Synthesis and Decomposition Reactions Lesson Outline Synthesis reactions







- Slides: 15

Synthesis and Decomposition Reactions

Lesson Outline Synthesis reactions Types of Synthesis reactions How to determine the products of synthesis reactions Decomposition reactions How to determine the products of decomposition reactions

Specific expectations C 2. 2 Write balanced chemical equations to represent synthesis, decomposition, single displacement, double displacement, and combustion reactions, using the IUPAC nomenclature system. C 2. 4 predict the products of different types of synthesis and decomposition reactions. C 3. 1 identify various types of chemical reactions, including synthesis, decomposition, single displacement, double displacement, and combustion.

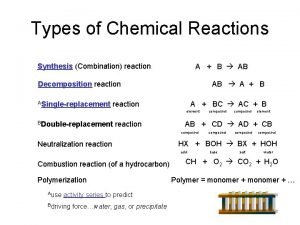
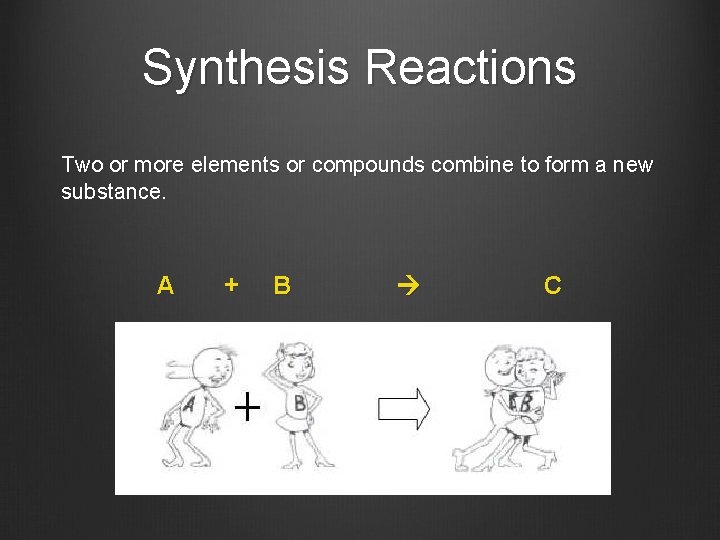
Synthesis Reactions Two or more elements or compounds combine to form a new substance. A + B C

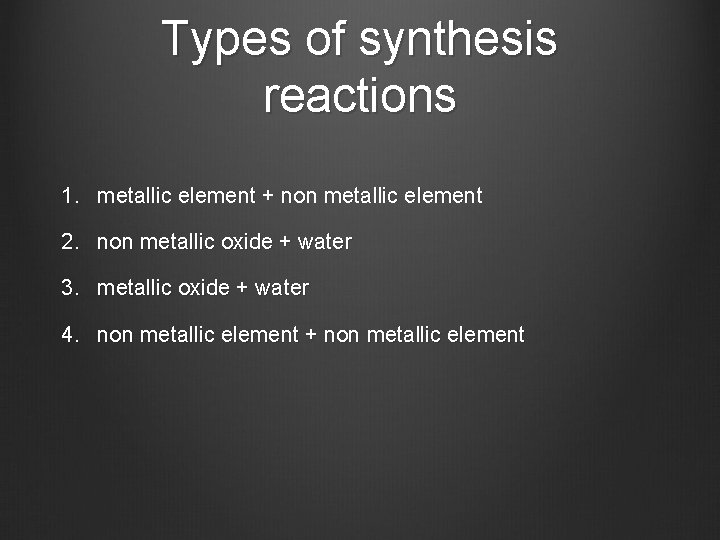
Types of synthesis reactions 1. metallic element + non metallic element 2. non metallic oxide + water 3. metallic oxide + water 4. non metallic element + non metallic element

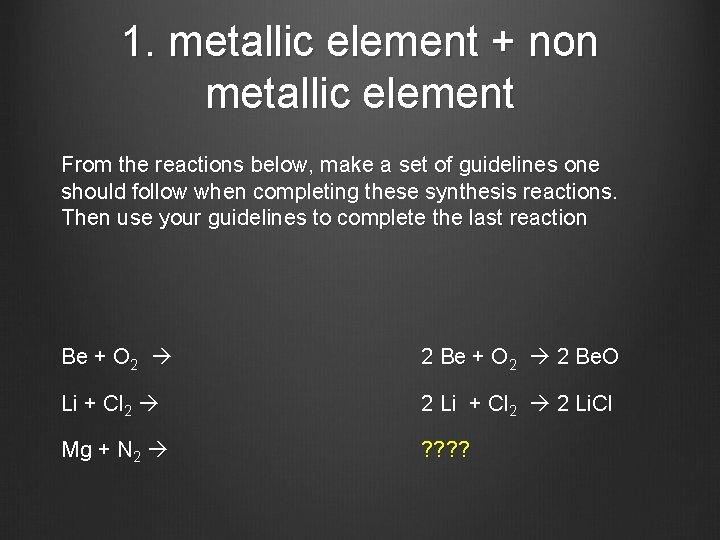
1. metallic element + non metallic element From the reactions below, make a set of guidelines one should follow when completing these synthesis reactions. Then use your guidelines to complete the last reaction Be + O 2 2 Be. O Li + Cl 2 2 Li. Cl Mg + N 2 ? ?

1. metallic element + non metallic element How to determine the reaction products 1. Combine both reactants to form the product. 2. Use the zero sum rule (drop the charges) to determine the element ratio of the product. 3. Balance the equation. Ex. Fe + O 2

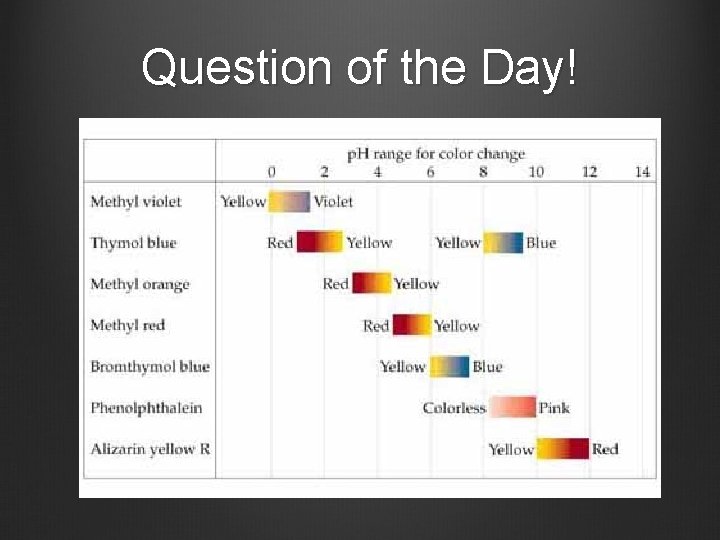
Question of the Day!

2. non metallic oxide + water Non metallic oxide + water acid hydrogen cation + polyatomic anion SO 3 + H 20 H 2 SO 4 How to determine the reaction products: 1. Determine the polyatomic anion formed. (usually addition oxygen atom to polyatomic ion) 2. Determine the ratio between the hydrogen cation and polyatomic anion (zero sum) 3. Balance the equation. Ex. SO 2 + H 20

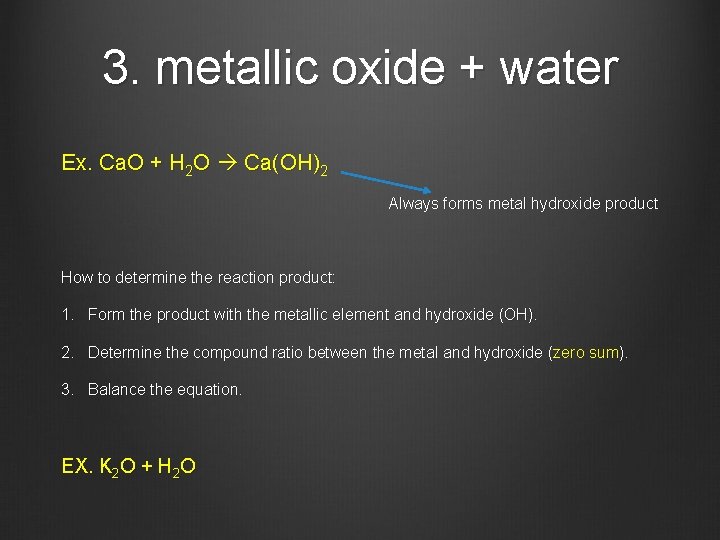
3. metallic oxide + water Ex. Ca. O + H 2 O Ca(OH)2 Always forms metal hydroxide product How to determine the reaction product: 1. Form the product with the metallic element and hydroxide (OH). 2. Determine the compound ratio between the metal and hydroxide (zero sum). 3. Balance the equation. EX. K 2 O + H 2 O

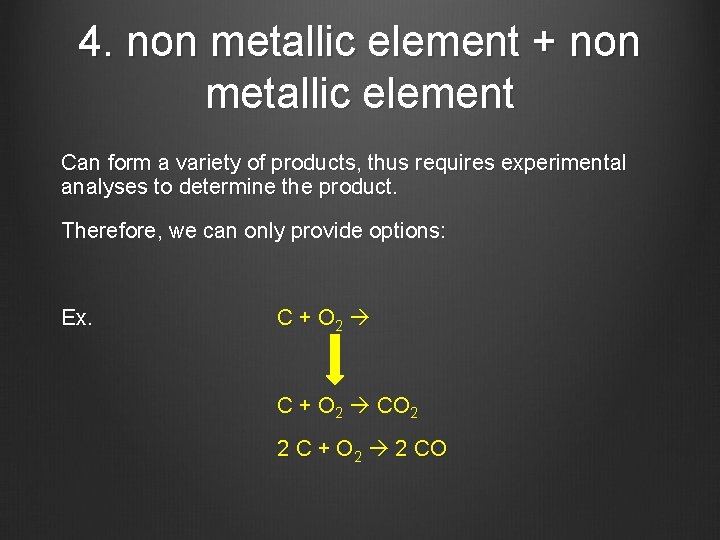
4. non metallic element + non metallic element Can form a variety of products, thus requires experimental analyses to determine the product. Therefore, we can only provide options: Ex. C + O 2 CO 2 2 C + O 2 2 CO

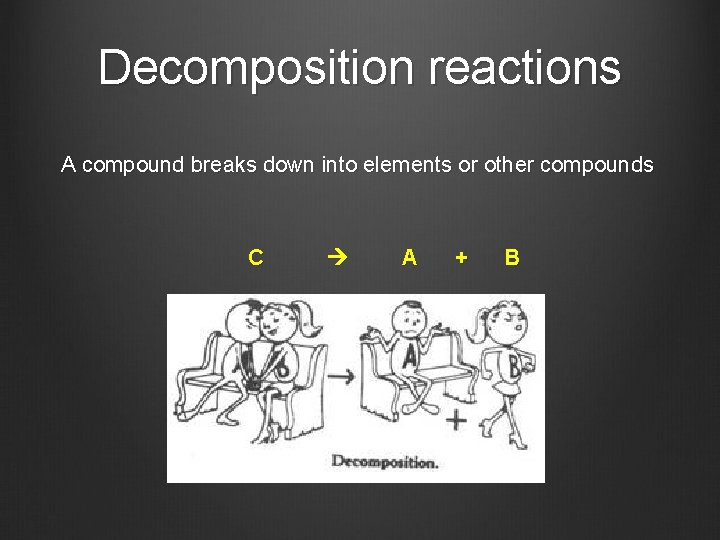
Decomposition reactions A compound breaks down into elements or other compounds C A + B

Decomposition reactions 1. What is extremely important to remember when forming the products of a decomposition reaction? 2. Complete the last reaction! think-pair-share activity 2 H 2 O 2 H 2 + O 2 2 Al. Cl 3 2 Al +3 Cl 2 Mg. O ? ? ?

Decomposition reactions How to determine the products: 1. Break apart the reactant into the resulting elements. 2. Ensure that all diatomic atoms have a subscript of 2 (H 2). 3. Balance the equation. Example: Ag 2 O

Homework Define the following terms: Exothermic reactions Endothermic reactions
 Lesson outline lesson 1 solids liquids and gases answer key
Lesson outline lesson 1 solids liquids and gases answer key Lesson outline lesson 1 magnets and magnetic fields
Lesson outline lesson 1 magnets and magnetic fields The sun-earth-moon system worksheet answers lesson 1
The sun-earth-moon system worksheet answers lesson 1 Combustion definition chemistry
Combustion definition chemistry Reaction type synthesis
Reaction type synthesis Opposite of synthesis reaction
Opposite of synthesis reaction Examples combination reaction
Examples combination reaction Tetrahydroxoaluminate
Tetrahydroxoaluminate Synthesis decomposition
Synthesis decomposition Lesson outline lesson 3 describing circuits answers
Lesson outline lesson 3 describing circuits answers Mountain building
Mountain building Lesson outline lesson 2 aquatic ecosystems answer key
Lesson outline lesson 2 aquatic ecosystems answer key Weather forecast lesson 3 outline answers
Weather forecast lesson 3 outline answers Lesson outline physical properties lesson 2
Lesson outline physical properties lesson 2 Unit 6 lesson 1 climates of the world
Unit 6 lesson 1 climates of the world Lesson outline lesson 2
Lesson outline lesson 2