Component preparation and storage When Deciding to Transfuse

















































- Slides: 75


Component preparation and storage


When Deciding to Transfuse

• What improvement in the patients condition am I aiming to achieve? • Can I minimize blood loss to reduce the patients need for transfusion? • Are there any other treatments I should give before making the decision to transfuse? • What are the specific indications for transfusing this patient?

• What are the risks of transmitting infectious agents through the available blood products? It should also be borne in mind that the rate of non-infectious complications is probably higher than infectious complications. • Do the benefits of transfusion outweigh the risks to this particular patient? • Has the patient been given a clear explanation of the potential risks and benefits of blood component therapy in his or her particular case?

• Have I recorded my decision to transfuse and the reasons for transfusion on the patients chart and any documentation used in the ordering or administration of blood components? • Will a trained person monitor this patient and respond immediately if any acute transfusion reactions occur? • Has crossmatching and other relevant testing been carried out?

Uses and Contraindications Whole Blood Packed RBC

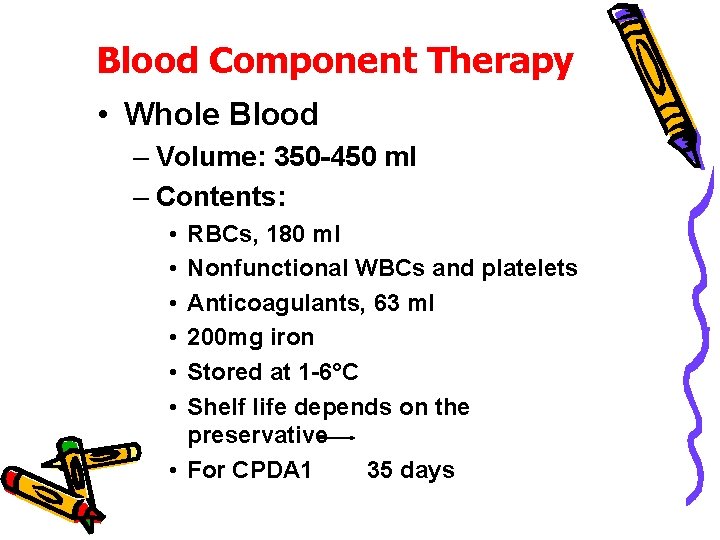
Blood Component Therapy • Whole Blood – Volume: 350 -450 ml – Contents: • • • RBCs, 180 ml Nonfunctional WBCs and platelets Anticoagulants, 63 ml 200 mg iron Stored at 1 -6°C Shelf life depends on the preservative • For CPDA 1 35 days


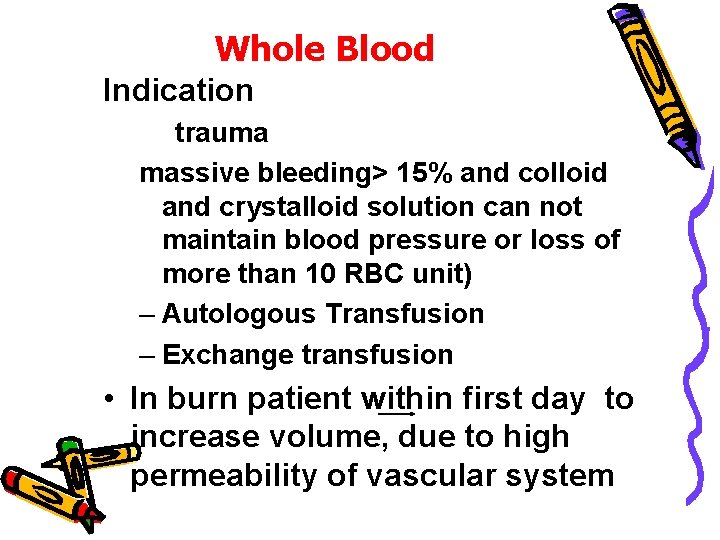
Whole Blood Indication trauma massive bleeding> 15% and colloid and crystalloid solution can not maintain blood pressure or loss of more than 10 RBC unit) – Autologous Transfusion – Exchange transfusion • In burn patient within first day to increase volume, due to high permeability of vascular system

Packed red cell transfusion • One unit of PRBC vol 250 ml hct 65% Hb 20 g/100 raise Hb by 1 gm/dl 200 mg iron 70% post transfusion survival • Age of blood concerns regarding K level 2, 3 DPG level decreased post transfusion survival • Specific conditions intrauterine transfusion thalassemics open heart surgery < 3 days old < 5 days < 10 days


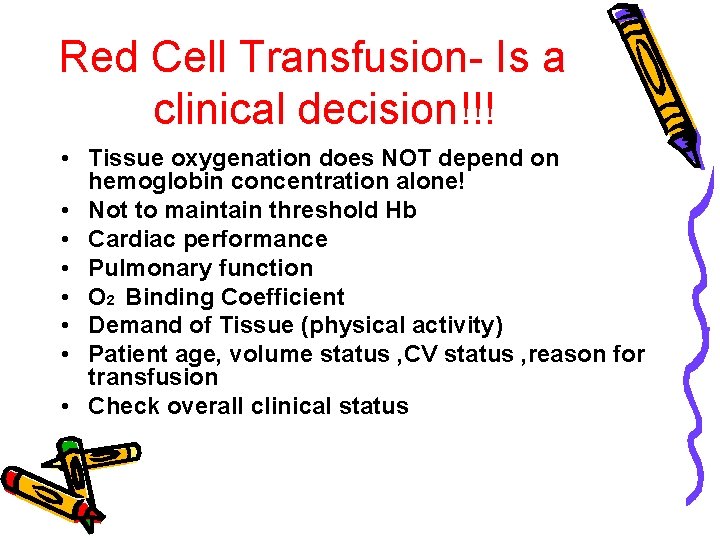
Red Cell Transfusion- Is a clinical decision!!! • Tissue oxygenation does NOT depend on hemoglobin concentration alone! • Not to maintain threshold Hb • Cardiac performance • Pulmonary function • O 2 Binding Coefficient • Demand of Tissue (physical activity) • Patient age, volume status , CV status , reason for transfusion • Check overall clinical status

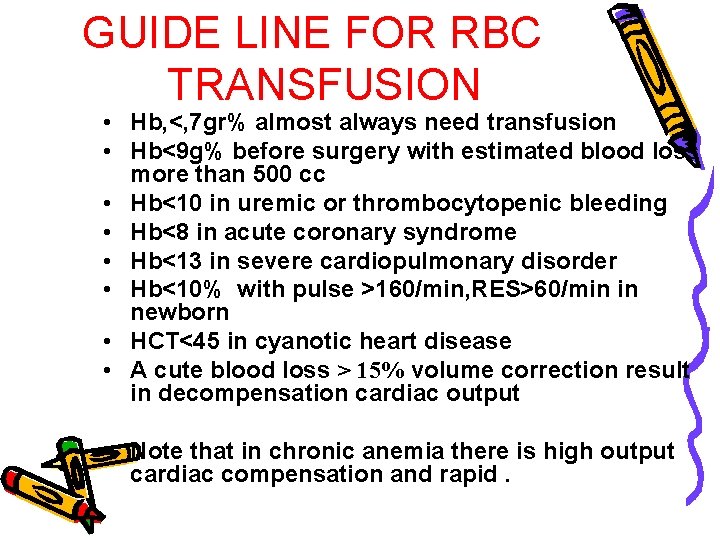
GUIDE LINE FOR RBC TRANSFUSION • Hb, <, 7 gr% almost always need transfusion • Hb<9 g% before surgery with estimated blood loss more than 500 cc • Hb<10 in uremic or thrombocytopenic bleeding • Hb<8 in acute coronary syndrome • Hb<13 in severe cardiopulmonary disorder • Hb<10% with pulse >160/min, RES>60/min in newborn • HCT<45 in cyanotic heart disease • A cute blood loss > 15% volume correction result in decompensation cardiac output • Note that in chronic anemia there is high output cardiac compensation and rapid.


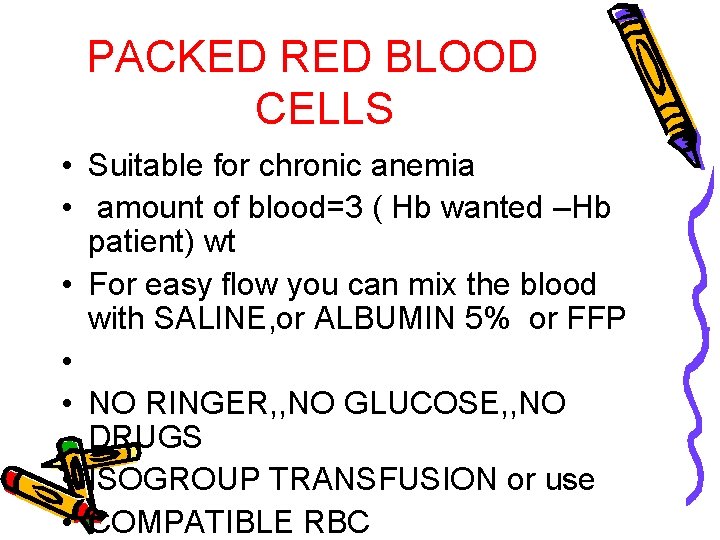
PACKED RED BLOOD CELLS • Suitable for chronic anemia • amount of blood=3 ( Hb wanted –Hb patient) wt • For easy flow you can mix the blood with SALINE, or ALBUMIN 5% or FFP • • NO RINGER, , NO GLUCOSE, , NO DRUGS • ISOGROUP TRANSFUSION or use • COMPATIBLE RBC

Expected Effects of packed PRBC – May be seen immediately, but often takes up to 24 hours – Usually order a 4 -hour posttransfusion HB to manage patient

RBC should not be used: : • when anemia can be corrected with specific medications, e. g. , iron, B 12, folic acid, erythropoietin, etc • for volume replacement


Guideline and Policies for Transfusion • Considerations – Must be crossmatch compatible – Must be administered through a filter – Iron load of -250 mg per unit

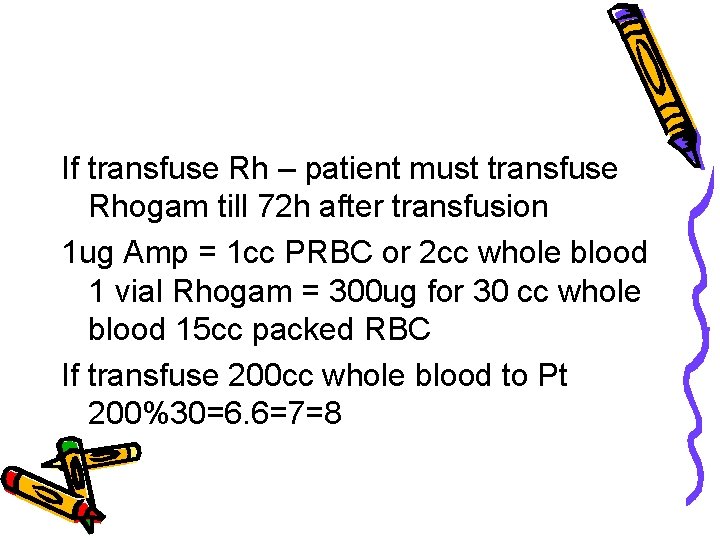
If transfuse Rh – patient must transfuse Rhogam till 72 h after transfusion 1 ug Amp = 1 cc PRBC or 2 cc whole blood 1 vial Rhogam = 300 ug for 30 cc whole blood 15 cc packed RBC If transfuse 200 cc whole blood to Pt 200%30=6. 6=7=8

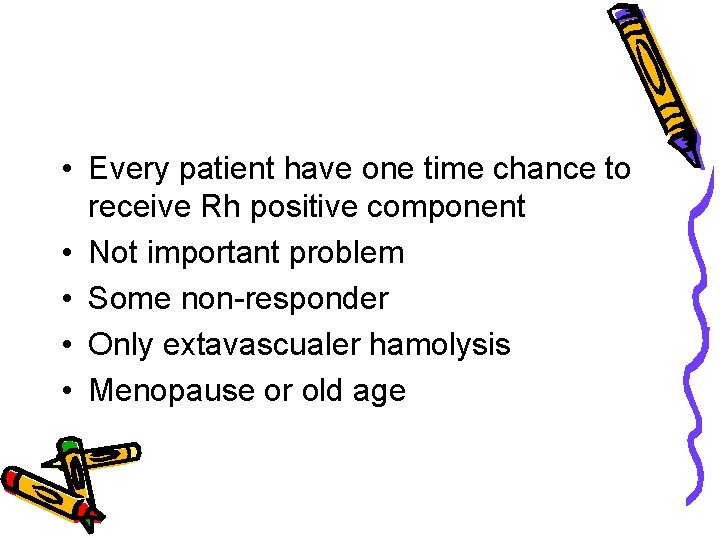
• Every patient have one time chance to receive Rh positive component • Not important problem • Some non-responder • Only extavascualer hamolysis • Menopause or old age


Leukocyte-reduced RBCs – Definition: Produced with less than 70% of original white cells and more than 80% original red cells. – Leukocyte less than < 5× 106 – Methods • • • Centrifugation Washing Micro aggregate filter, 90 -99% removal Fourth generation filter, 99. 9% removal Prestorage leuko depletion lower level of cytokine generation in blood bag during storage and lower risk of FNHTR • Bedside leukoreduction hypotention

Indications For Leucodepleted Blood Established indications • Prevention of recurrent FNHTR • Prevention of alloimmunization to HLA in multiply transfused pts or multiple pregnancy • Prevention of CMV & HTLV transmission To reduce the immunmodulatory effect of blood transfusion. Wound infection in surgeric patient Indications under review • Prevention of latent HIV reactivation Not prevent GVHD

Leukocyte Reduces RBCs Minimizes white cell immunization in patients receiving repeated transfusions but, to achieve this, all blood components given to the patient must be leucocyte-depleted Prevention of CMV transmission • CMV is carried in WBCs (probably neutrophils) only • Filtered products appear equivalent to CMV seronegative products in prevention of CMV seroconversion • CMV seronegative products – Infant /neonatal, Organ transplant patients – If pt is CMV positive, give CMV positive products – HIV patients should be given CMV positive products, because CMV (-) can stimulates further immune reaction

Washed RBCs – Removes plasma; 1 -2 hours process – Must used washed RBC within 24 hours of preparation because the system was opened

Indication of Washed Packed Red Blood Cells • to reduce the recurrence of severe allergic or anaphylactic transfusion reactions to be caused by plasma proteins • Ig. A deficient patients • Post-transfusion purpura. • Repeated febrile nonhemolytic transfusion reactions • In hyperkalemic patient

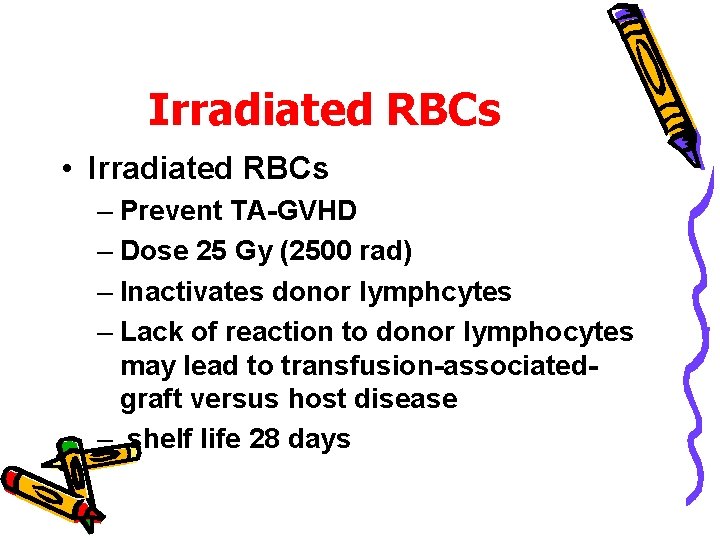
Irradiated RBCs • Irradiated RBCs – Prevent TA-GVHD – Dose 25 Gy (2500 rad) – Inactivates donor lymphcytes – Lack of reaction to donor lymphocytes may lead to transfusion-associatedgraft versus host disease – shelf life 28 days

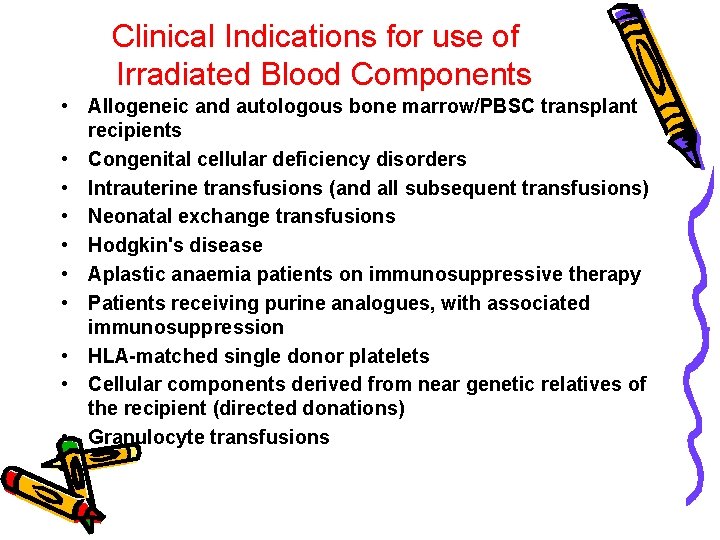
Clinical Indications for use of Irradiated Blood Components • Allogeneic and autologous bone marrow/PBSC transplant recipients • Congenital cellular deficiency disorders • Intrauterine transfusions (and all subsequent transfusions) • Neonatal exchange transfusions • Hodgkin's disease • Aplastic anaemia patients on immunosuppressive therapy • Patients receiving purine analogues, with associated immunosuppression • HLA-matched single donor platelets • Cellular components derived from near genetic relatives of the recipient (directed donations) • Granulocyte transfusions

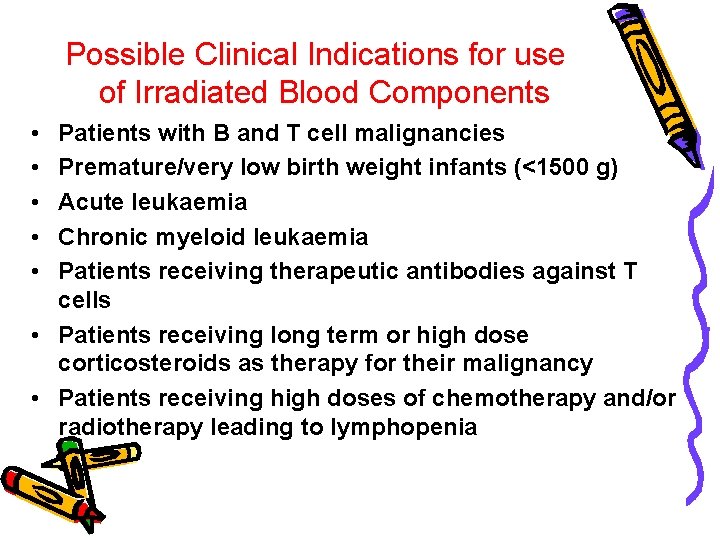
Possible Clinical Indications for use of Irradiated Blood Components • • • Patients with B and T cell malignancies Premature/very low birth weight infants (<1500 g) Acute leukaemia Chronic myeloid leukaemia Patients receiving therapeutic antibodies against T cells • Patients receiving long term or high dose corticosteroids as therapy for their malignancy • Patients receiving high doses of chemotherapy and/or radiotherapy leading to lymphopenia

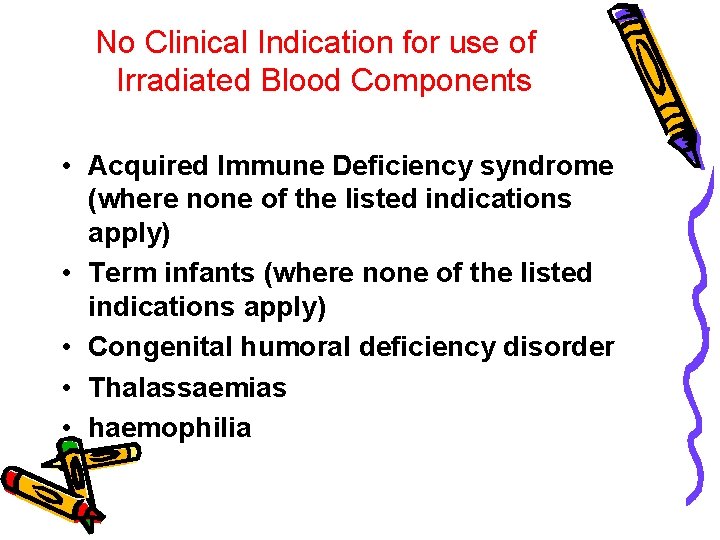
No Clinical Indication for use of Irradiated Blood Components • Acquired Immune Deficiency syndrome (where none of the listed indications apply) • Term infants (where none of the listed indications apply) • Congenital humoral deficiency disorder • Thalassaemias • haemophilia





Frozen, Deglycerolized Packed Red Blood Cells

F/D -PRBCs are indicated in the following circumstances 1. For use by those patients with alloantibodies to high frequency red blood cells antigens, rare, antigen-negative PRBC units are stored in repositories. 2. Autologous donation may be frozen if the time to the surgical procedure is extended past the storage date for the collected WB.

Fresh Frozen Plasma infusion • plasma separated & frozen within 8 hours of collection • composition 200 ml (450 ml blood) contain all clotting proteins level of factors 1 IU/ml • indications - broad spectrum coagulation defects (PT/a. PTT > 1. 5 x normal, INR > 2) • liver failure • DIC • exchange transfusion in neonates • vit K deficiency, reversal of warfarin • massive transfusion - plasma infusion for T T P - protein C deficiency










Cryoprecipitate (Cryo) Cryoprecipitate Pooling Cryoprecipitate










پﻼکﺖ ﻣﺘﺮﺍکﻢ Random donor Platelets • Whole blood 1 unit →Platelet Concentrate 1 unit↓ ≥ 5. 5 x 10¹º platelets in 50 -70 ml of plasma 3 days

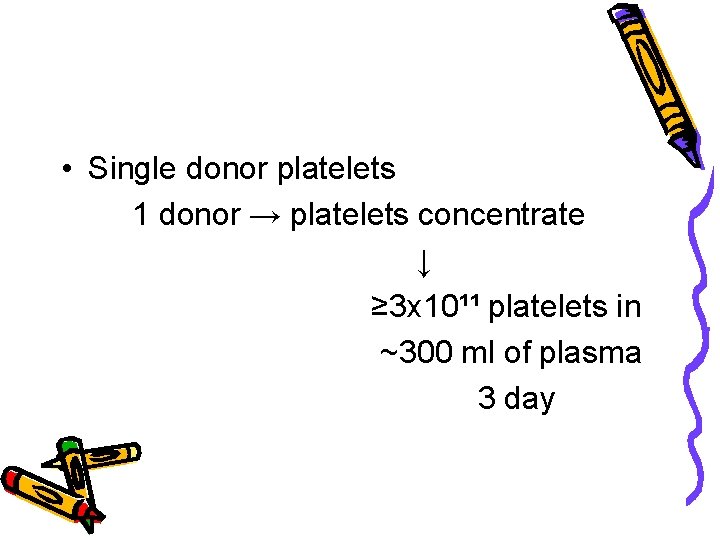
• Single donor platelets 1 donor → platelets concentrate ↓ ≥ 3 x 10¹¹ platelets in ~300 ml of plasma 3 day







Issues in platelet transfusion • Type of platelet product--- • random PC 50 -70 ml 5. 5 x 1010/unit one dose = 6 -8 units = 6 -8 donor exposures • single donor PC one dose = one unit = one donor exposure 3. 5 x 1011 200 -400 cc Indications • production - platelet transfusion effective acute leukemia, aplastic anemia, chemotherapy • consumption - platelet transfusion ineffective ITP, TTP, HUS, DIC, splenomegaly, drugs if pooled at open system must be used with 4 hours

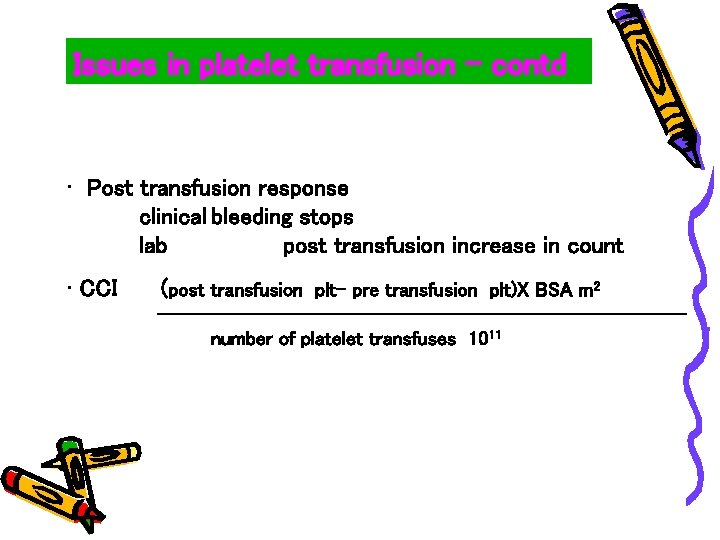
Issues in platelet transfusion - contd • Post transfusion response clinical bleeding stops lab post transfusion increase in count • CCI (post transfusion plt- pre transfusion plt)X BSA m 2 --------------------------number of platelet transfuses 1011

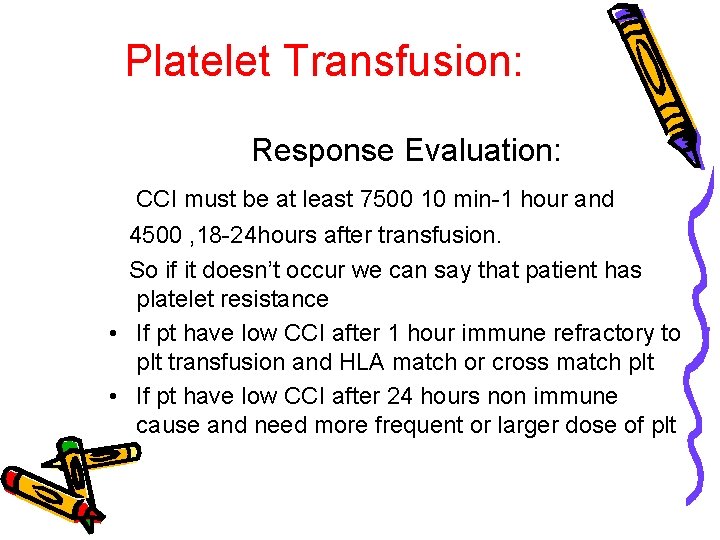
Platelet Transfusion: Response Evaluation: CCI must be at least 7500 10 min-1 hour and 4500 , 18 -24 hours after transfusion. So if it doesn’t occur we can say that patient has platelet resistance • If pt have low CCI after 1 hour immune refractory to plt transfusion and HLA match or cross match plt • If pt have low CCI after 24 hours non immune cause and need more frequent or larger dose of plt



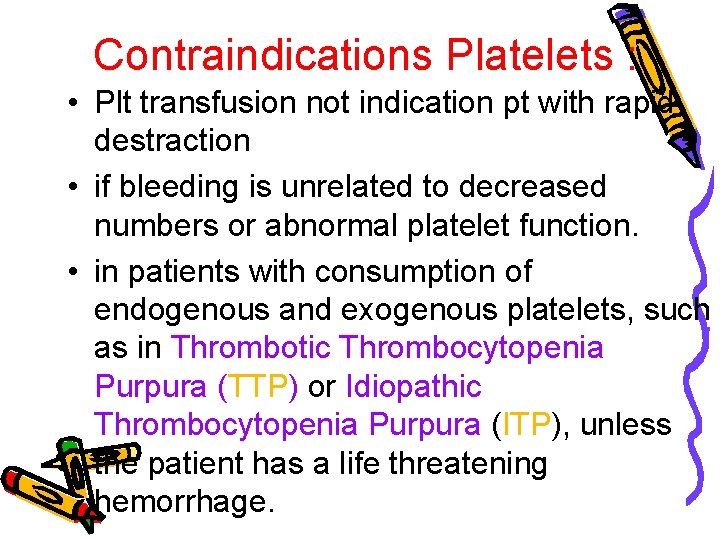
Contraindications Platelets : • Plt transfusion not indication pt with rapid destraction • if bleeding is unrelated to decreased numbers or abnormal platelet function. • in patients with consumption of endogenous and exogenous platelets, such as in Thrombotic Thrombocytopenia Purpura (TTP) or Idiopathic Thrombocytopenia Purpura (ITP), unless the patient has a life threatening hemorrhage.

Contraindications and Precautions Platelets • • Immune Thrombocytopenia Purpura ITP Heparin-Induced Thrombocytopenia HIT Thrombotic Thrombocytopenic Purpura TTP Untreated Disseminated Intravascular coagulation DIC • HLA/HPA Alloimmunized- apheresis platelet • “Platelet Glue” • Rh- patients with child bearing potential receiving Rh+ platelet

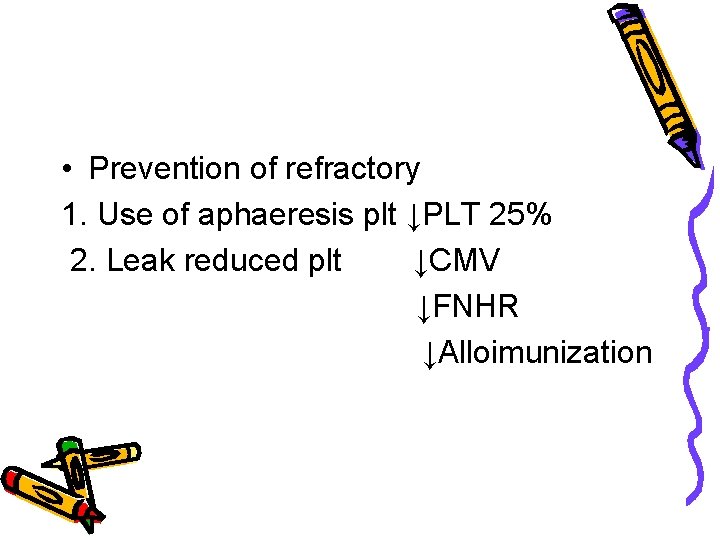
• Prevention of refractory 1. Use of aphaeresis plt ↓PLT 25% 2. Leak reduced plt ↓CMV ↓FNHR ↓Alloimunization