Chapter 7 Cyclic Compounds Stereochemistry of Reactions Monocyclic
- Slides: 23

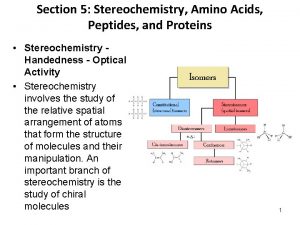
Chapter 7 Cyclic Compounds. Stereochemistry of Reactions

Monocyclic Compounds • Compounds containing a single ring • Relative stabilities are determined from heats of formation (DHf°) • All have the same empirical formula: CH 2 • Thus, stabilities can be readily compared on a per carbon basis 7. 1 Relative Stabilities of the Monocyclic Alkanes 2

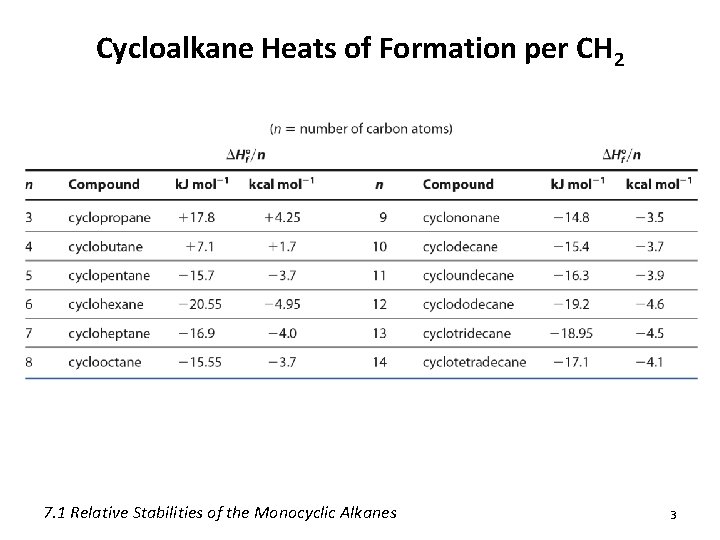
Cycloalkane Heats of Formation per CH 2 7. 1 Relative Stabilities of the Monocyclic Alkanes 3

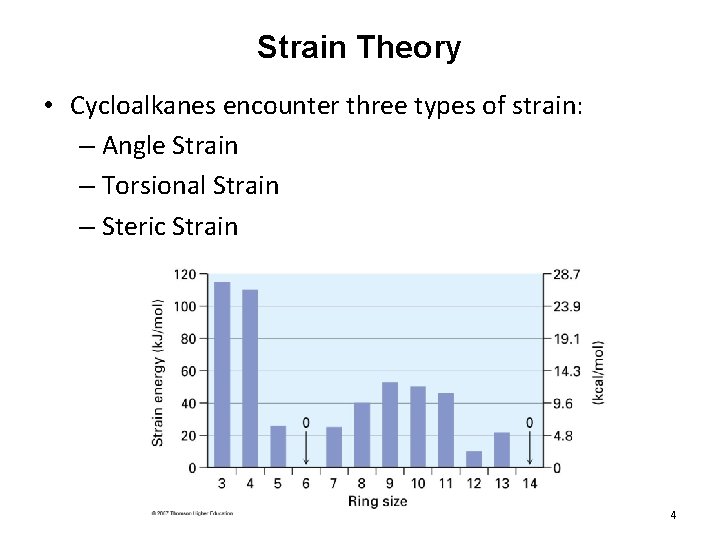
Strain Theory • Cycloalkanes encounter three types of strain: – Angle Strain – Torsional Strain – Steric Strain 4

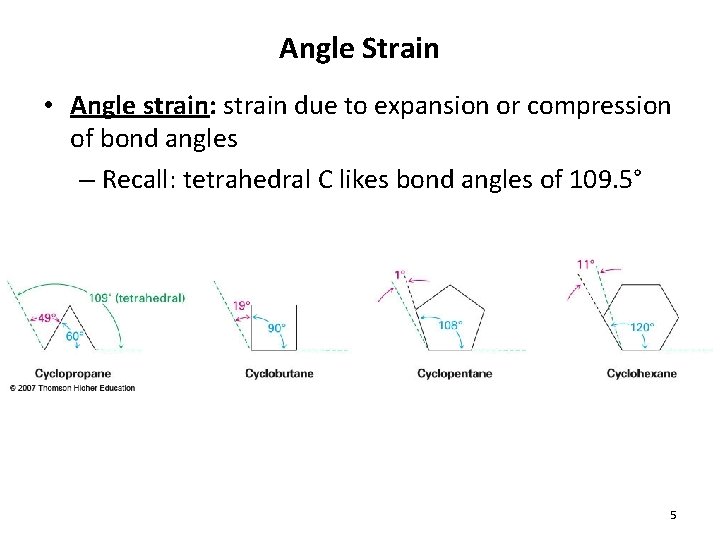
Angle Strain • Angle strain: strain due to expansion or compression of bond angles – Recall: tetrahedral C likes bond angles of 109. 5° 5

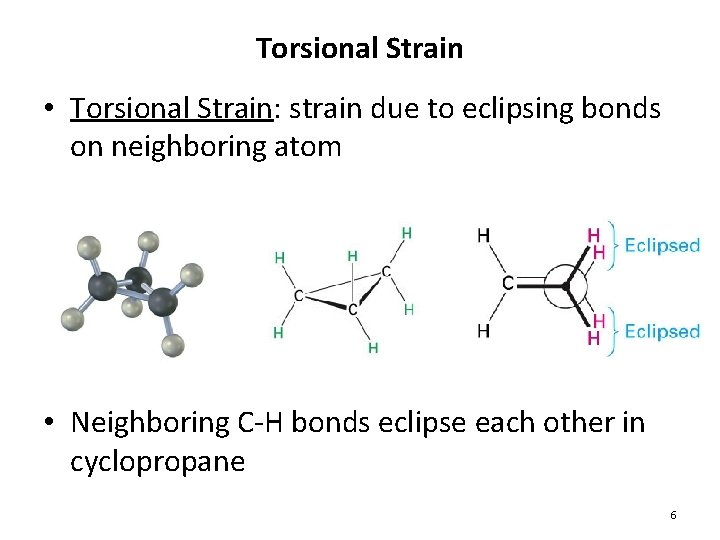
Torsional Strain • Torsional Strain: strain due to eclipsing bonds on neighboring atom • Neighboring C-H bonds eclipse each other in cyclopropane 6

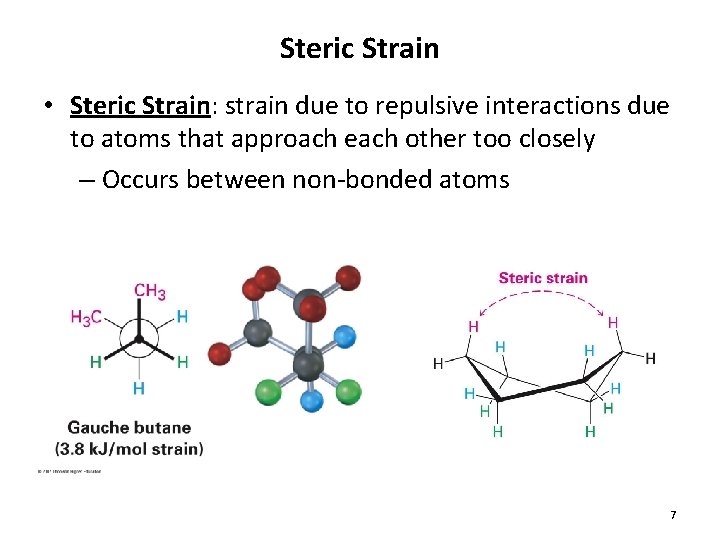
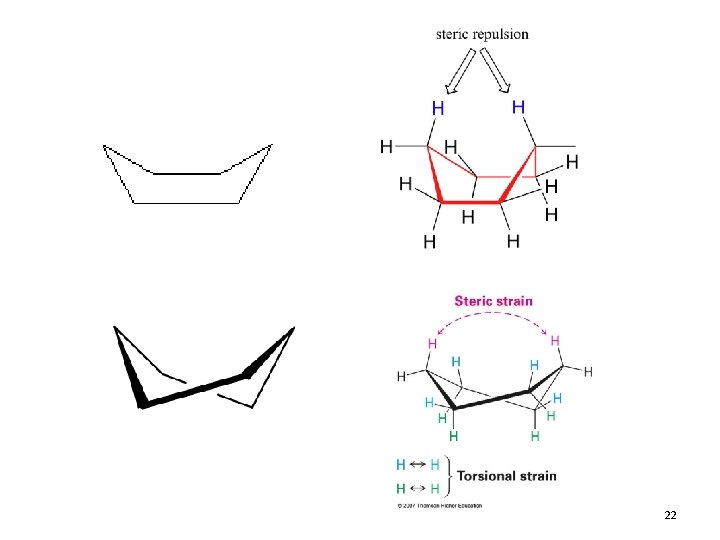
Steric Strain • Steric Strain: strain due to repulsive interactions due to atoms that approach each other too closely – Occurs between non-bonded atoms 7

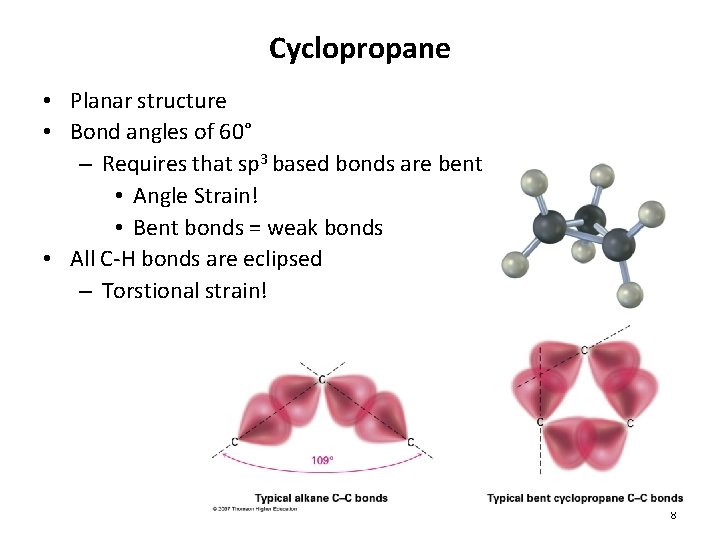
Cyclopropane • Planar structure • Bond angles of 60° – Requires that sp 3 based bonds are bent • Angle Strain! • Bent bonds = weak bonds • All C-H bonds are eclipsed – Torstional strain! 8

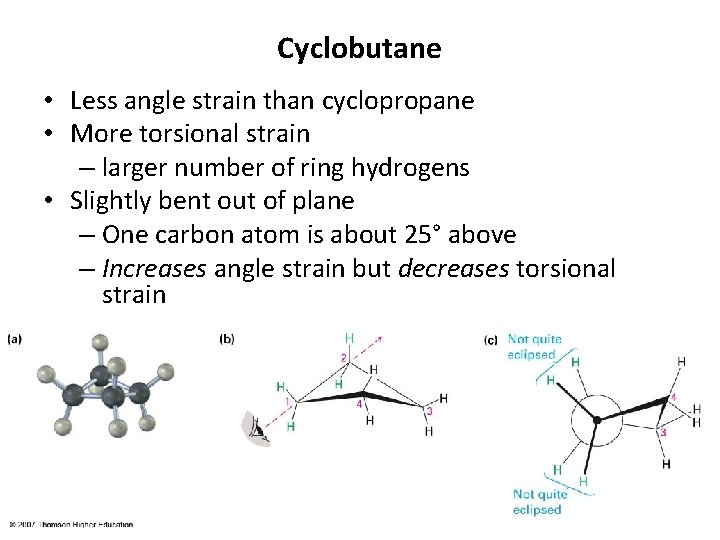
Cyclobutane • Less angle strain than cyclopropane • More torsional strain – larger number of ring hydrogens • Slightly bent out of plane – One carbon atom is about 25° above – Increases angle strain but decreases torsional strain 9

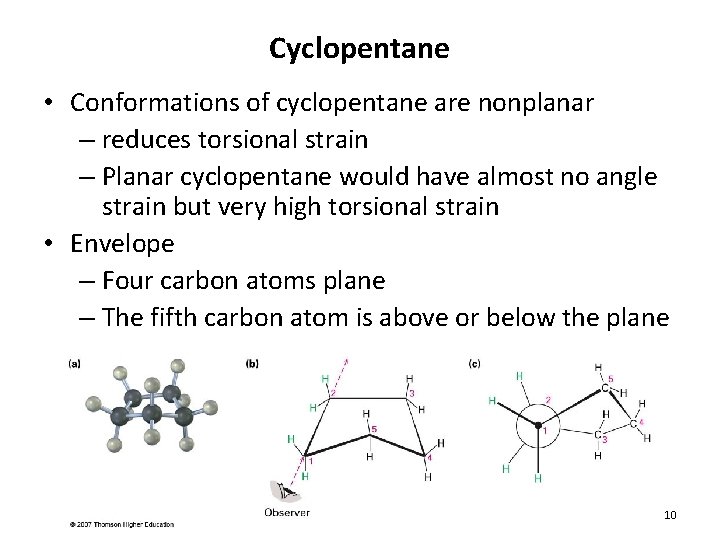
Cyclopentane • Conformations of cyclopentane are nonplanar – reduces torsional strain – Planar cyclopentane would have almost no angle strain but very high torsional strain • Envelope – Four carbon atoms plane – The fifth carbon atom is above or below the plane 10

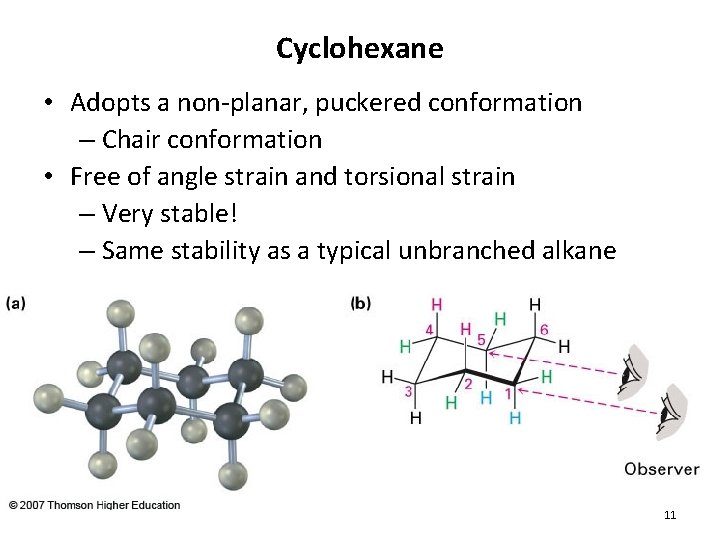
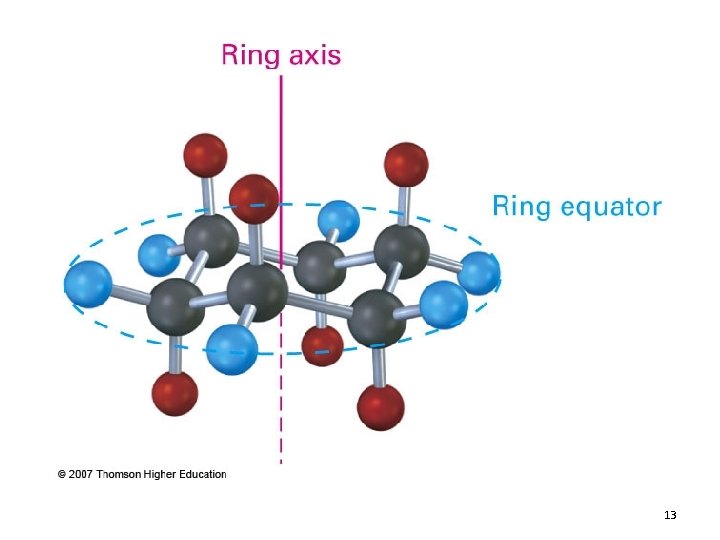
Cyclohexane • Adopts a non-planar, puckered conformation – Chair conformation • Free of angle strain and torsional strain – Very stable! – Same stability as a typical unbranched alkane 11

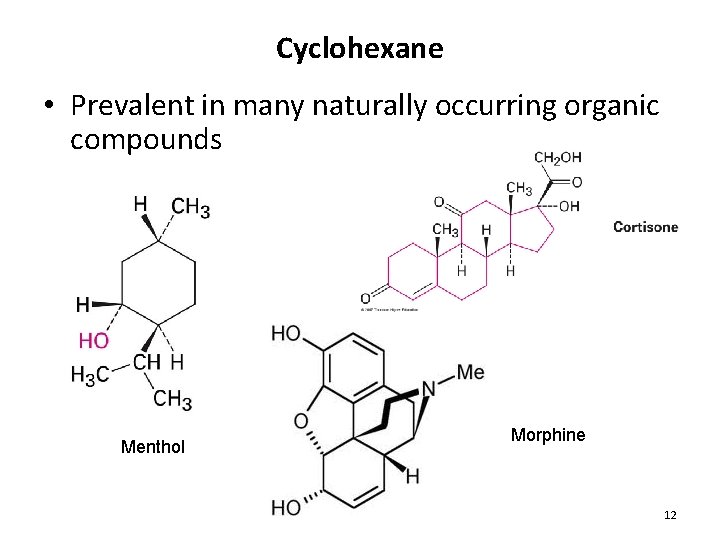
Cyclohexane • Prevalent in many naturally occurring organic compounds Menthol Morphine 12

13

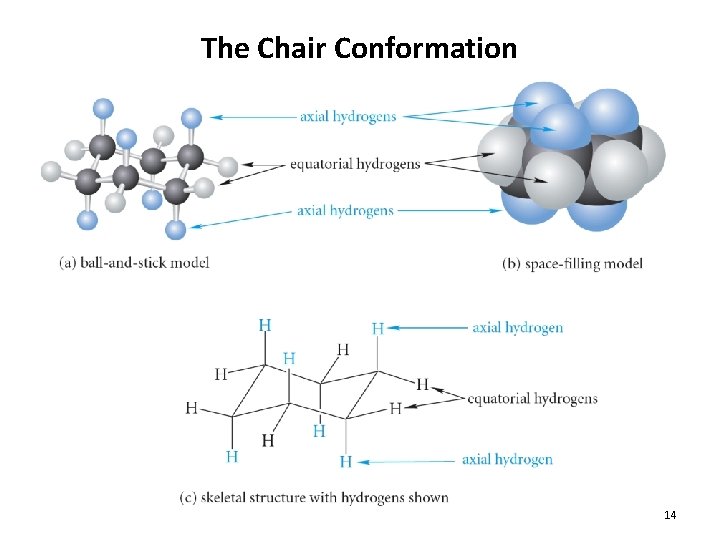
The Chair Conformation 14

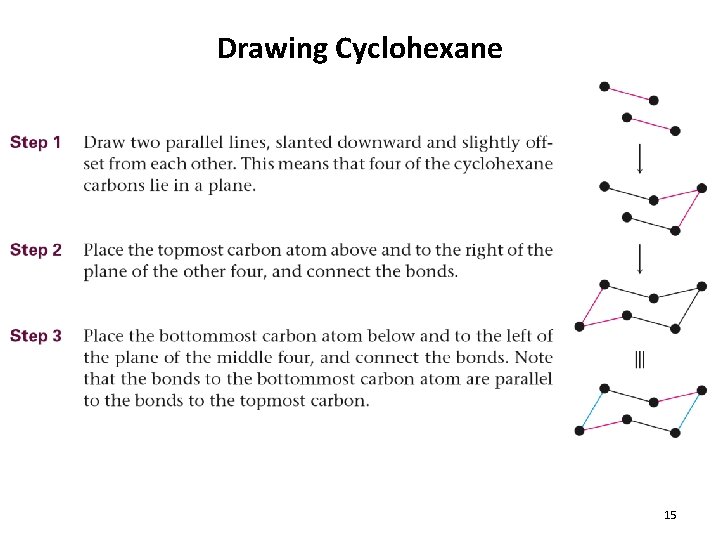
Drawing Cyclohexane 15

Problems • Practice drawing both chair conformations of cyclohexane 16

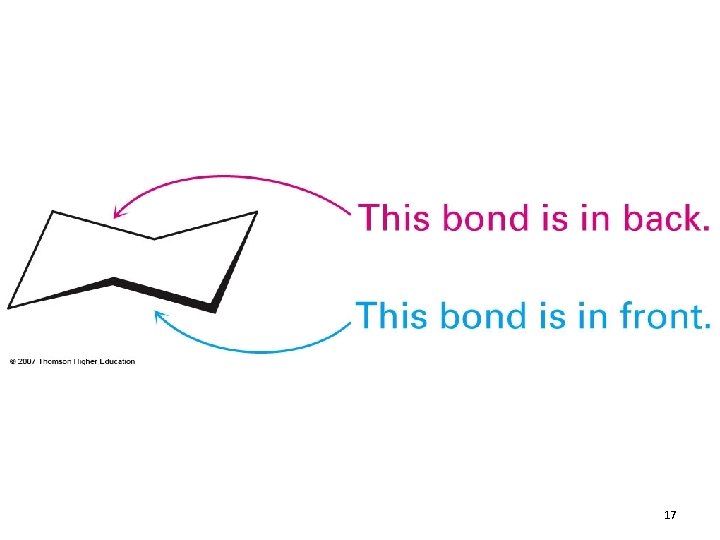
17

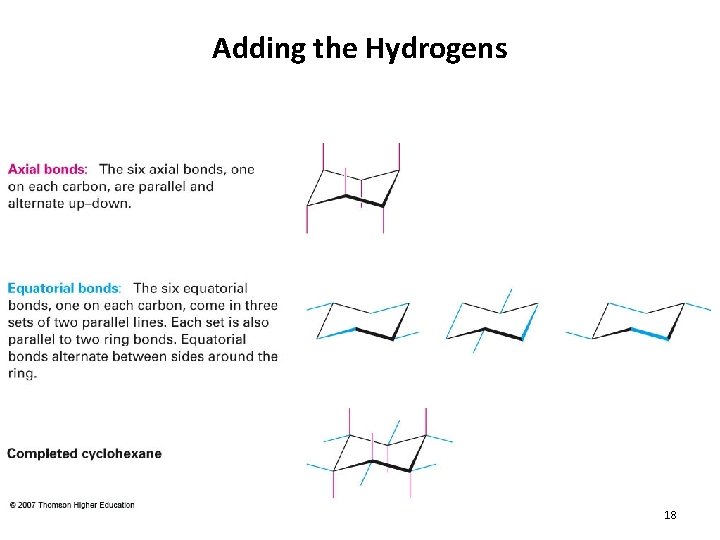
Adding the Hydrogens 18

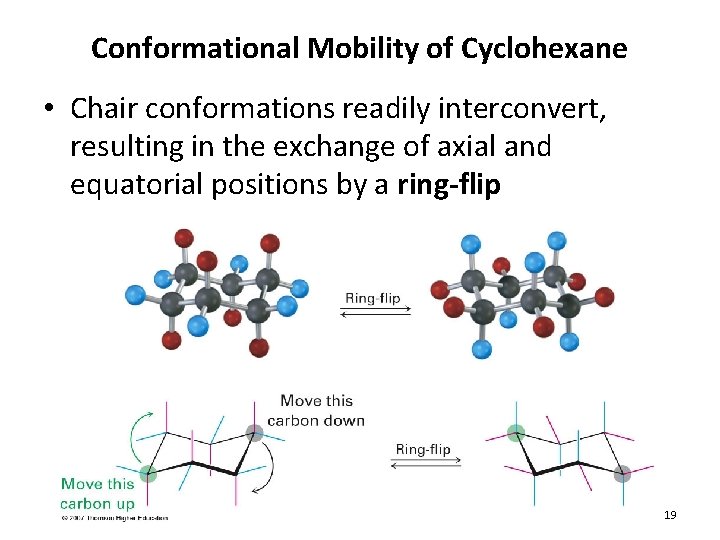
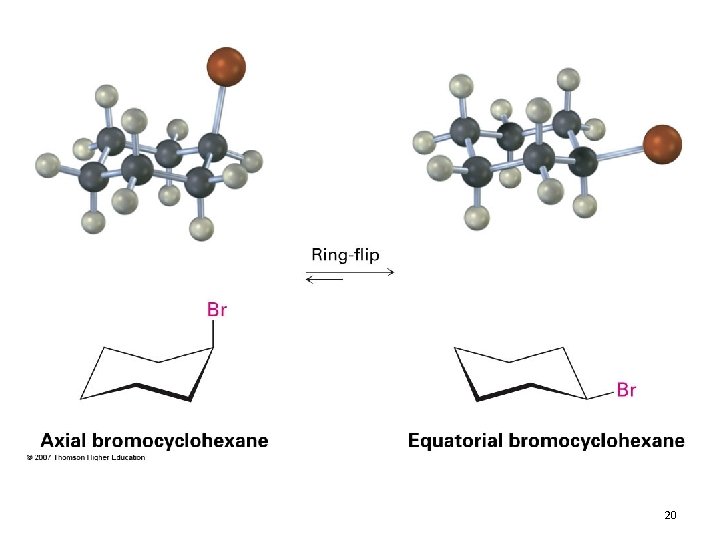
Conformational Mobility of Cyclohexane • Chair conformations readily interconvert, resulting in the exchange of axial and equatorial positions by a ring-flip 19

20

21

22

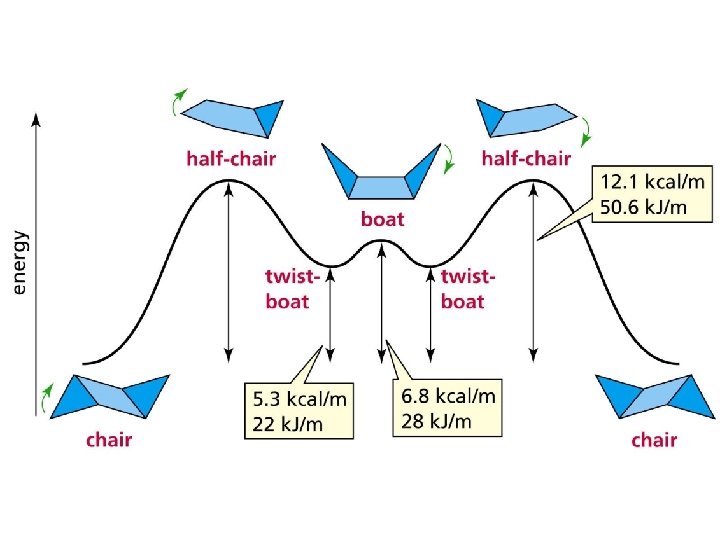
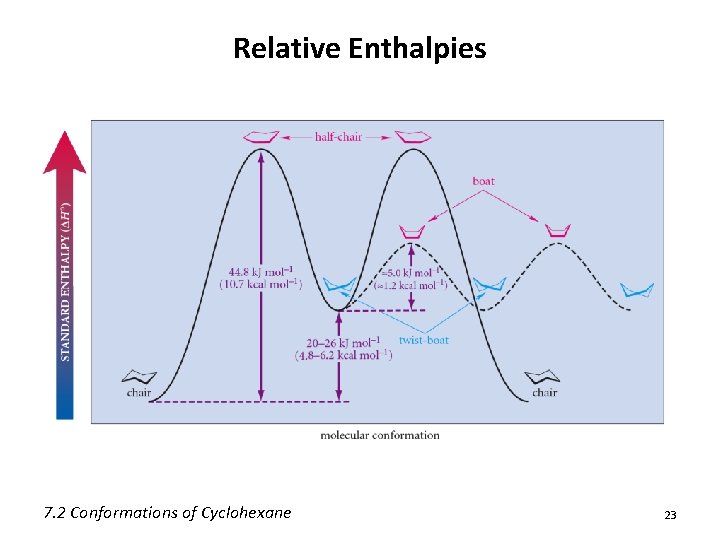
Relative Enthalpies 7. 2 Conformations of Cyclohexane 23
 Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Examples of redox reaction
Examples of redox reaction Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Peptide stereochemistry
Peptide stereochemistry R and s configuration
R and s configuration Cyclopentane cis trans isomers
Cyclopentane cis trans isomers Stereochemistry quiz
Stereochemistry quiz Father of stereochemistry
Father of stereochemistry Chiral vs achiral
Chiral vs achiral Naproxen stereochemistry
Naproxen stereochemistry Stereochemistry of alkanes and cycloalkanes
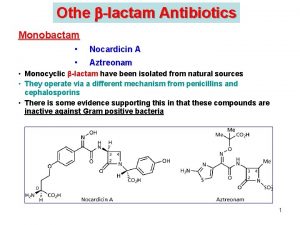
Stereochemistry of alkanes and cycloalkanes Stereochemistry of cephalosporin
Stereochemistry of cephalosporin Finding stereocenters
Finding stereocenters Pyranoses
Pyranoses Non metals examples
Non metals examples Covalent and ionic bonds venn diagram
Covalent and ionic bonds venn diagram Chapter 10 chapter assessment chemical reactions answers
Chapter 10 chapter assessment chemical reactions answers Chapter 9 study guide chemical reactions
Chapter 9 study guide chemical reactions Ionic compounds
Ionic compounds Chapter 7 chapter assessment ionic compounds and metals
Chapter 7 chapter assessment ionic compounds and metals Oxidation reduction reactions chapter 19 review
Oxidation reduction reactions chapter 19 review Chapter 9 chemical reactions
Chapter 9 chemical reactions