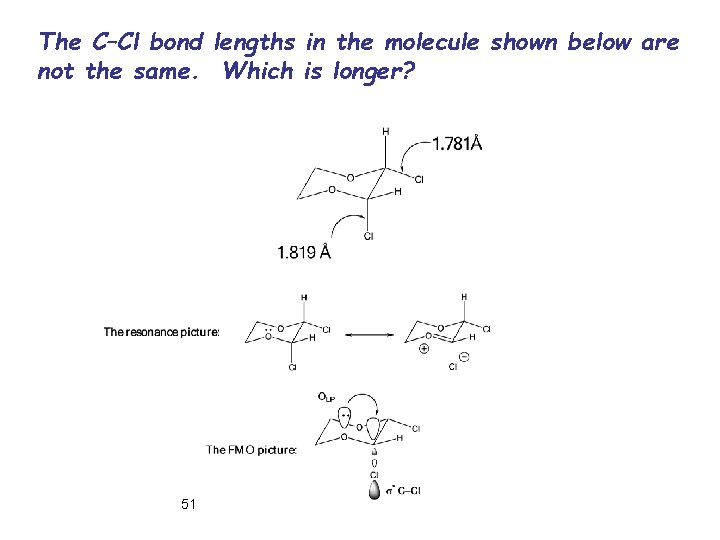
Origin of Anomeric Effect: a) VBT: There is a larger dipole-dipole repulsion between the polar bonds at the anomeric carbon in the equatorial conformation. Dipole-dipole interaction between polar bonds at the anomeric carbon is reduced in axial conformation (solvent effect). b) MOT: Anomeric effect is resulting from an interaction between oxygen LP and the s* orbital of C-2 substituent. When the C-X bond is axial this interaction is possible. 46

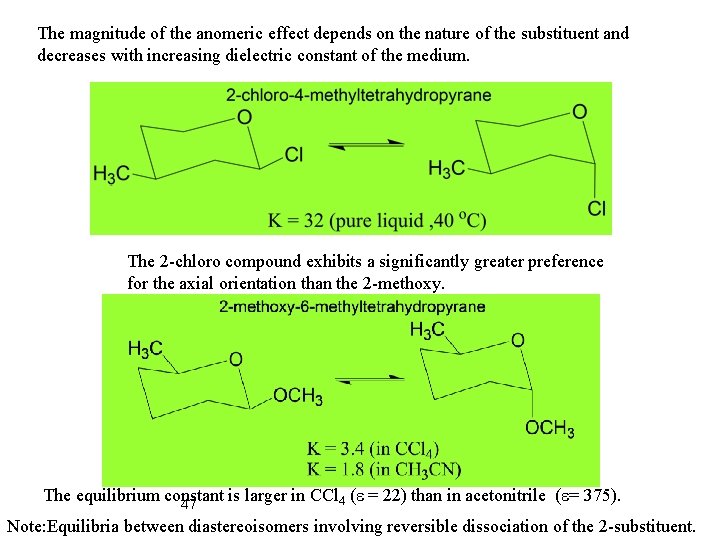
The magnitude of the anomeric effect depends on the nature of the substituent and decreases with increasing dielectric constant of the medium. The 2 -chloro compound exhibits a significantly greater preference for the axial orientation than the 2 -methoxy. The equilibrium constant is larger in CCl 4 (e = 22) than in acetonitrile (e= 375). 47 Note: Equilibria between diastereoisomers involving reversible dissociation of the 2 -substituent.

48

The anomeric effect of a single chlorine is sufficient to drive the equilibrium in favor of the conformation that puts the three acetoxy groups in axial positions. 49

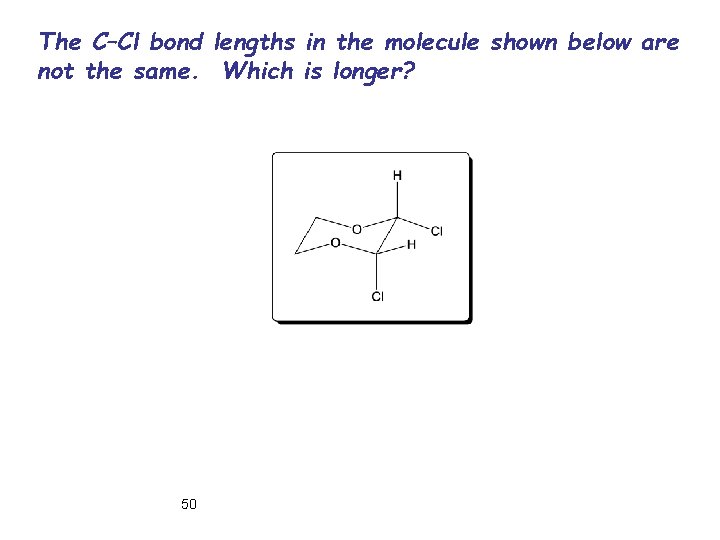
The C–Cl bond lengths in the molecule shown below are not the same. Which is longer? 50

The C–Cl bond lengths in the molecule shown below are not the same. Which is longer? 51

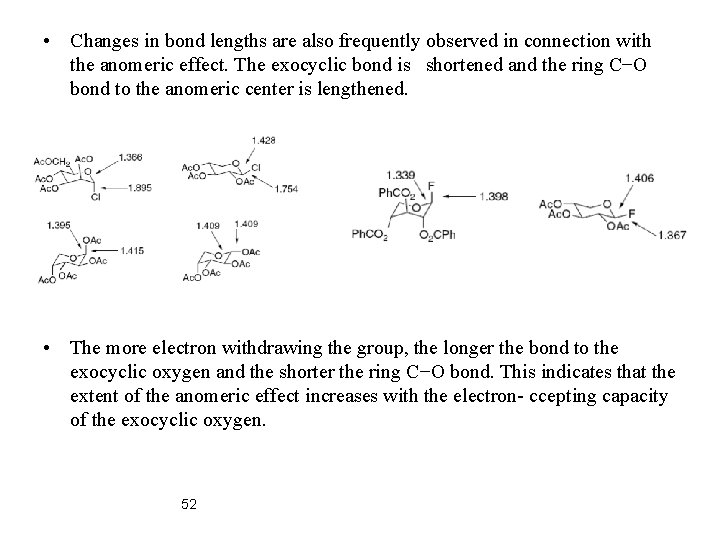
• Changes in bond lengths are also frequently observed in connection with the anomeric effect. The exocyclic bond is shortened and the ring C−O bond to the anomeric center is lengthened. • The more electron withdrawing the group, the longer the bond to the exocyclic oxygen and the shorter the ring C−O bond. This indicates that the extent of the anomeric effect increases with the electron- ccepting capacity of the exocyclic oxygen. 52

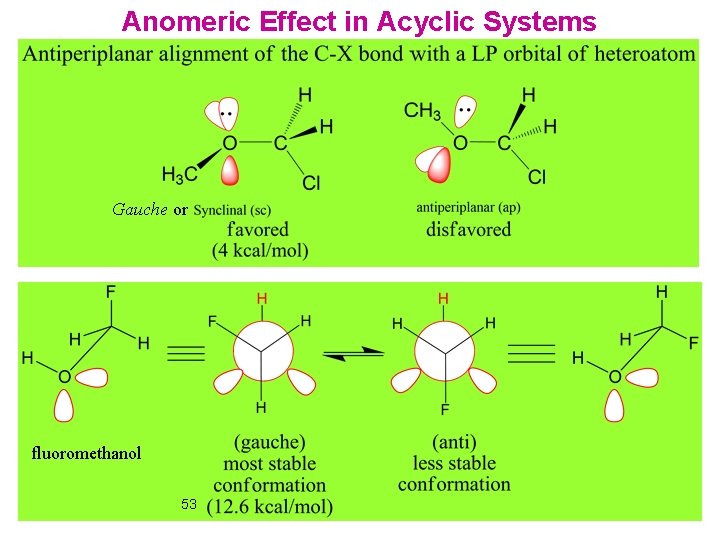
Anomeric Effect in Acyclic Systems Gauche or fluoromethanol 53

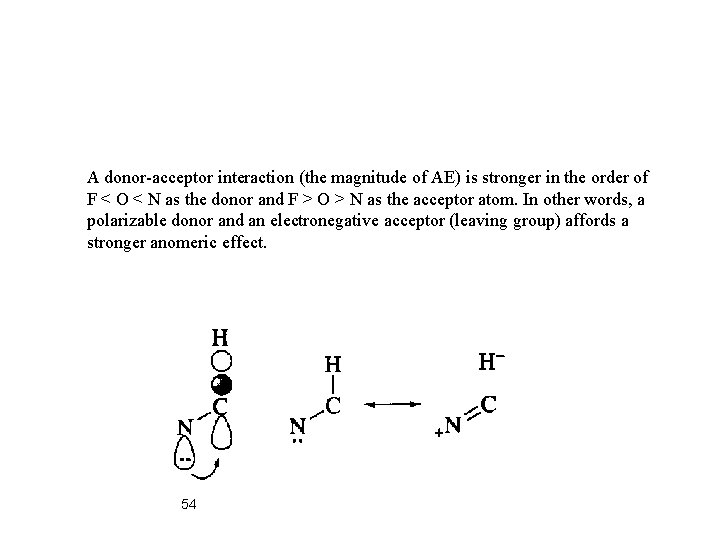
A donor-acceptor interaction (the magnitude of AE) is stronger in the order of F < O < N as the donor and F > O > N as the acceptor atom. In other words, a polarizable donor and an electronegative acceptor (leaving group) affords a stronger anomeric effect. 54

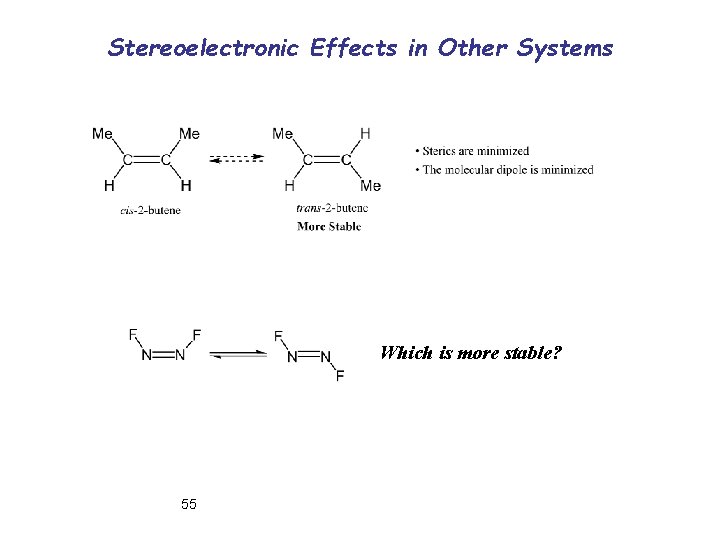
Stereoelectronic Effects in Other Systems Which is more stable? 55

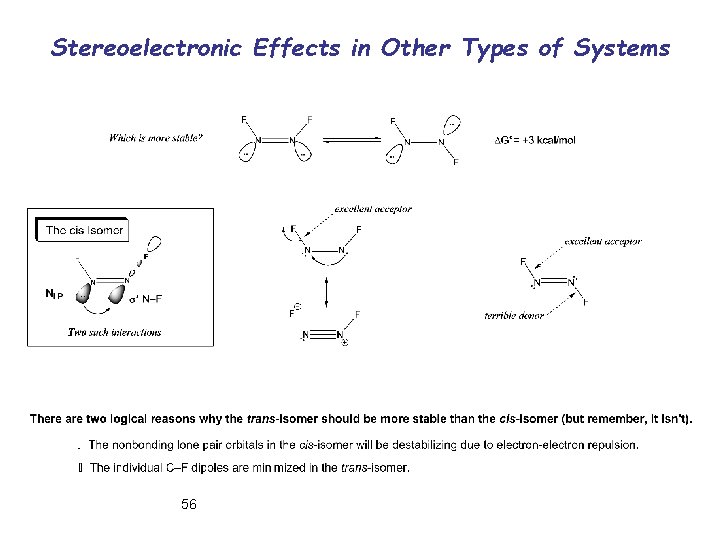
Stereoelectronic Effects in Other Types of Systems 56

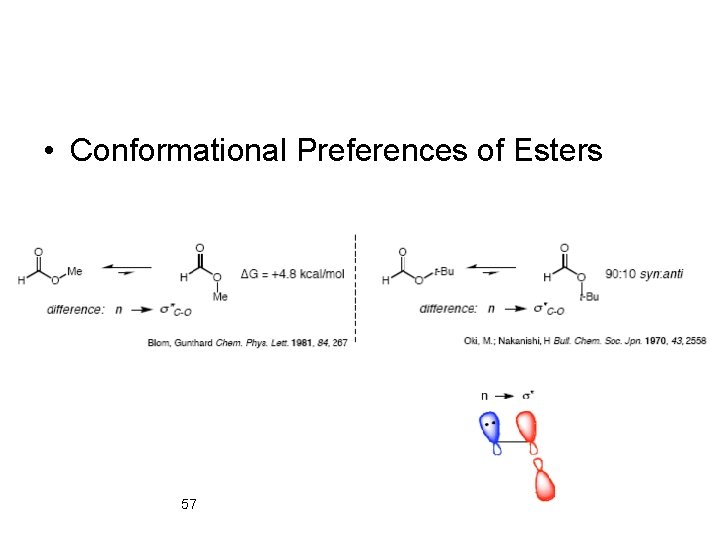
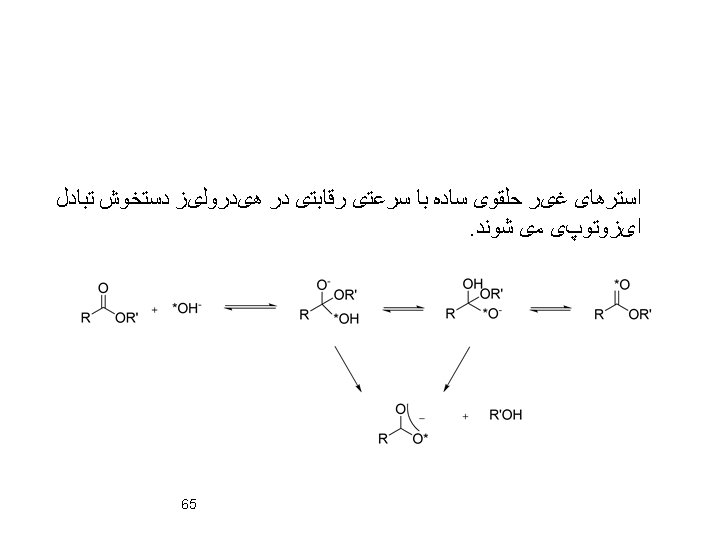
• Conformational Preferences of Esters 57


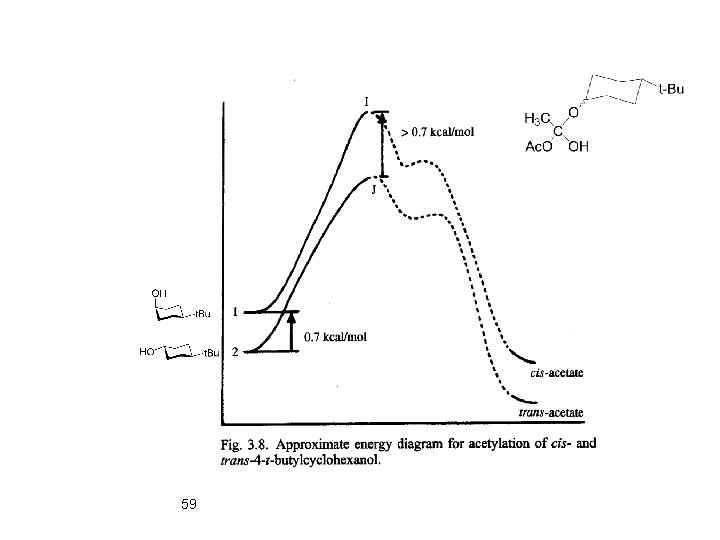
59

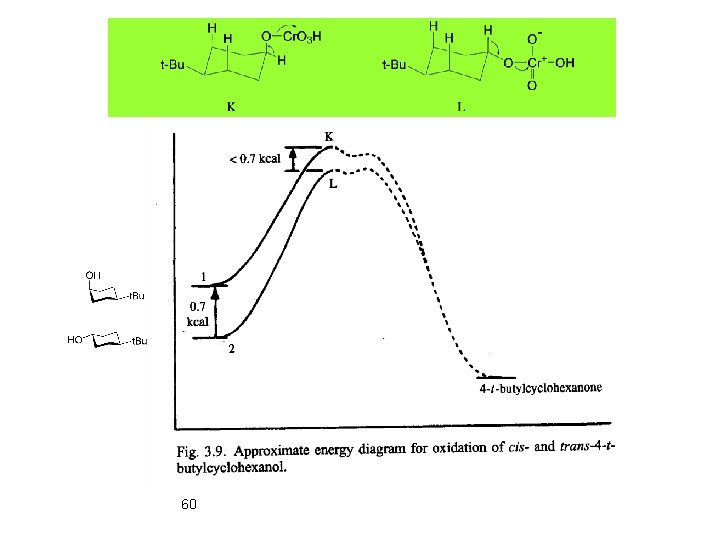
60

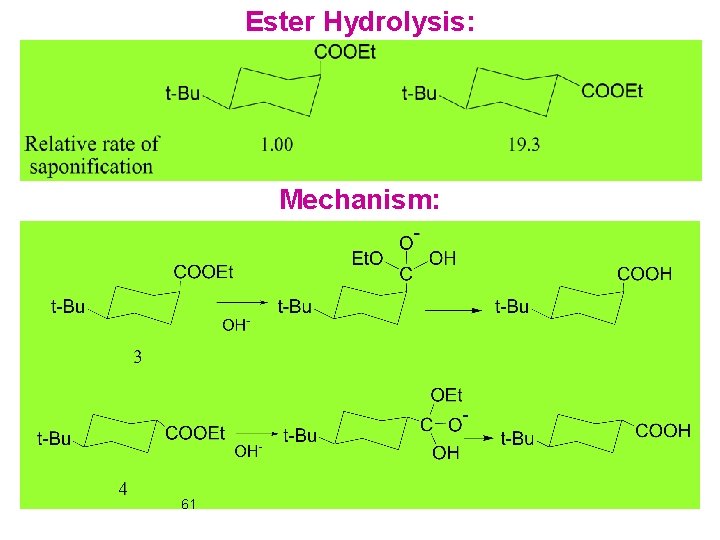
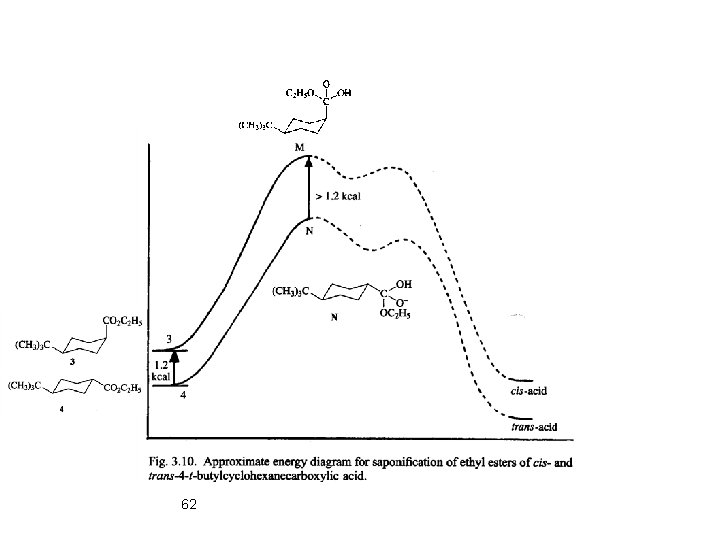
Ester Hydrolysis: Mechanism: 61

62

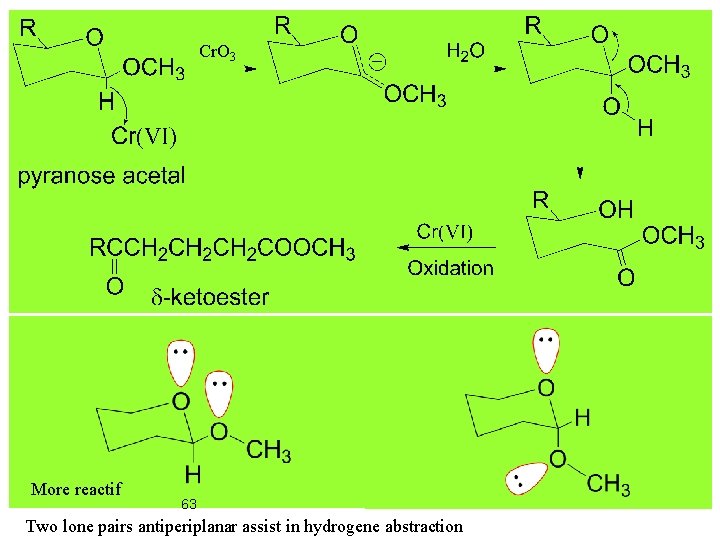
Cr. O 3 More reactif 63 Two lone pairs antiperiplanar assist in hydrogene abstraction

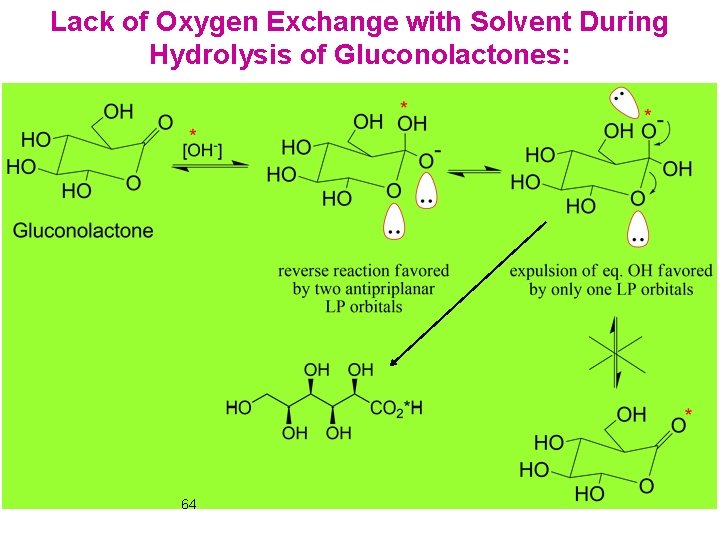
Lack of Oxygen Exchange with Solvent During Hydrolysis of Gluconolactones: 64


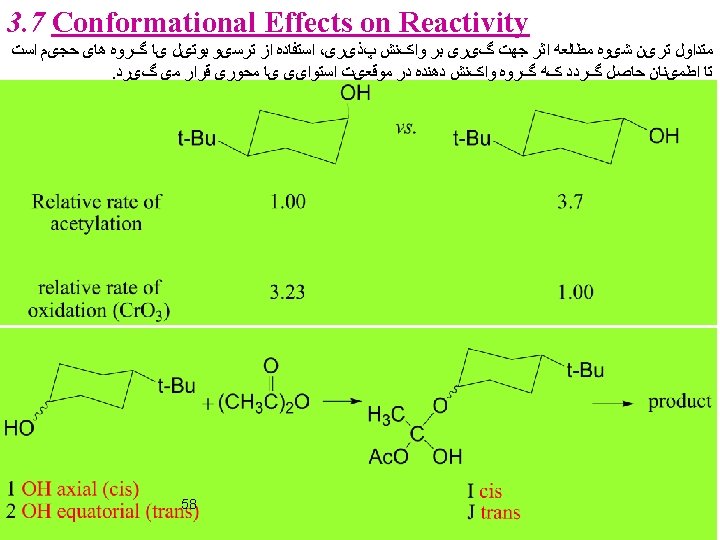
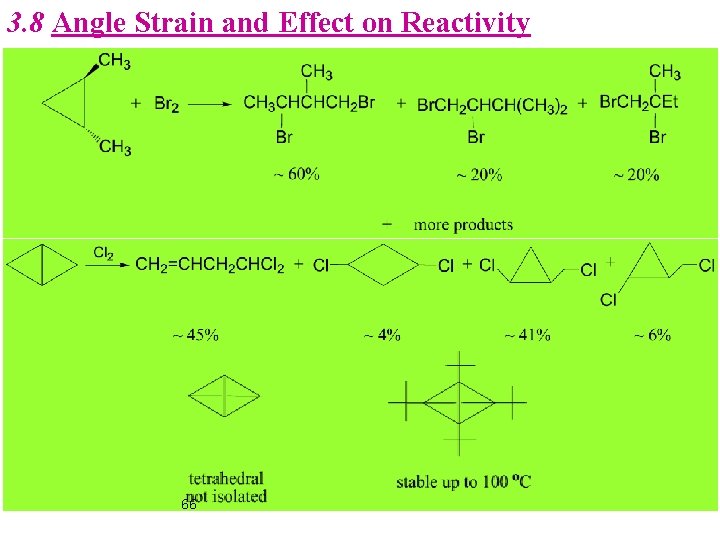
3. 8 Angle Strain and Effect on Reactivity 66

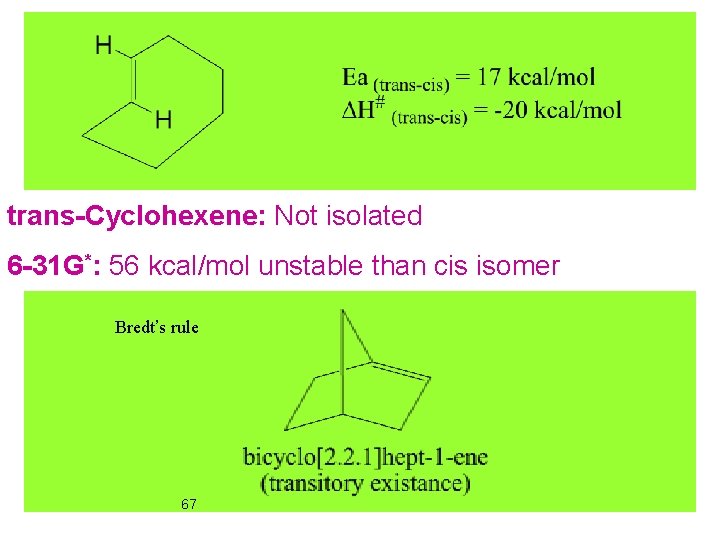
trans-Cyclohexene: Not isolated 6 -31 G*: 56 kcal/mol unstable than cis isomer Bredt’s rule 67

3. 9 Relationship Between Ring Size and Facility of Ring Closure 5 > 6 > 3 > 7 > 4 > 8 -10 Baldwin’s Rule for Ring Closure: a) Ring size b) Hybridization of the carbon at the reaction center. c) The relationship of the reacting bond to the forming ring (endocyclic or exocyclic). 68

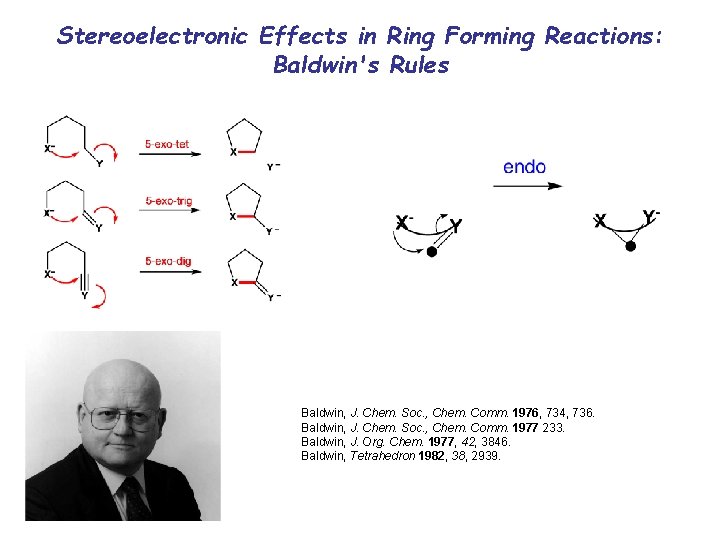
Stereoelectronic Effects in Ring Forming Reactions: Baldwin's Rules Baldwin, J. Chem. Soc. , Chem. Comm. 1976, 734, 736. Baldwin, J. Chem. Soc. , Chem. Comm. 1977 233. Baldwin, J. Org. Chem. 1977, 42, 3846. Baldwin, Tetrahedron 1982, 38, 2939. 69

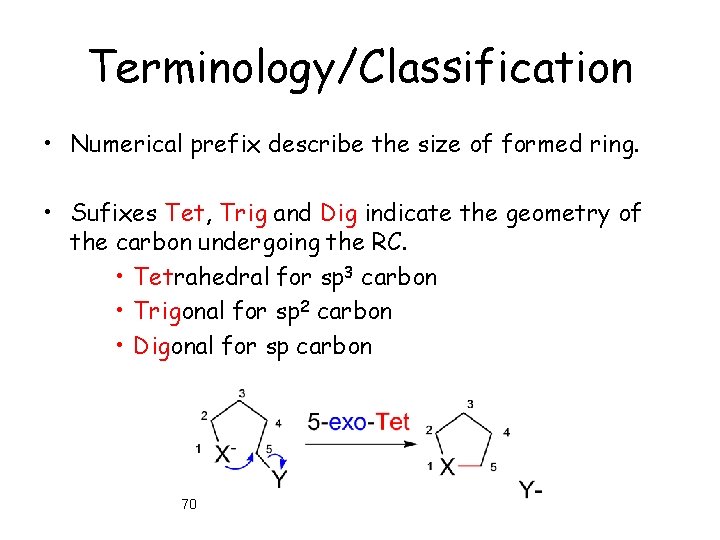
Terminology/Classification • Numerical prefix describe the size of formed ring. • Sufixes Tet, Trig and Dig indicate the geometry of the carbon undergoing the RC. • Tetrahedral for sp 3 carbon • Trigonal for sp 2 carbon • Digonal for sp carbon 70

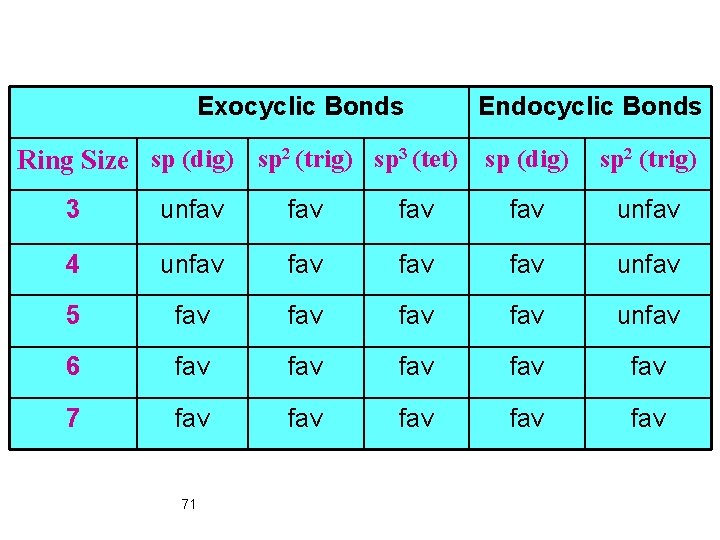
Exocyclic Bonds Endocyclic Bonds Ring Size sp (dig) sp 2 (trig) sp 3 (tet) sp (dig) sp 2 (trig) 3 unfav fav unfav 4 unfav fav unfav 5 fav fav unfav 6 fav fav fav 71

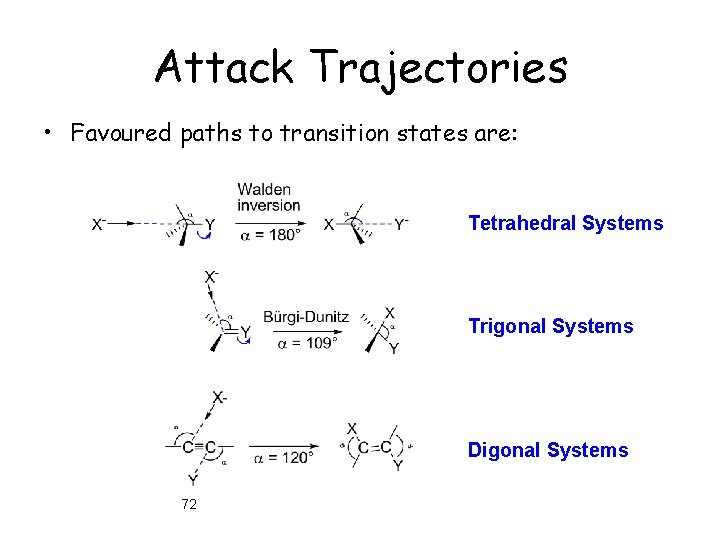
Attack Trajectories • Favoured paths to transition states are: Tetrahedral Systems Trigonal Systems Digonal Systems 72

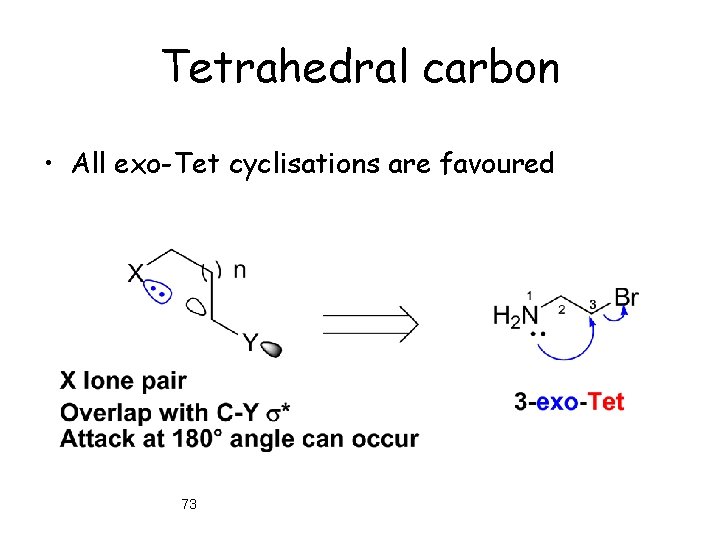
Tetrahedral carbon • All exo-Tet cyclisations are favoured 73

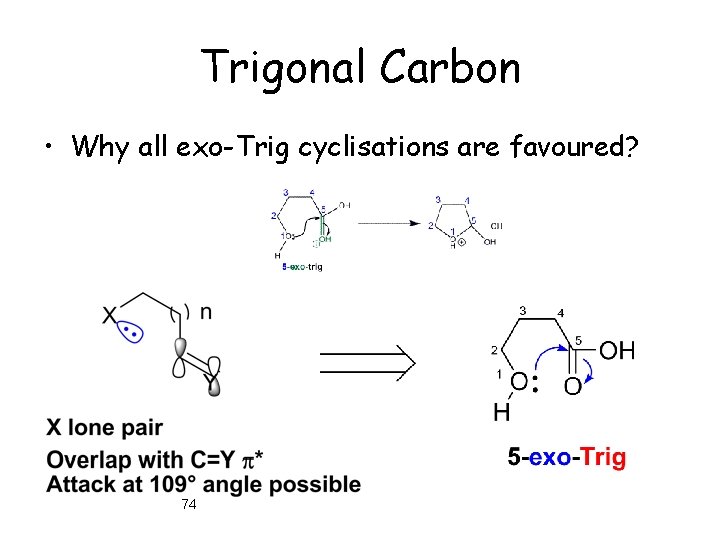
Trigonal Carbon • Why all exo-Trig cyclisations are favoured? 74

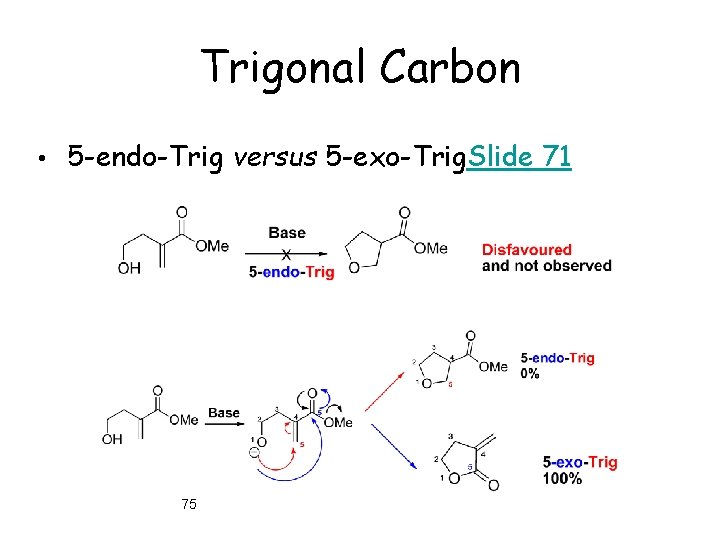
Trigonal Carbon • 5 -endo-Trig versus 5 -exo-Trig. Slide 71 75

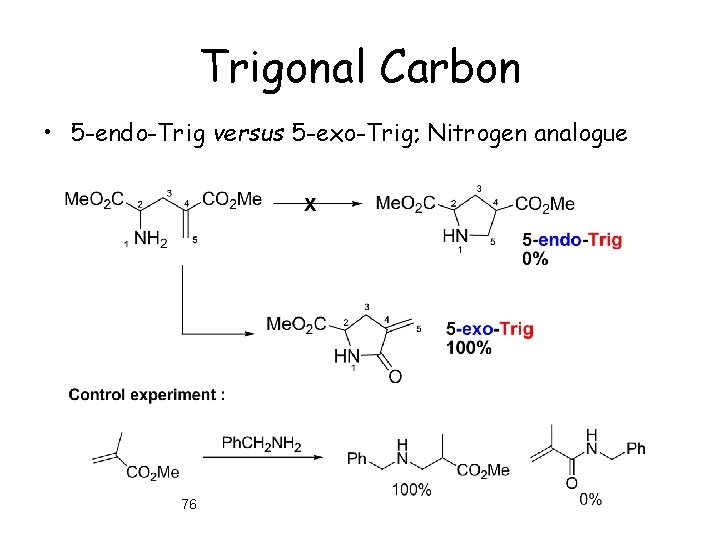
Trigonal Carbon • 5 -endo-Trig versus 5 -exo-Trig; Nitrogen analogue 76

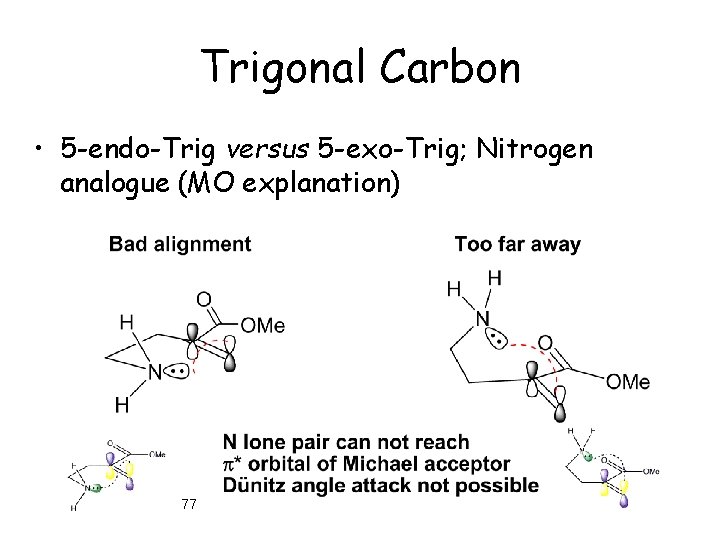
Trigonal Carbon • 5 -endo-Trig versus 5 -exo-Trig; Nitrogen analogue (MO explanation) 77

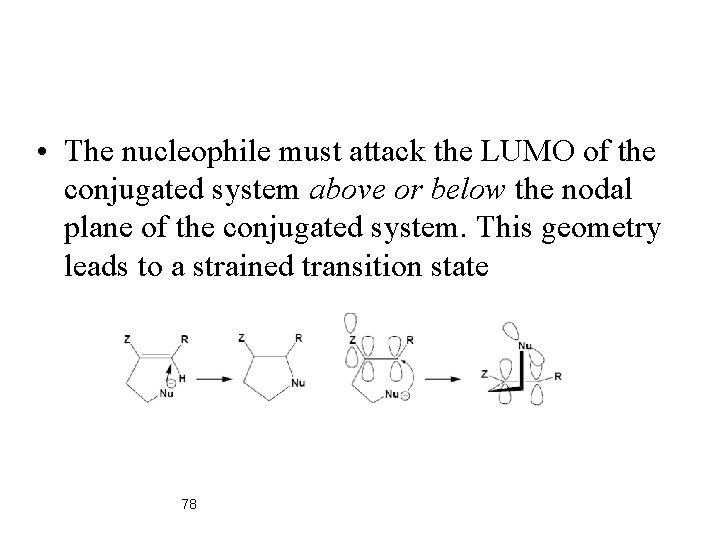
• The nucleophile must attack the LUMO of the conjugated system above or below the nodal plane of the conjugated system. This geometry leads to a strained transition state 78

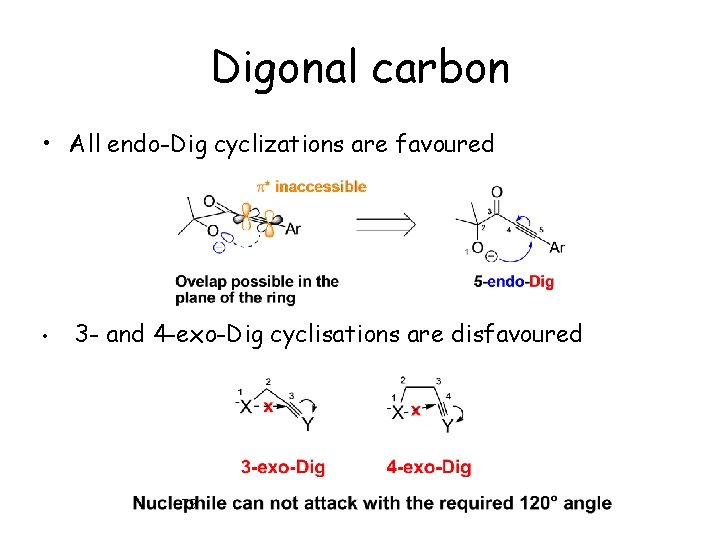
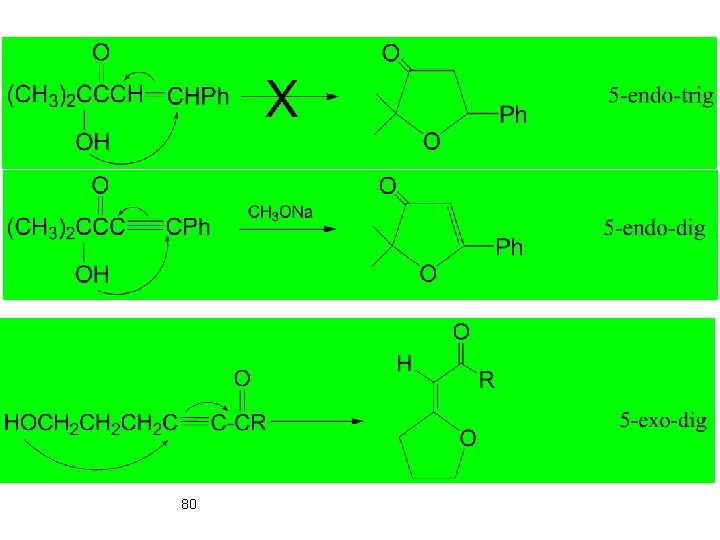
Digonal carbon • All endo-Dig cyclizations are favoured • 3 - and 4 -exo-Dig cyclisations are disfavoured 79

80

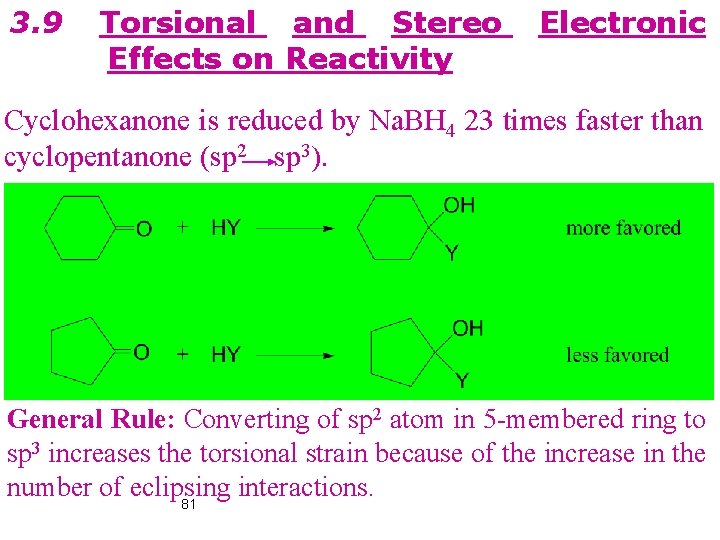
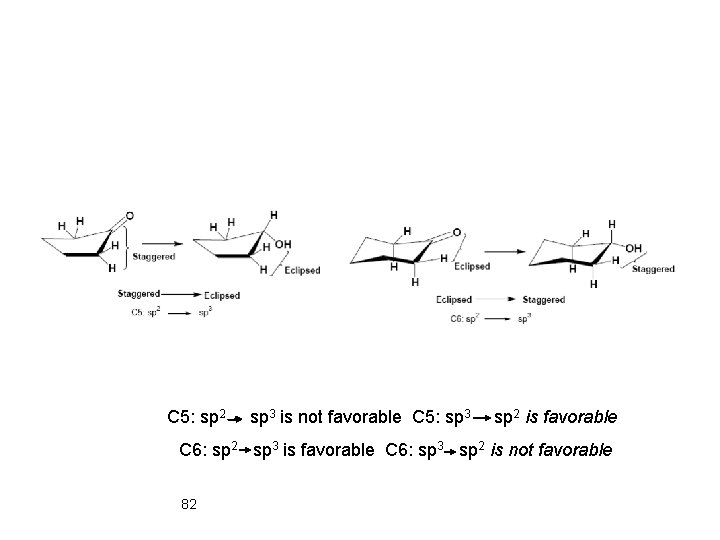
3. 9 Torsional and Stereo Effects on Reactivity Electronic Cyclohexanone is reduced by Na. BH 4 23 times faster than cyclopentanone (sp 2 sp 3). General Rule: Converting of sp 2 atom in 5 -membered ring to sp 3 increases the torsional strain because of the increase in the number of eclipsing interactions. 81

C 5: sp 2 sp 3 is not favorable C 5: sp 3 sp 2 is favorable C 6: sp 2 sp 3 is favorable C 6: sp 3 sp 2 is not favorable 82

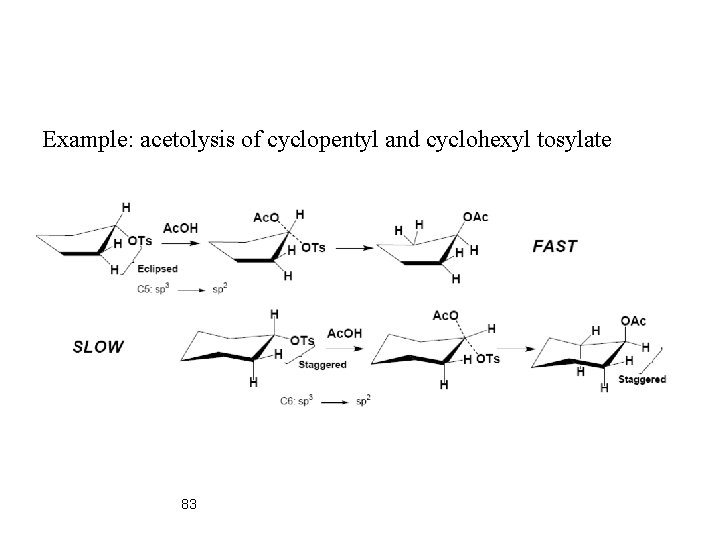
Example: acetolysis of cyclopentyl and cyclohexyl tosylate 83

84

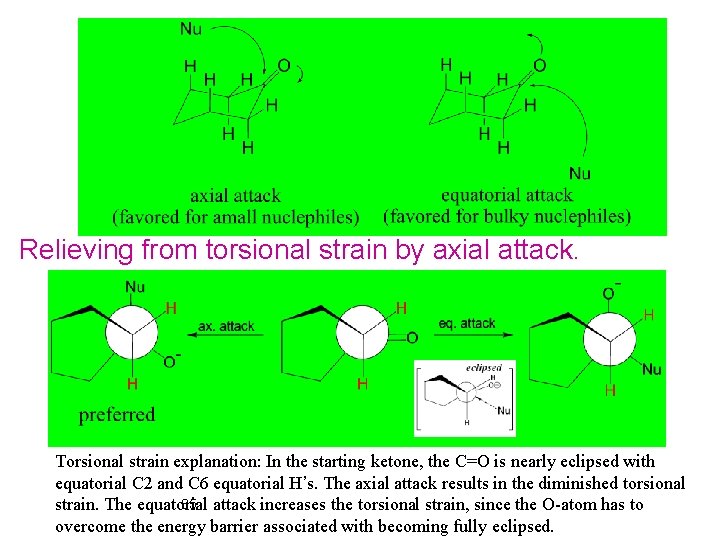
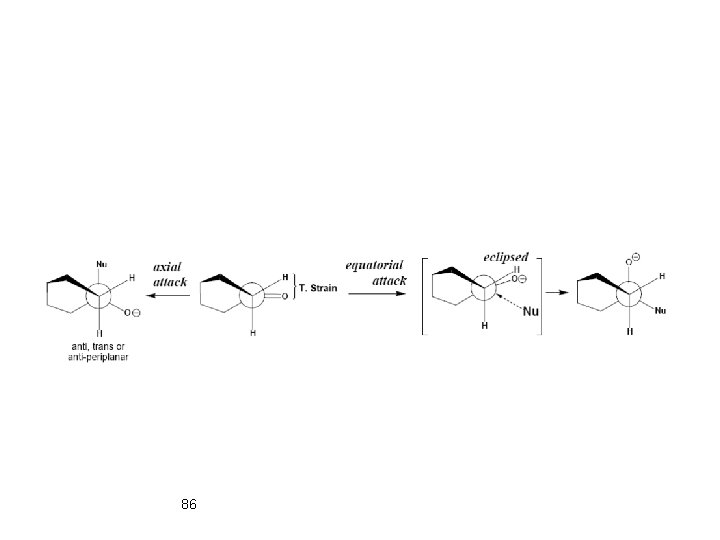
Relieving from torsional strain by axial attack. Torsional strain explanation: In the starting ketone, the C=O is nearly eclipsed with equatorial C 2 and C 6 equatorial H’s. The axial attack results in the diminished torsional 85 attack increases the torsional strain, since the O-atom has to strain. The equatorial overcome the energy barrier associated with becoming fully eclipsed.

86

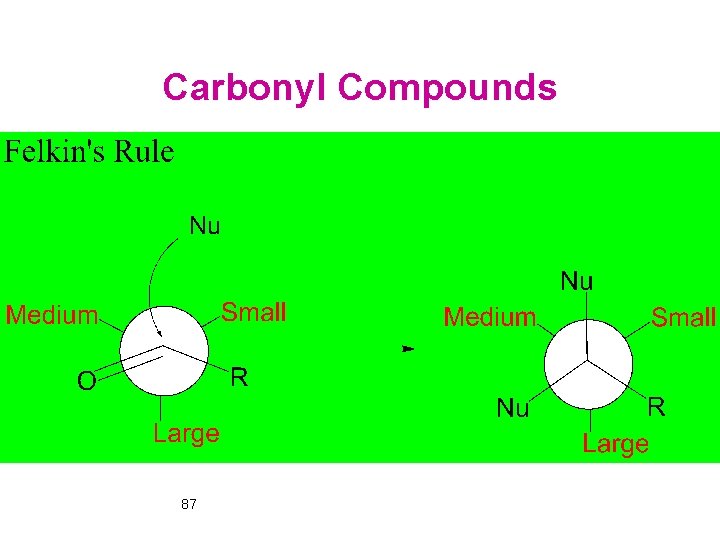
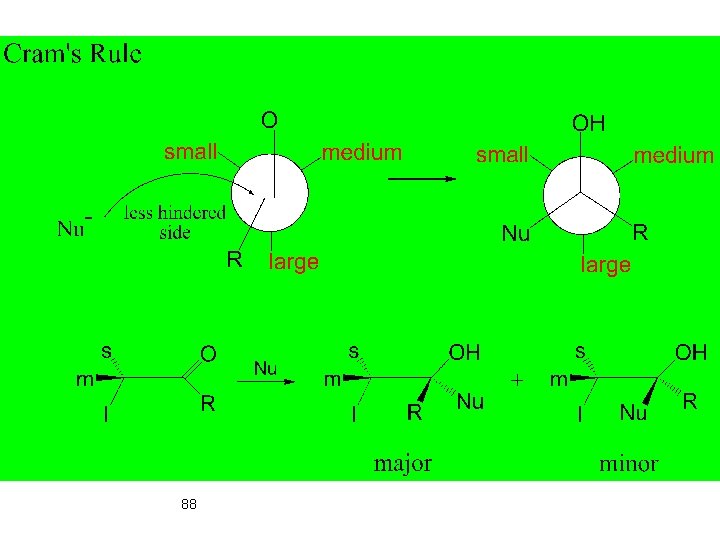
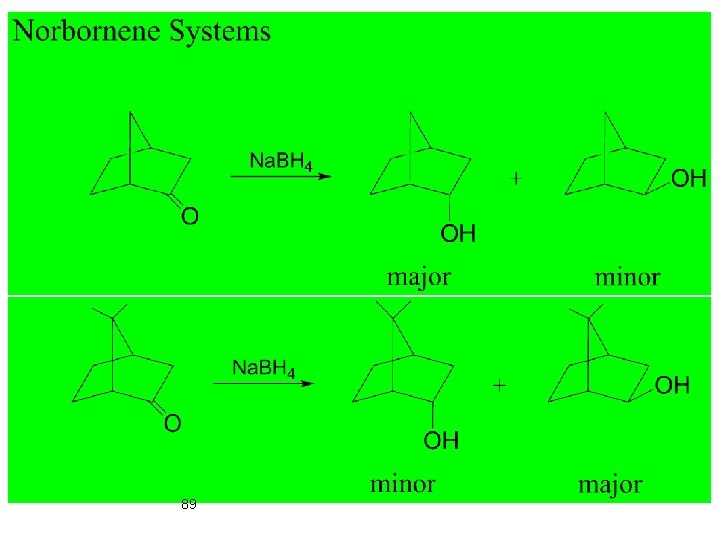
Carbonyl Compounds 87

88

89

90