Classifying Chemical Reactions Types Synthesis Reaction Decomposition Reactions









- Slides: 18

Classifying Chemical Reactions Types • Synthesis Reaction • Decomposition Reactions • Single-Replacement Reactions • Double-replacement Reactions • Combustion Reactions

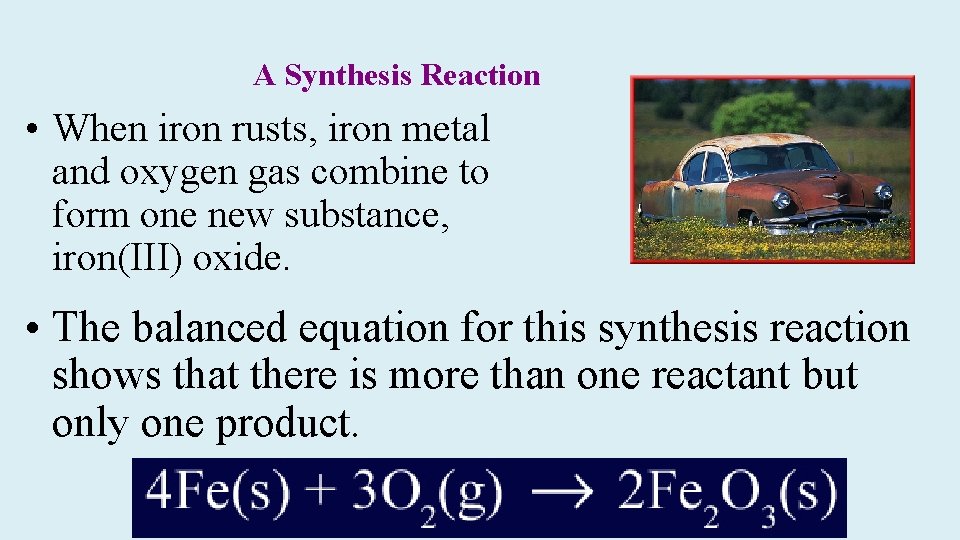
A Synthesis Reaction • When iron rusts, iron metal and oxygen gas combine to form one new substance, iron(III) oxide. • The balanced equation for this synthesis reaction shows that there is more than one reactant but only one product.

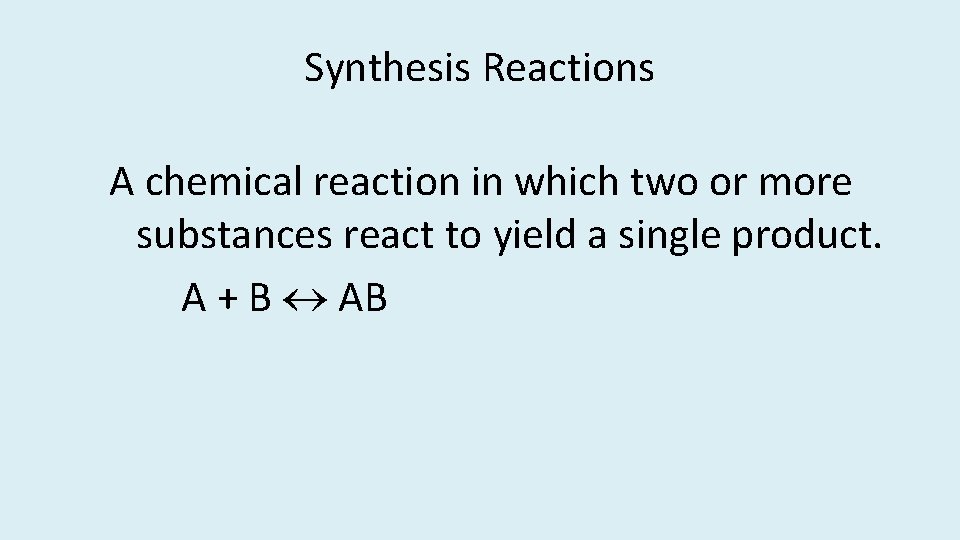
Synthesis Reactions A chemical reaction in which two or more substances react to yield a single product. A + B AB

• Zinc and Iodide synthesis reaction

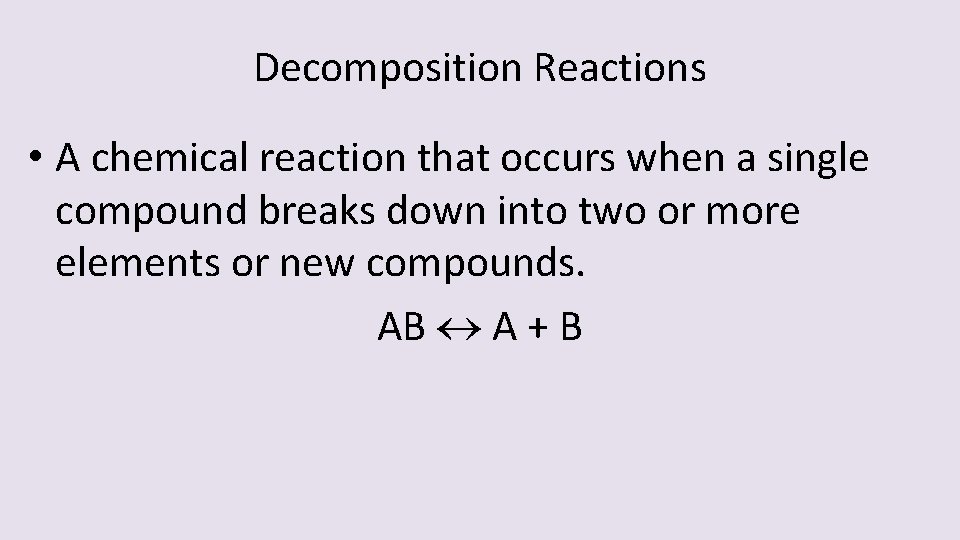
Decomposition Reactions • A chemical reaction that occurs when a single compound breaks down into two or more elements or new compounds. AB A + B

A Decomposition Reaction • When ammonium nitrate is heated to a high temperature, it explosively breaks down into dinitrogen monoxide and water. • The decomposition reaction taking place is represented by a balanced equation that shows one reactant and more than one product.

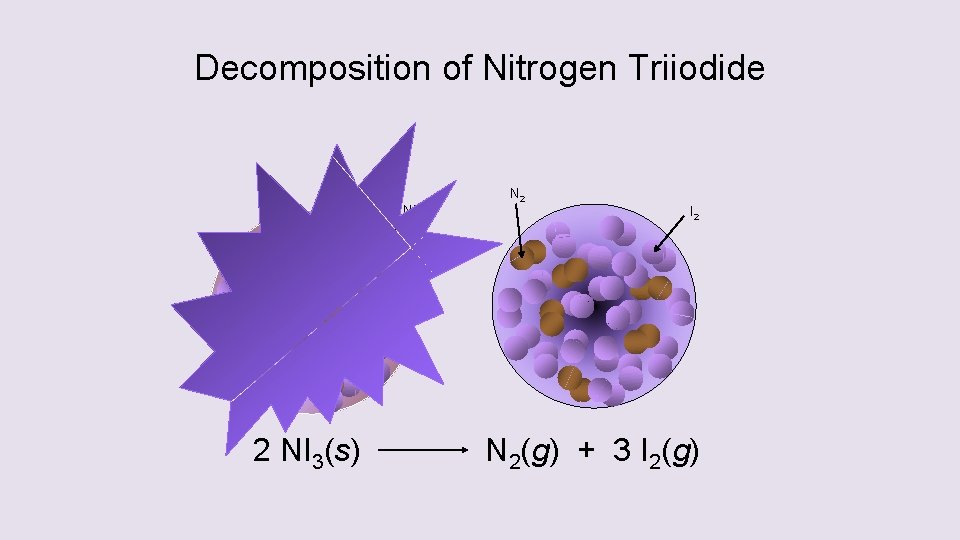
Decomposition of Nitrogen Triiodide

Decomposition of Nitrogen Triiodide NI 3 2 NI 3(s) N 2 I 2 N 2(g) + 3 I 2(g)

• You. Tube - Elementary Productions: Ammonium Dichromate

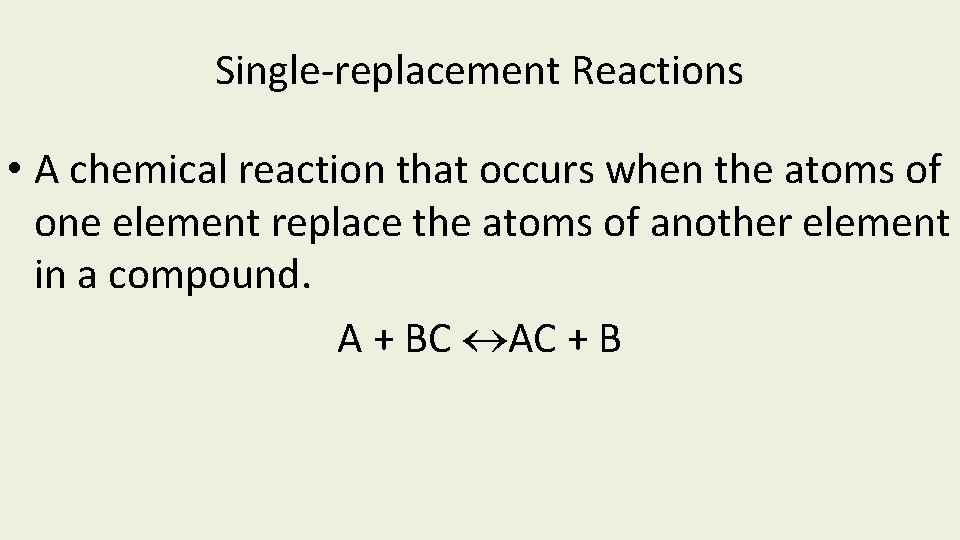
Single-replacement Reactions • A chemical reaction that occurs when the atoms of one element replace the atoms of another element in a compound. A + BC AC + B

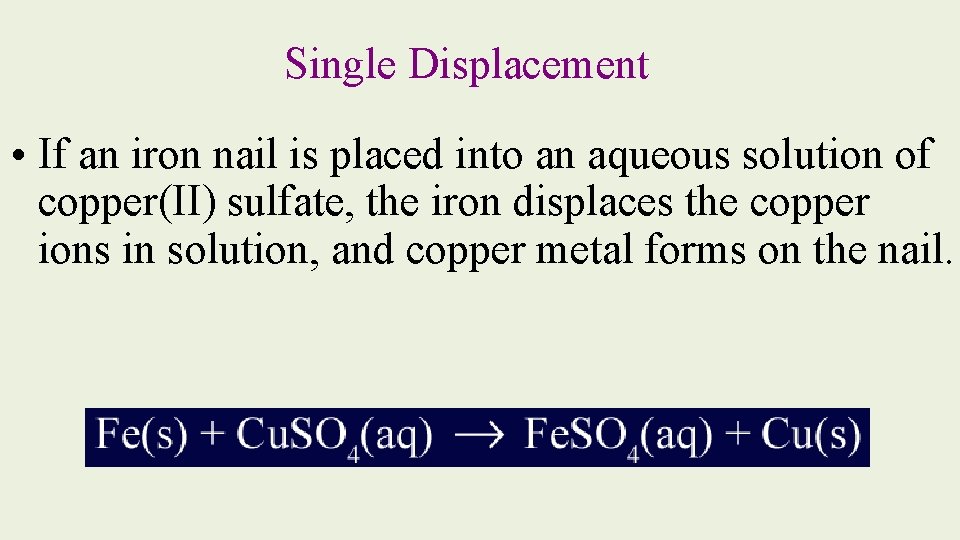
Single Displacement • If an iron nail is placed into an aqueous solution of copper(II) sulfate, the iron displaces the copper ions in solution, and copper metal forms on the nail.

• You. Tube - Single Replacement Reactions

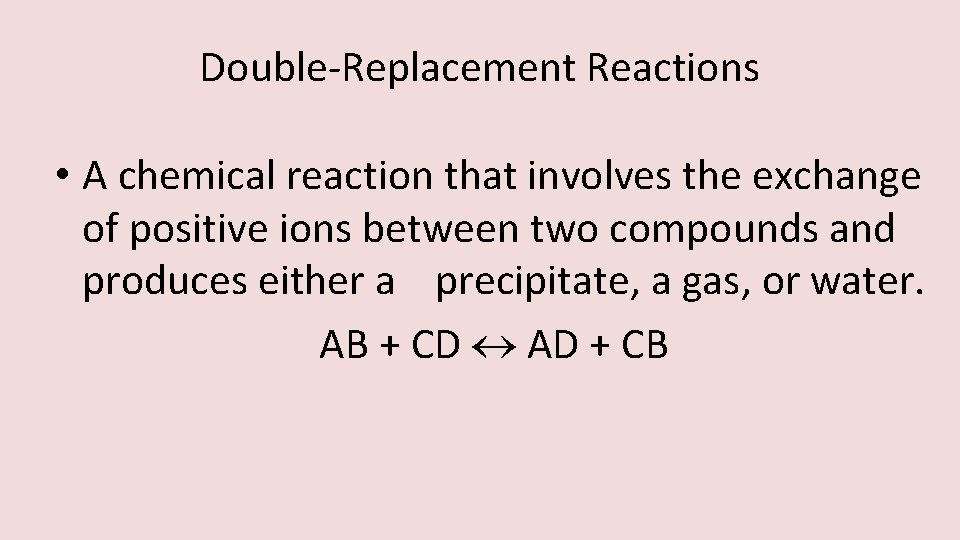
Double-Replacement Reactions • A chemical reaction that involves the exchange of positive ions between two compounds and produces either a precipitate, a gas, or water. AB + CD AD + CB

Double Replacement • When clear aqueous solutions of lead(II) nitrate and potassium iodine are mixed, a doubledisplacement reaction takes place and a yellow solid appears in the mixture. • This solid is lead(II) iodine, and it precipitates out because it is insoluble in water, unlike the two reactants and the other product.

• You. Tube - Double Replacement Reactions

Combustion Reactions • A chemical reaction that occurs when a substance reacts with oxygen, releasing energy in the form of heat and light. Fuel + Oxygen → Carbon dioxide + Water + Heat + Light

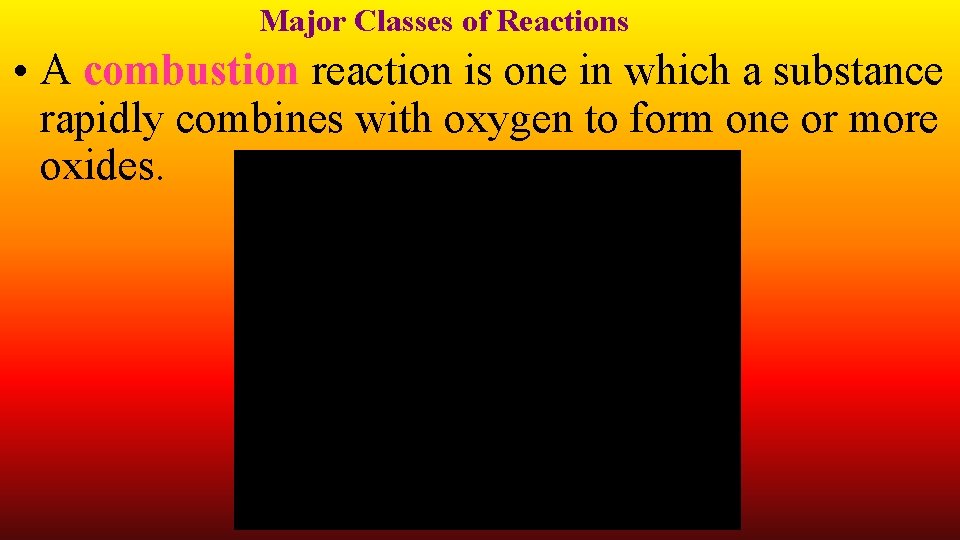
Major Classes of Reactions • A combustion reaction is one in which a substance rapidly combines with oxygen to form one or more oxides.

Combustion • When welding is done with an acetylene torch, acetylene combines with oxygen to form carbon dioxide and water. • This combustion reaction is exothermic, and enough energy is released to melt metal.