Clinical Document Architecture previously Patient Record Architecture HL








- Slides: 53

Clinical Document Architecture (previously "Patient Record Architecture") HL 7 Meeting St. Louis, Missouri September 13, 2000 Bob Dolin, MD Kaiser Permanente 1

Outline • Objectives • Overview of August 2000 Clinical Document Architecture ballot proposal • Introduction • What is the CDA? • The "A" in "CDA" • Relationship of CDA to HL 7 Messaging Standards • Technical Specifications • CDA Header • CDA Body • HL 7 V 3 Data Types 2

Disclaimer • The models and examples presented here are based on the August 2000 membership-level ballot draft. While the ballot did pass, there will be some minor edits before finalizing the standard. • The examples displayed in the slides leave out required components and introduce modifications to illustrate a point. The examples in the other handout are complete and accurate. • The exact representation of CDA instances is contingent on the outcome of the HL 7 V 3 data type DTD ballot. 3

Introduction • Objectives • Overview of August 2000 Clinical Document Architecture ballot proposal • Introduction • What is the CDA? • The "A" in "CDA" • Relationship of CDA to HL 7 Messaging Standards • Technical Specifications • CDA Header • CDA Body • HL 7 V 3 Data Types 4

Introduction • What is the CDA? The CDA is a document markup standard for the structure and semantics of exchanged "clinical documents". A clinical document is a documentation of observations and other services with the following characteristics: • Persistence • Stewardship • Potential for authentication • Wholeness • Human readability A CDA document is a defined and complete information object that can exist outside of a message, and can include text, images, sounds, and other multimedia content. 5

Introduction • What is the CDA? (cont. ) Key aspects of the CDA: • CDA documents are encoded in Extensible Markup Language (XML). • CDA documents derive their meaning from the HL 7 Reference Information Model (RIM ) and use HL 7 V 3 data types. • The complete CDA will include a hierarchical set of document specifications. This hierarchy is referred to as an "architecture". 6

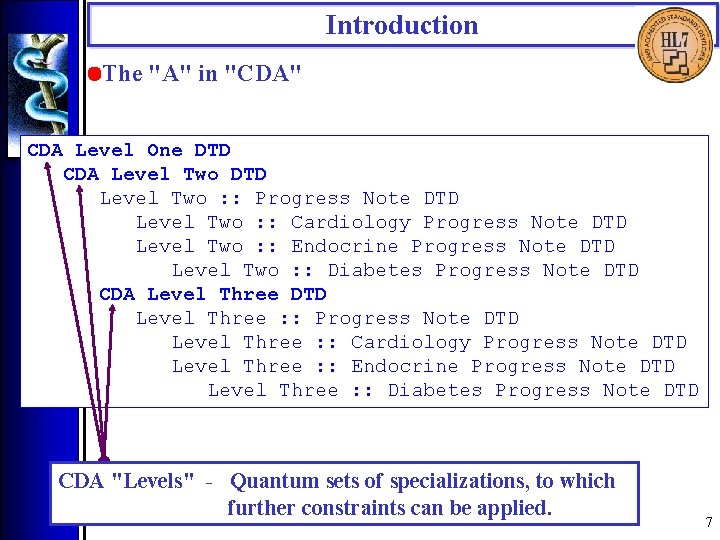
Introduction • The "A" in "CDA" CDA Level One DTD CDA Level Two DTD Level Two : : Progress Note DTD Level Two : : Cardiology Progress Note DTD Level Two : : Endocrine Progress Note DTD Level Two : : Diabetes Progress Note DTD CDA Level Three DTD Level Three : : Progress Note DTD Level Three : : Cardiology Progress Note DTD Level Three : : Endocrine Progress Note DTD Level Three : : Diabetes Progress Note DTD CDA "Levels" - Quantum sets of specializations, to which further constraints can be applied. 7

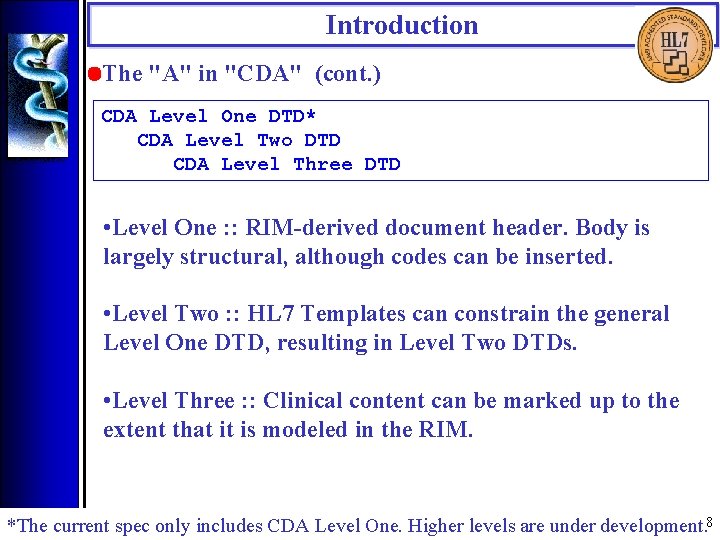
Introduction • The "A" in "CDA" (cont. ) CDA Level One DTD* CDA Level Two DTD CDA Level Three DTD • Level One : : RIM-derived document header. Body is largely structural, although codes can be inserted. • Level Two : : HL 7 Templates can constrain the general Level One DTD, resulting in Level Two DTDs. • Level Three : : Clinical content can be marked up to the extent that it is modeled in the RIM. *The current spec only includes CDA Level One. Higher levels are under development. 8

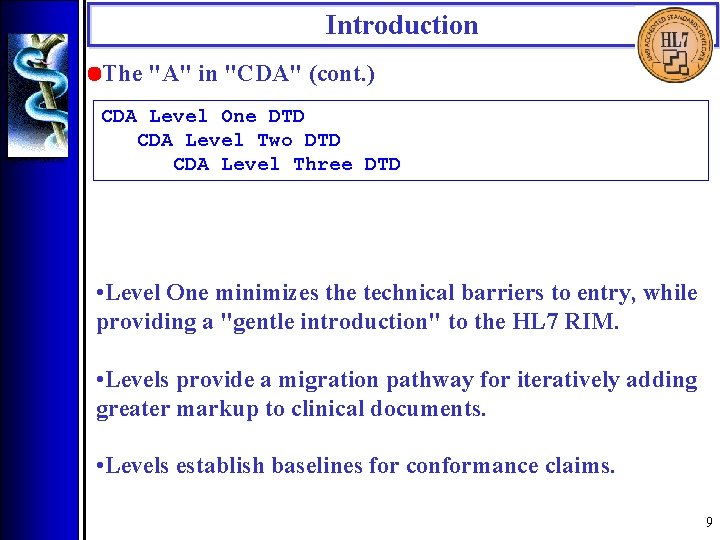
Introduction • The "A" in "CDA" (cont. ) CDA Level One DTD CDA Level Two DTD CDA Level Three DTD • Level One minimizes the technical barriers to entry, while providing a "gentle introduction" to the HL 7 RIM. • Levels provide a migration pathway for iteratively adding greater markup to clinical documents. • Levels establish baselines for conformance claims. 9

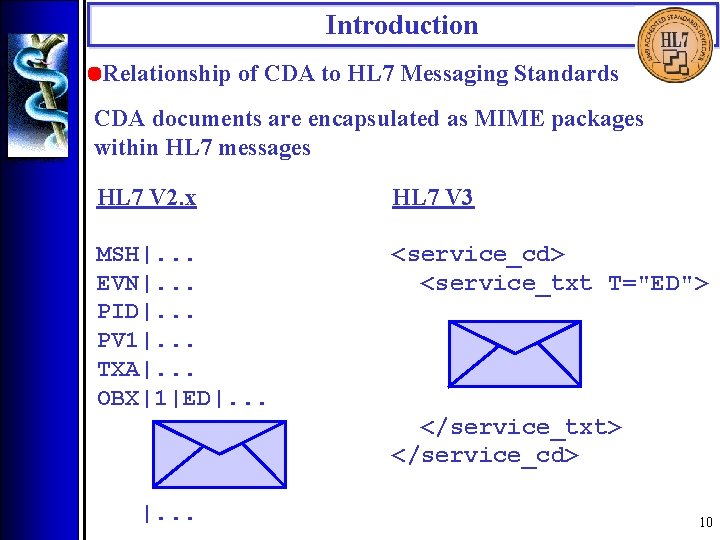
Introduction • Relationship of CDA to HL 7 Messaging Standards CDA documents are encapsulated as MIME packages within HL 7 messages HL 7 V 2. x HL 7 V 3 MSH|. . . EVN|. . . PID|. . . PV 1|. . . TXA|. . . OBX|1|ED|. . . <service_cd> <service_txt T="ED"> </service_txt> </service_cd> |. . . 10

Outline • Objectives • Overview of August 2000 Clinical Document Architecture ballot proposal • Introduction • What is the CDA? • The "A" in "CDA" • Relationship of CDA to HL 7 Messaging Standards • Technical Specifications • CDA Header • CDA Body • HL 7 V 3 Data Types 11

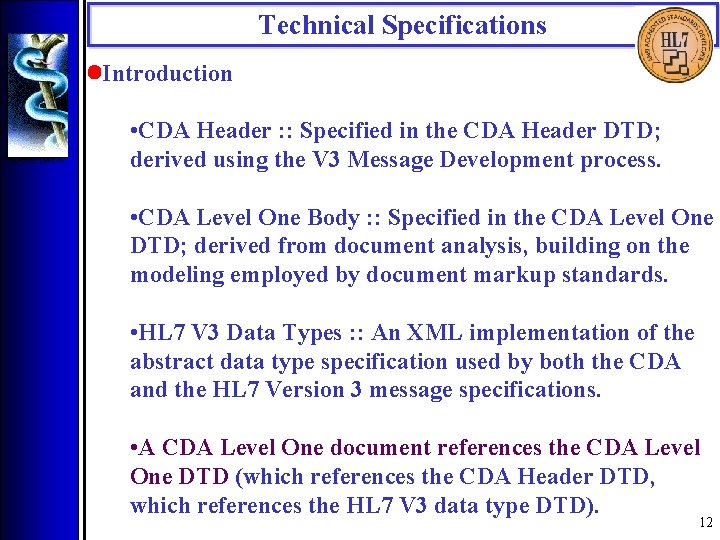
Technical Specifications • Introduction • CDA Header : : Specified in the CDA Header DTD; derived using the V 3 Message Development process. • CDA Level One Body : : Specified in the CDA Level One DTD; derived from document analysis, building on the modeling employed by document markup standards. • HL 7 V 3 Data Types : : An XML implementation of the abstract data type specification used by both the CDA and the HL 7 Version 3 message specifications. • A CDA Level One document references the CDA Level One DTD (which references the CDA Header DTD, which references the HL 7 V 3 data type DTD). 12

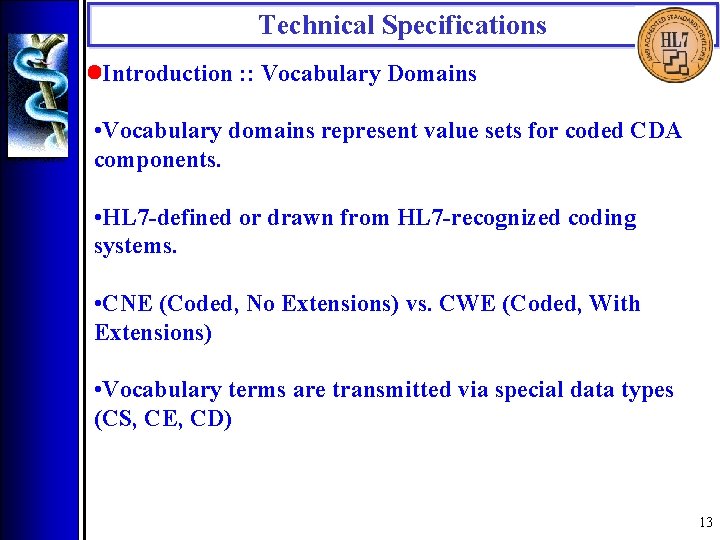
Technical Specifications • Introduction : : Vocabulary Domains • Vocabulary domains represent value sets for coded CDA components. • HL 7 -defined or drawn from HL 7 -recognized coding systems. • CNE (Coded, No Extensions) vs. CWE (Coded, With Extensions) • Vocabulary terms are transmitted via special data types (CS, CE, CD) 13

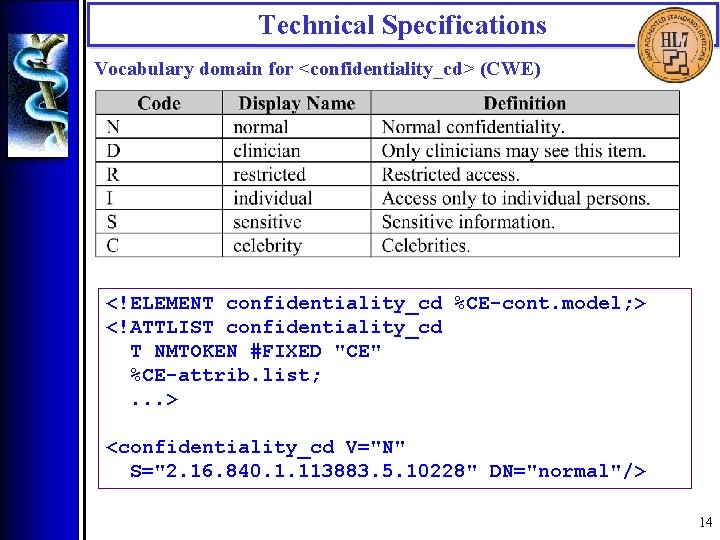
Technical Specifications Vocabulary domain for <confidentiality_cd> (CWE) <!ELEMENT confidentiality_cd %CE-cont. model; > <!ATTLIST confidentiality_cd T NMTOKEN #FIXED "CE" %CE-attrib. list; . . . > <confidentiality_cd V="N" S="2. 16. 840. 1. 113883. 5. 10228" DN="normal"/> 14

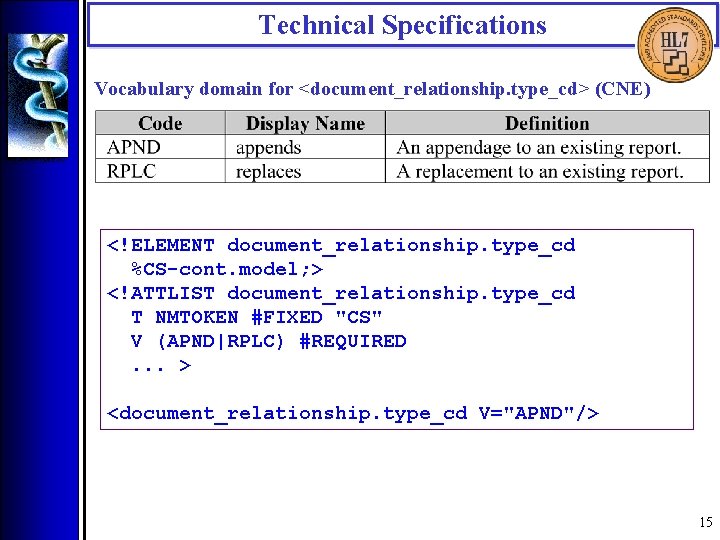
Technical Specifications Vocabulary domain for <document_relationship. type_cd> (CNE) <!ELEMENT document_relationship. type_cd %CS-cont. model; > <!ATTLIST document_relationship. type_cd T NMTOKEN #FIXED "CS" V (APND|RPLC) #REQUIRED. . . > <document_relationship. type_cd V="APND"/> 15

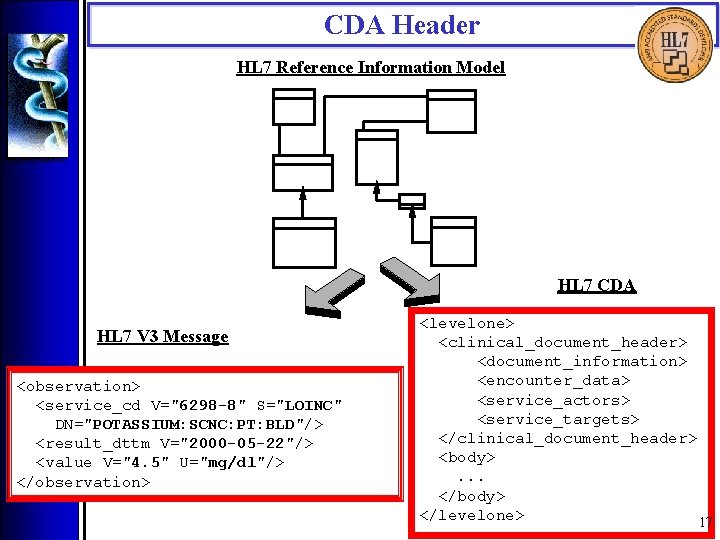
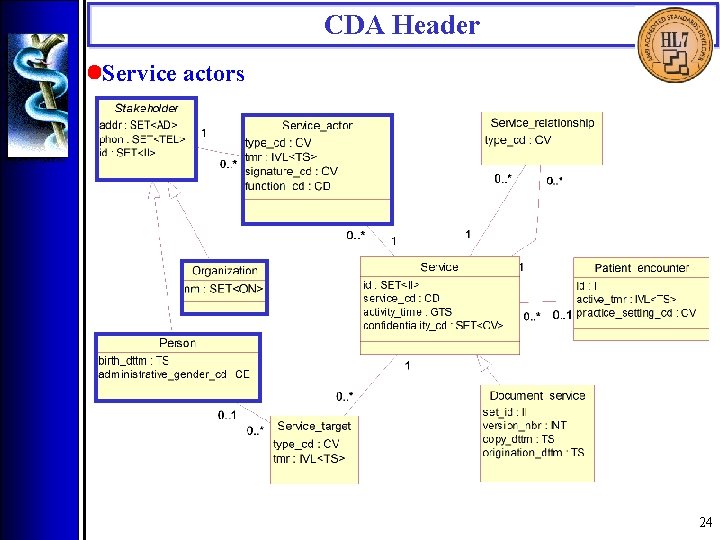
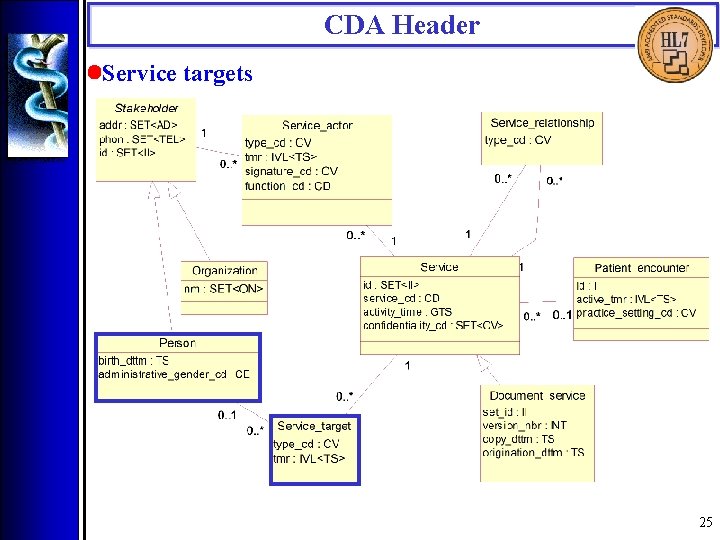
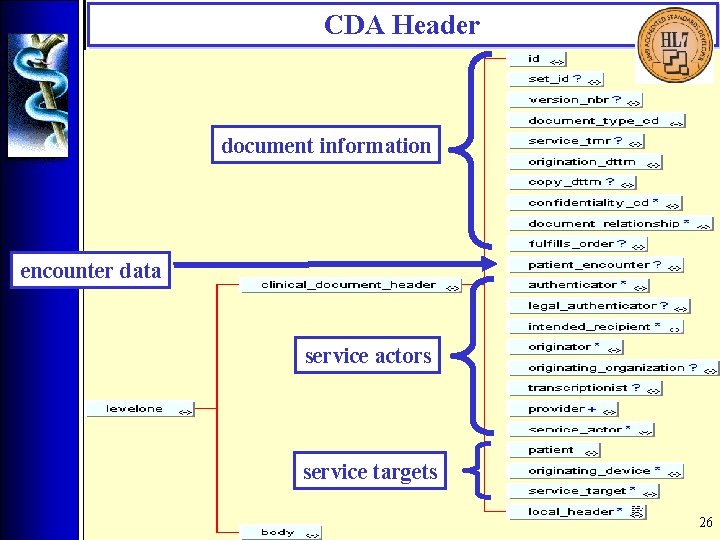
CDA Header • Overview The CDA Header is specified by the CDA Header DTD which is derived from a Hierarchical Description (HD), using a method that closely parallels the V 3 Message Development Framework. There are four logical components of the CDA Header: 1. Document information; 2. Encounter data; 3. Service actors (such as providers); 4. Service targets (such as patients). 16

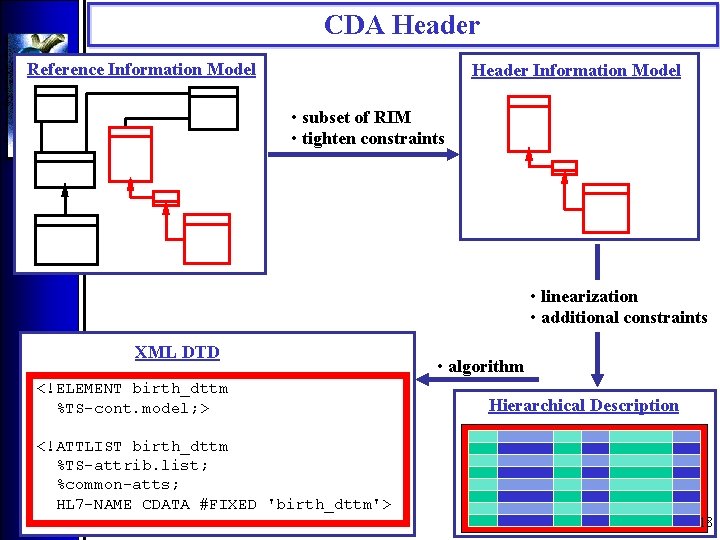
CDA Header HL 7 Reference Information Model HL 7 CDA HL 7 V 3 Message <observation> <service_cd V="6298 -8" S="LOINC" DN="POTASSIUM: SCNC: PT: BLD"/> <result_dttm V="2000 -05 -22"/> <value V="4. 5" U="mg/dl"/> </observation> <levelone> <clinical_document_header> <document_information> <encounter_data> <service_actors> <service_targets> </clinical_document_header> <body>. . . </body> </levelone> 17

CDA Header Reference Information Model Header Information Model • subset of RIM • tighten constraints • linearization • additional constraints XML DTD <!ELEMENT birth_dttm %TS-cont. model; > • algorithm Hierarchical Description <!ATTLIST birth_dttm %TS-attrib. list; %common-atts; HL 7 -NAME CDATA #FIXED 'birth_dttm'> 18

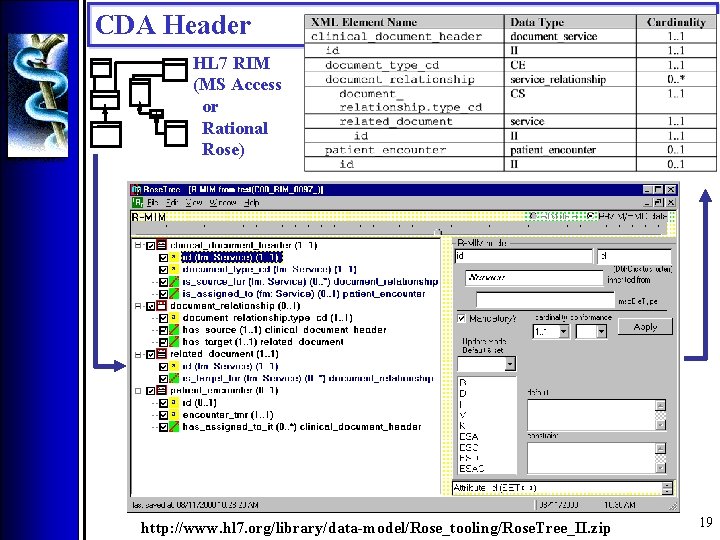
CDA Header HL 7 RIM (MS Access or Rational Rose) http: //www. hl 7. org/library/data-model/Rose_tooling/Rose. Tree_II. zip 19

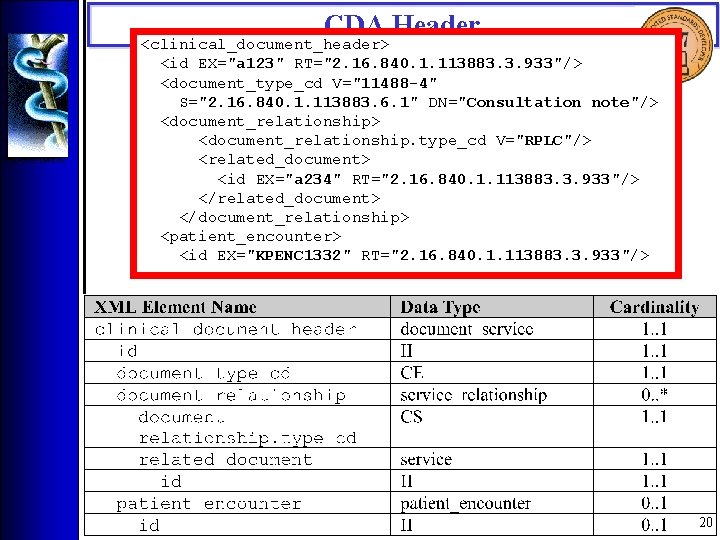
CDA Header <clinical_document_header> <id EX="a 123" RT="2. 16. 840. 1. 113883. 3. 933"/> <document_type_cd V="11488 -4" S="2. 16. 840. 1. 113883. 6. 1" DN="Consultation note"/> <document_relationship. type_cd V="RPLC"/> <related_document> <id EX="a 234" RT="2. 16. 840. 1. 113883. 3. 933"/> </related_document> </document_relationship> <patient_encounter> <id EX="KPENC 1332" RT="2. 16. 840. 1. 113883. 3. 933"/> 20

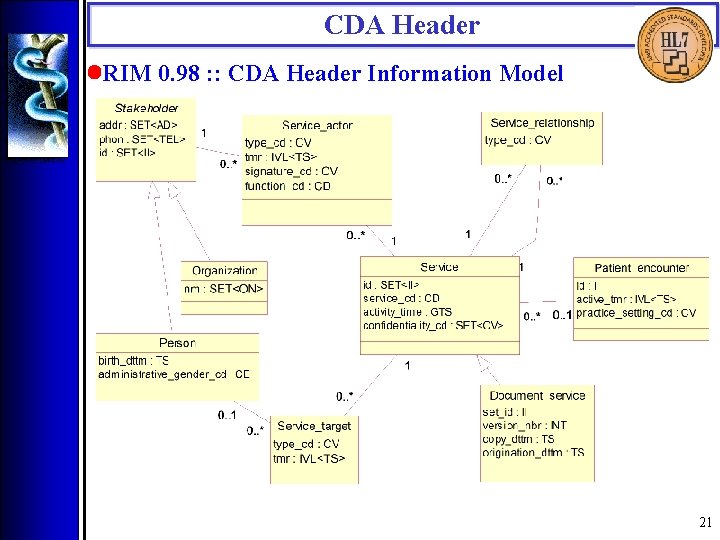
CDA Header • RIM 0. 98 : : CDA Header Information Model 21

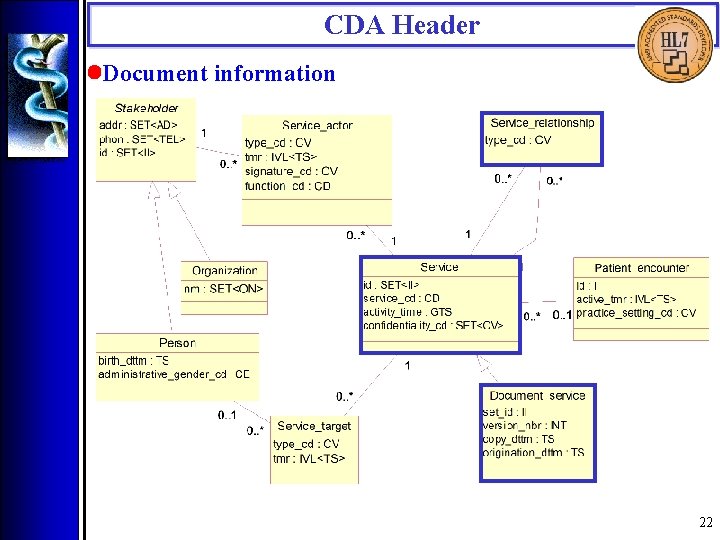
CDA Header • Document information 22

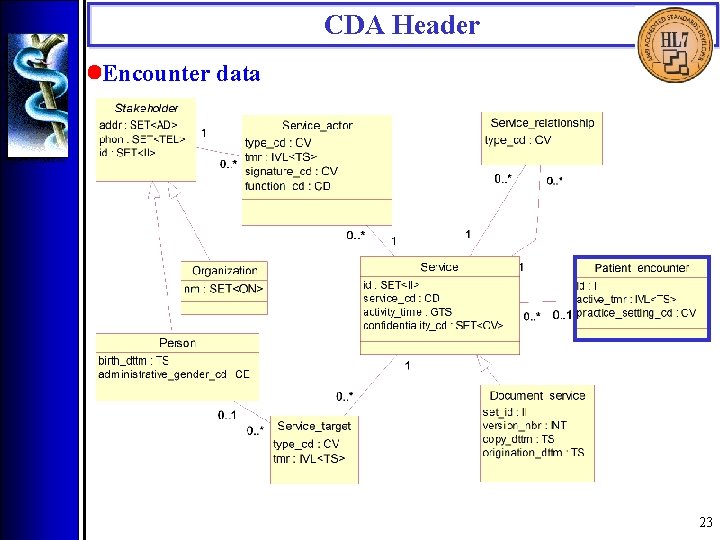
CDA Header • Encounter data 23

CDA Header • Service actors 24

CDA Header • Service targets 25

CDA Header document information encounter data service actors service targets 26

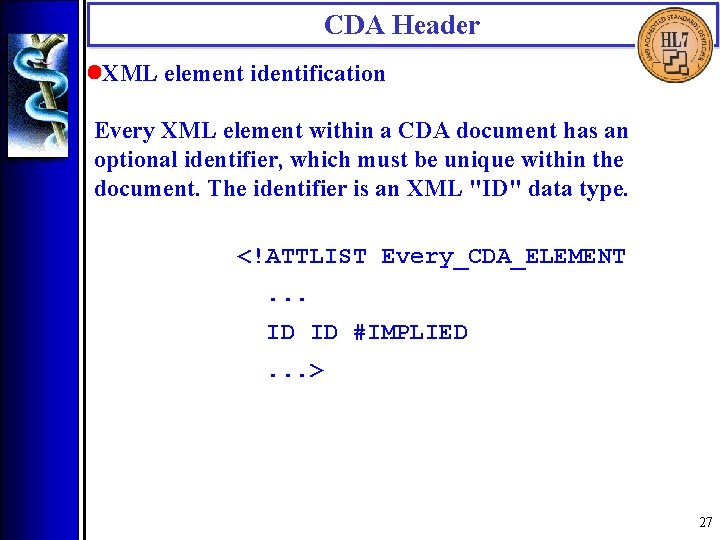
CDA Header • XML element identification Every XML element within a CDA document has an optional identifier, which must be unique within the document. The identifier is an XML "ID" data type. <!ATTLIST Every_CDA_ELEMENT. . . ID ID #IMPLIED. . . > 27

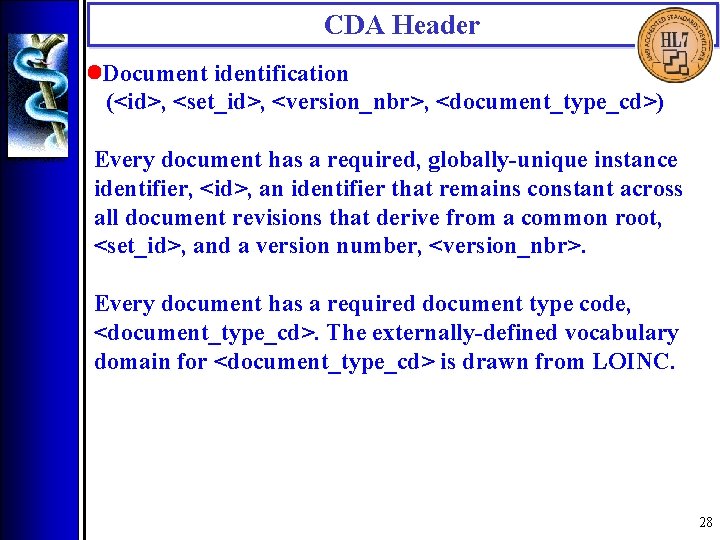
CDA Header • Document identification (<id>, <set_id>, <version_nbr>, <document_type_cd>) Every document has a required, globally-unique instance identifier, <id>, an identifier that remains constant across all document revisions that derive from a common root, <set_id>, and a version number, <version_nbr>. Every document has a required document type code, <document_type_cd>. The externally-defined vocabulary domain for <document_type_cd> is drawn from LOINC. 28

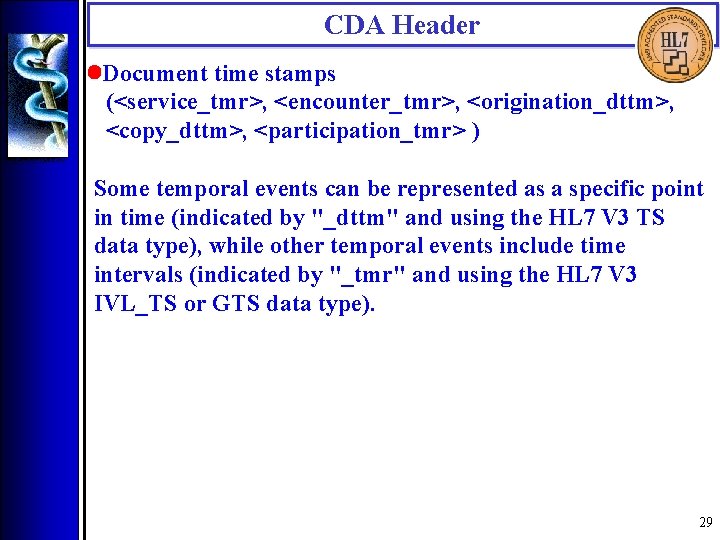
CDA Header • Document time stamps (<service_tmr>, <encounter_tmr>, <origination_dttm>, <copy_dttm>, <participation_tmr> ) Some temporal events can be represented as a specific point in time (indicated by "_dttm" and using the HL 7 V 3 TS data type), while other temporal events include time intervals (indicated by "_tmr" and using the HL 7 V 3 IVL_TS or GTS data type). 29

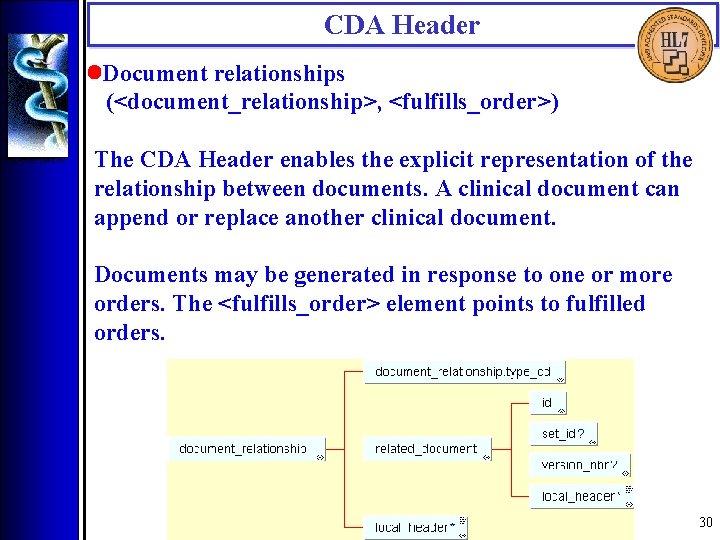
CDA Header • Document relationships (<document_relationship>, <fulfills_order>) The CDA Header enables the explicit representation of the relationship between documents. A clinical document can append or replace another clinical document. Documents may be generated in response to one or more orders. The <fulfills_order> element points to fulfilled orders. 30

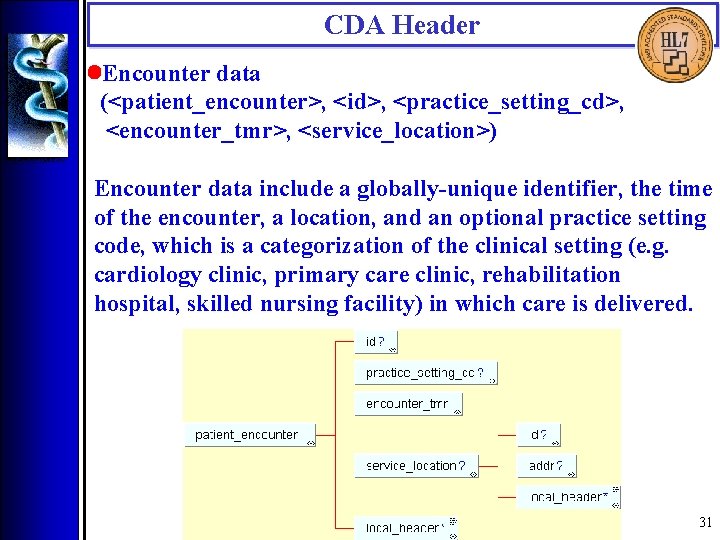
CDA Header • Encounter data (<patient_encounter>, <id>, <practice_setting_cd>, <encounter_tmr>, <service_location>) Encounter data include a globally-unique identifier, the time of the encounter, a location, and an optional practice setting code, which is a categorization of the clinical setting (e. g. cardiology clinic, primary care clinic, rehabilitation hospital, skilled nursing facility) in which care is delivered. 31

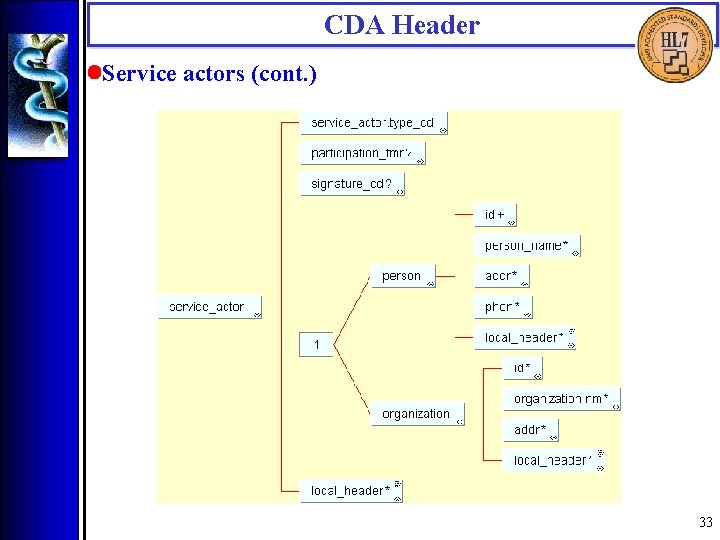
CDA Header • Service actors (<authenticator>, <legal_authenticator>, <intended_recipient>, <originator>, <originating_organization>, <transcriptionist>, <provider>, <service_actor>) Service actors include those who authenticate the document, those intended to receive a copy of the document, document originators and transcriptionists, and health care providers who participated in the service(s) being documented. Service actors are capable of and accountable for their independent decisions. 32

CDA Header • Service actors (cont. ) 33

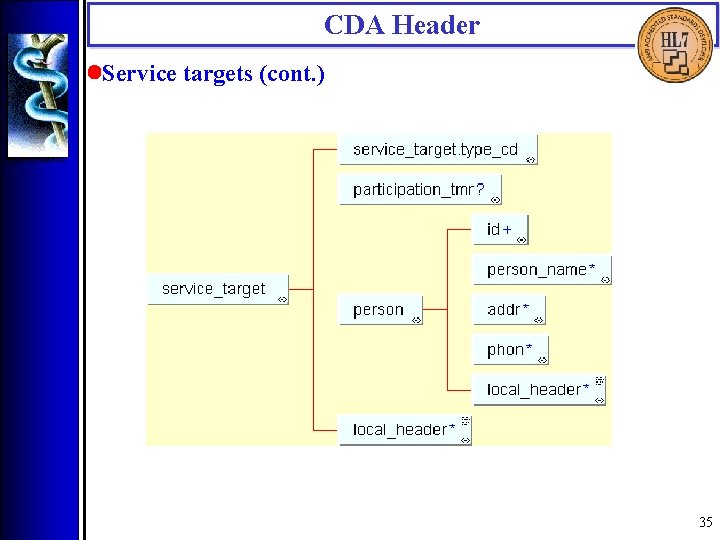
CDA Header • Service targets (<patient>, <originating_device>, <service_target>) Service targets are physical entities, including living subjects and inanimaterial, that are typically the object of services being documented. Service targets include the patient, other significant participants (such as family members), and those devices that may have originated portions of the document. 34

CDA Header • Service targets (cont. ) 35

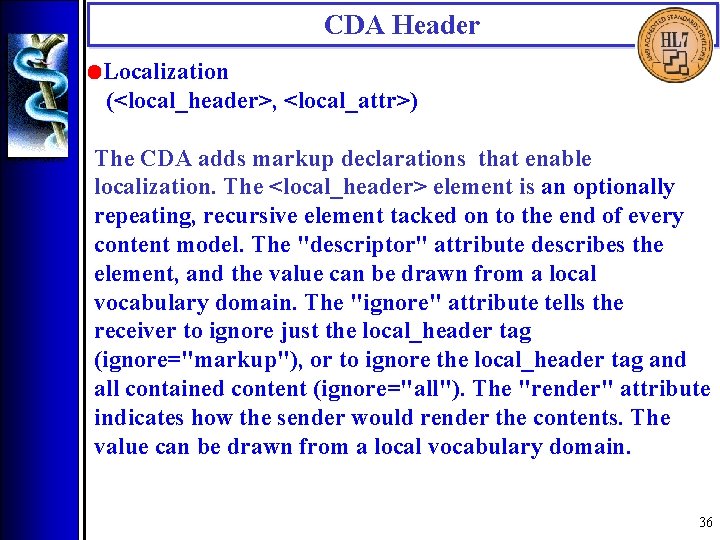
CDA Header • Localization (<local_header>, <local_attr>) The CDA adds markup declarations that enable localization. The <local_header> element is an optionally repeating, recursive element tacked on to the end of every content model. The "descriptor" attribute describes the element, and the value can be drawn from a local vocabulary domain. The "ignore" attribute tells the receiver to ignore just the local_header tag (ignore="markup"), or to ignore the local_header tag and all contained content (ignore="all"). The "render" attribute indicates how the sender would render the contents. The value can be drawn from a local vocabulary domain. 36

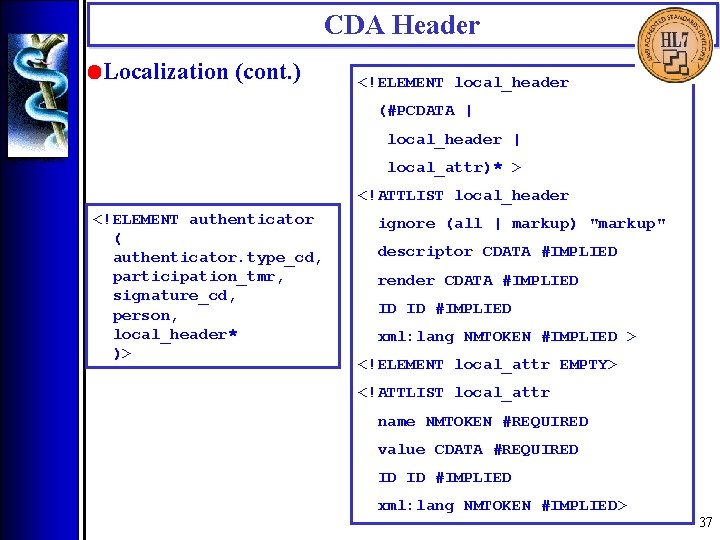
CDA Header • Localization (cont. ) <!ELEMENT local_header (#PCDATA | local_header | local_attr)* > <!ATTLIST local_header <!ELEMENT authenticator ( authenticator. type_cd, participation_tmr, signature_cd, person, local_header* )> ignore (all | markup) "markup" descriptor CDATA #IMPLIED render CDATA #IMPLIED ID ID #IMPLIED xml: lang NMTOKEN #IMPLIED > <!ELEMENT local_attr EMPTY> <!ATTLIST local_attr name NMTOKEN #REQUIRED value CDATA #REQUIRED ID ID #IMPLIED xml: lang NMTOKEN #IMPLIED> 37

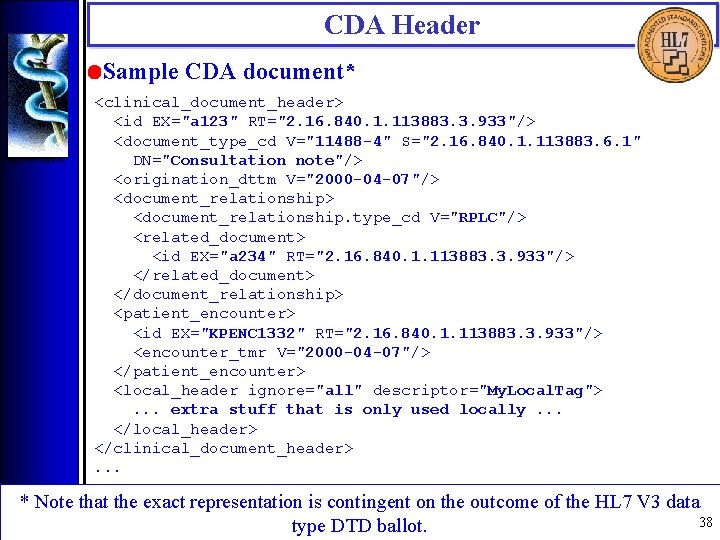
CDA Header • Sample CDA document* <clinical_document_header> <id EX="a 123" RT="2. 16. 840. 1. 113883. 3. 933"/> <document_type_cd V="11488 -4" S="2. 16. 840. 1. 113883. 6. 1" DN="Consultation note"/> <origination_dttm V="2000 -04 -07"/> <document_relationship. type_cd V="RPLC"/> <related_document> <id EX="a 234" RT="2. 16. 840. 1. 113883. 3. 933"/> </related_document> </document_relationship> <patient_encounter> <id EX="KPENC 1332" RT="2. 16. 840. 1. 113883. 3. 933"/> <encounter_tmr V="2000 -04 -07"/> </patient_encounter> <local_header ignore="all" descriptor="My. Local. Tag">. . . extra stuff that is only used locally. . . </local_header> </clinical_document_header>. . . * Note that the exact representation is contingent on the outcome of the HL 7 V 3 data 38 type DTD ballot.

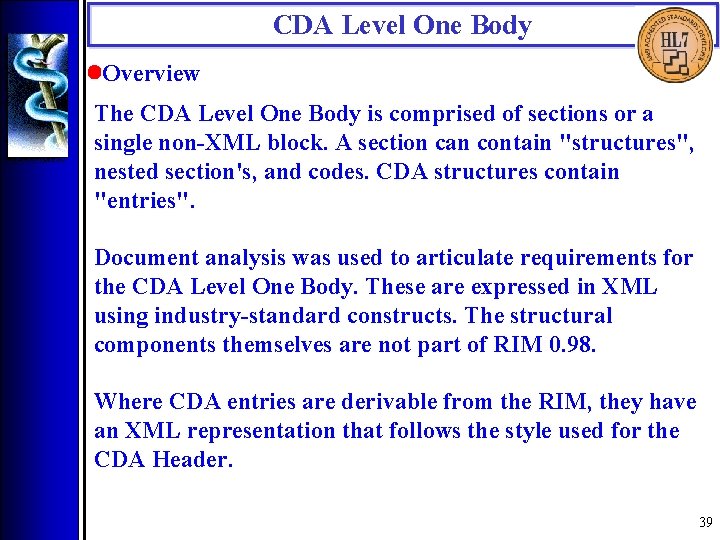
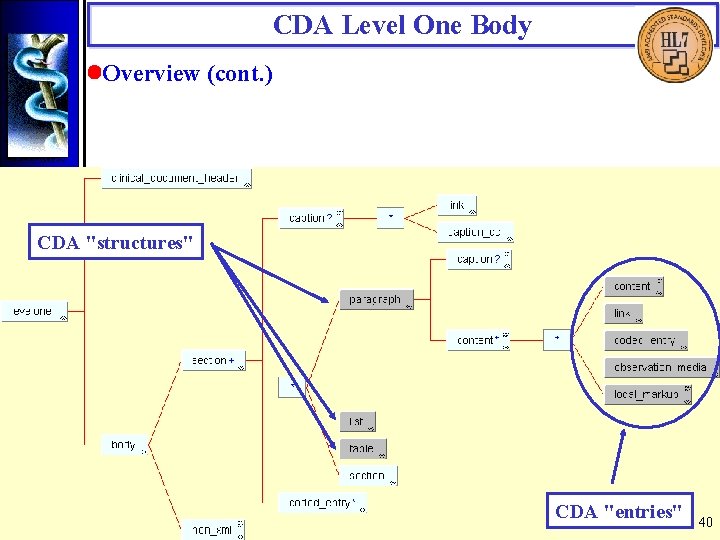
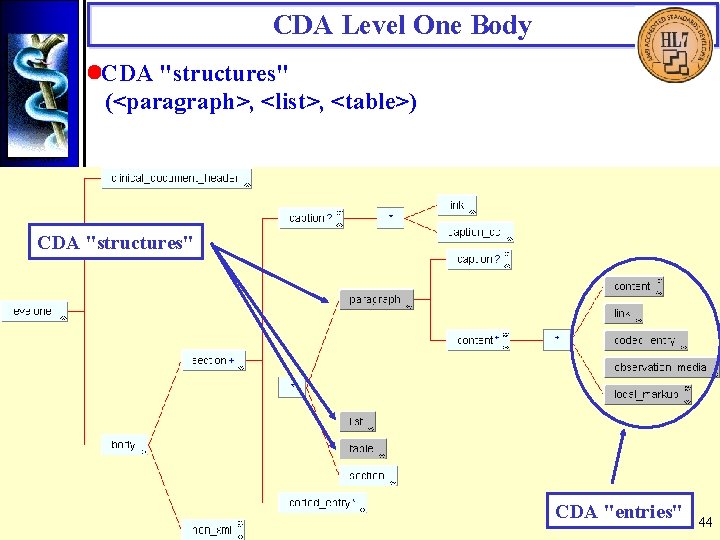
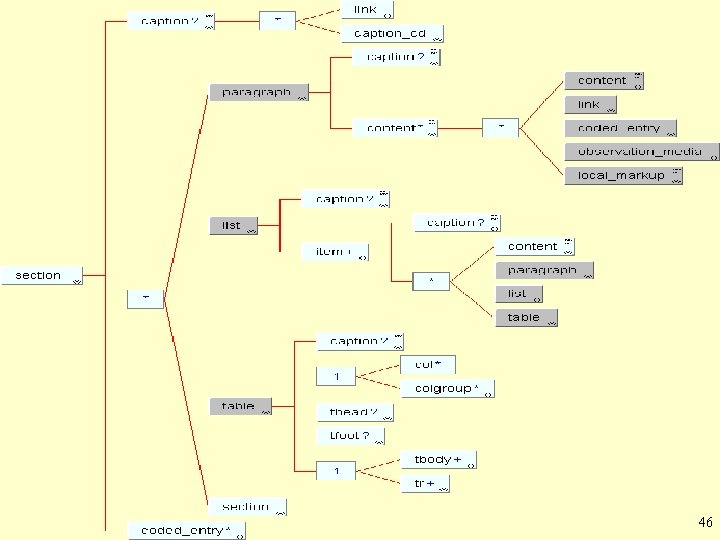
CDA Level One Body • Overview The CDA Level One Body is comprised of sections or a single non-XML block. A section can contain "structures", nested section's, and codes. CDA structures contain "entries". Document analysis was used to articulate requirements for the CDA Level One Body. These are expressed in XML using industry-standard constructs. The structural components themselves are not part of RIM 0. 98. Where CDA entries are derivable from the RIM, they have an XML representation that follows the style used for the CDA Header. 39

CDA Level One Body • Overview (cont. ) CDA "structures" CDA "entries" 40

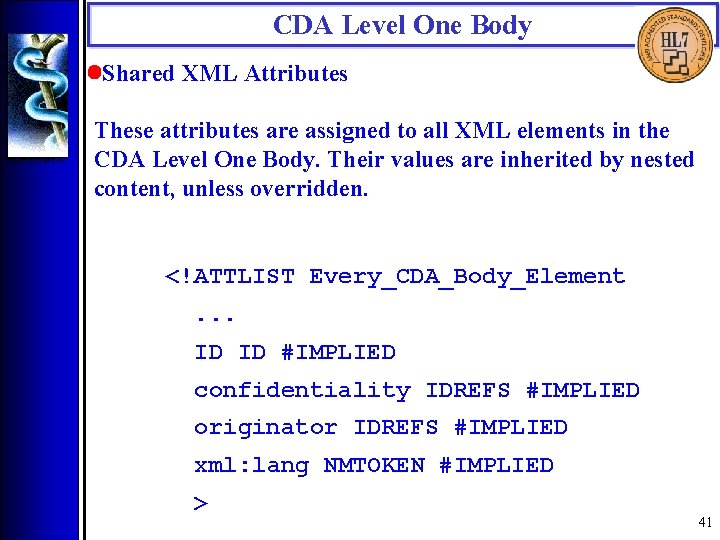
CDA Level One Body • Shared XML Attributes These attributes are assigned to all XML elements in the CDA Level One Body. Their values are inherited by nested content, unless overridden. <!ATTLIST Every_CDA_Body_Element. . . ID ID #IMPLIED confidentiality IDREFS #IMPLIED originator IDREFS #IMPLIED xml: lang NMTOKEN #IMPLIED > 41

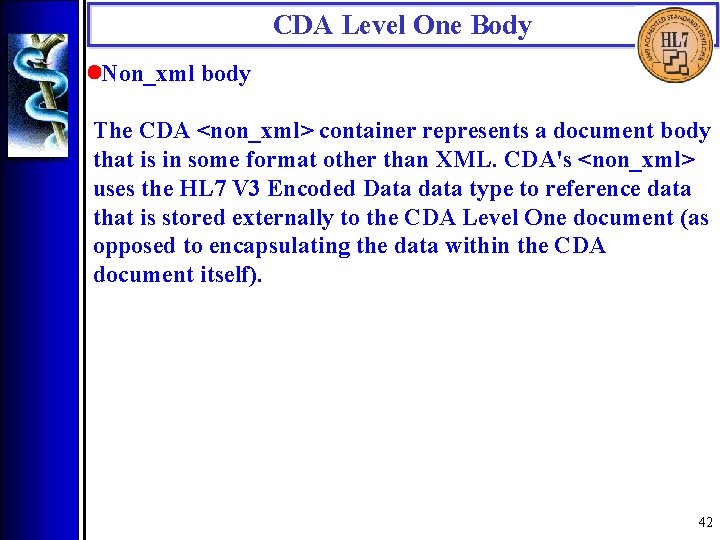
CDA Level One Body • Non_xml body The CDA <non_xml> container represents a document body that is in some format other than XML. CDA's <non_xml> uses the HL 7 V 3 Encoded Data data type to reference data that is stored externally to the CDA Level One document (as opposed to encapsulating the data within the CDA document itself). 42

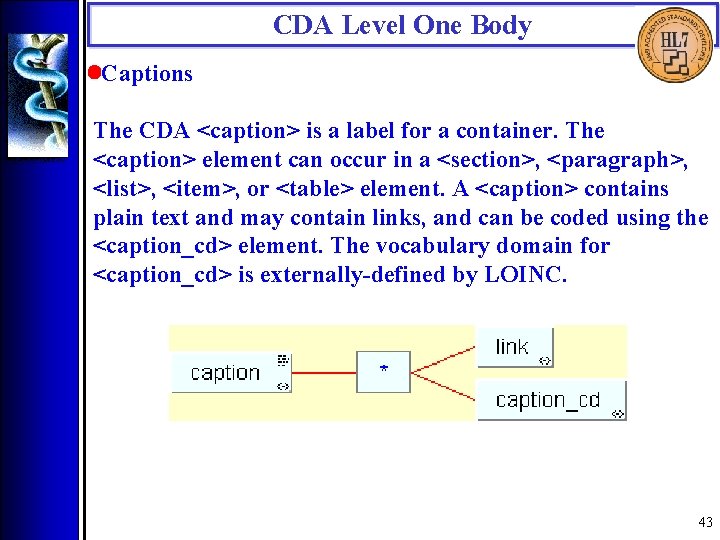
CDA Level One Body • Captions The CDA <caption> is a label for a container. The <caption> element can occur in a <section>, <paragraph>, <list>, <item>, or <table> element. A <caption> contains plain text and may contain links, and can be coded using the <caption_cd> element. The vocabulary domain for <caption_cd> is externally-defined by LOINC. 43

CDA Level One Body • CDA "structures" (<paragraph>, <list>, <table>) CDA "structures" CDA "entries" 44

CDA Level One Body • CDA "structures" (cont. ) Structures have optional captions. A <paragraph> contains CDA "entries". A <list> contains one or more <item>'s. An <item> contains entries and nested structures. The CDA <table> is a modification of the strict XHTML table model. In CDA Level One, any information can be presented as a table. The table markup is for presentation purposes only and, unlike a database table, does not possess meaningful field names. 45

CDA Level One Body • CDA "structures" (cont. ) 46

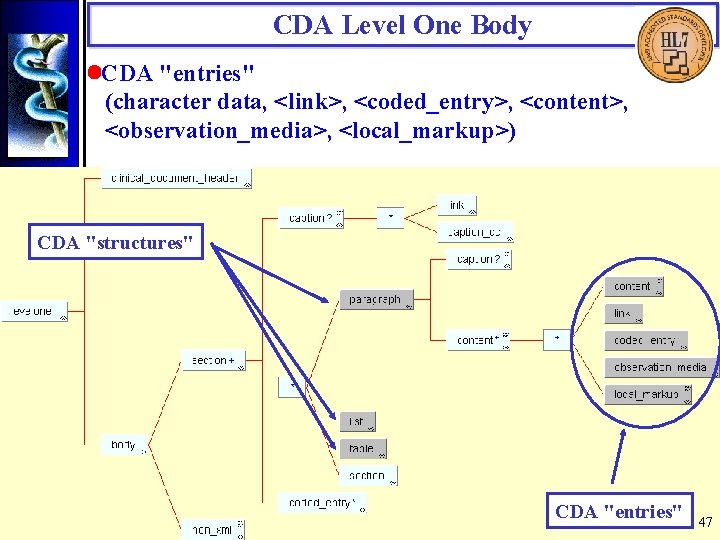
CDA Level One Body • CDA "entries" (character data, <link>, <coded_entry>, <content>, <observation_media>, <local_markup>) CDA "structures" CDA "entries" 47

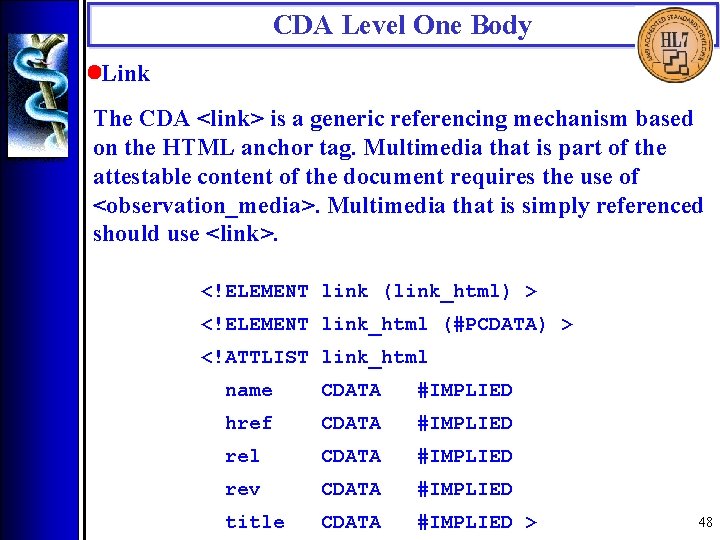
CDA Level One Body • Link The CDA <link> is a generic referencing mechanism based on the HTML anchor tag. Multimedia that is part of the attestable content of the document requires the use of <observation_media>. Multimedia that is simply referenced should use <link>. <!ELEMENT link (link_html) > <!ELEMENT link_html (#PCDATA) > <!ATTLIST link_html name CDATA #IMPLIED href CDATA #IMPLIED rel CDATA #IMPLIED rev CDATA #IMPLIED title CDATA #IMPLIED > 48

CDA Level One Body • Coded_entry The CDA <coded_entry> uses the HL 7 V 3 Concept Descriptor data type to insert codes from HL 7 -recognized coding schemes into CDA documents. Where there are no suitable HL 7 -recognized codes available, locally-defined codes can be used. <content> Asthma, with prior smoking history. Difficulty weaning off steroids. Will try gradual taper. <coded_entry> <coded_entry. value V="D 2 -51000" S="2. 16. 840. 1. 113883. 6. 5" DN="Asthma"/> </coded_entry> </content> 49

CDA Level One Body • Content The <content> element nests recursively, enabling wrapping a string of text down to as small a chunk as desired. These <content> elements can serve as anchors to be referenced by <coded_entry> elements to indicate the original text that supports the use of a code. <content> <content ID="String 001">Asthma</content>, with prior smoking history. Difficulty weaning off steroids. Will try gradual taper. <coded_entry> <coded_entry. value ORIGTXT="String 001" V="D 2 -51000" S="2. 16. 840. 1. 113883. 6. 5" DN="Asthma"/> </coded_entry> </content> 50

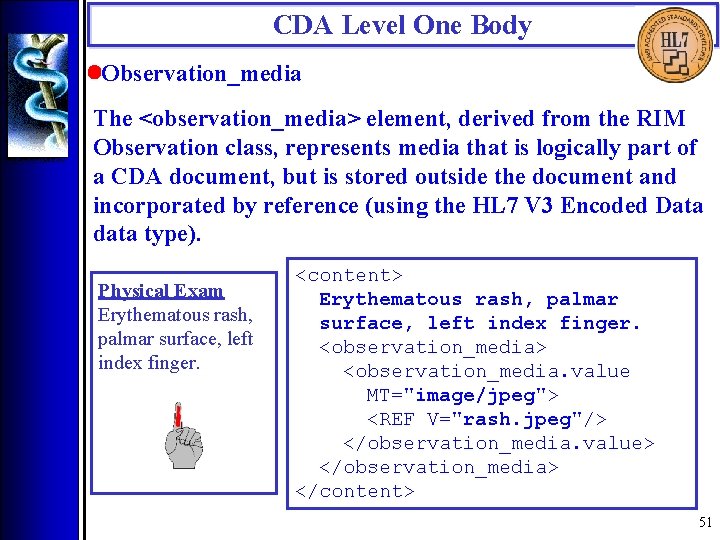
CDA Level One Body • Observation_media The <observation_media> element, derived from the RIM Observation class, represents media that is logically part of a CDA document, but is stored outside the document and incorporated by reference (using the HL 7 V 3 Encoded Data data type). Physical Exam Erythematous rash, palmar surface, left index finger. <content> Erythematous rash, palmar surface, left index finger. <observation_media> <observation_media. value MT="image/jpeg"> <REF V="rash. jpeg"/> </observation_media. value> </observation_media> </content> 51

CDA Level One Body • Localization The implementation of localization in the CDA Level One Body using the <local_markup> element parallels the approach used in the CDA Header. 52

Outline • Objectives • Overview of August 2000 Clinical Document Architecture ballot proposal • Introduction • What is the CDA? • The "A" in "CDA" • Relationship of CDA to HL 7 Messaging Standards • Technical Specifications • CDA Header • CDA Body • HL 7 V 3 Data Types 53