Chemistry 6e Steven S Zumdahl and Susan A

















- Slides: 28

Chemistry 6/e Steven S. Zumdahl and Susan A. Zumdahl Chapter 6: THERMOCHEMISTRY Copyright © Houghton Mifflin Company. All Rights Reserved. 1

Thermochemistry • The Nature of Energy • React 1 • React 2 • React 3 • React 4 • React 5 • React 6 • React 7 • React 8 • Enthalpy and Calorimetry • React 9 • React 10 • React 11 • Hess’s Law and Standard Enthalpies of Formation • React 12 • Present Sources of Energy Copyright © Houghton Mifflin Company. All Rights Reserved. 2

The Nature of Energy Copyright © Houghton Mifflin Company. All Rights Reserved. 3

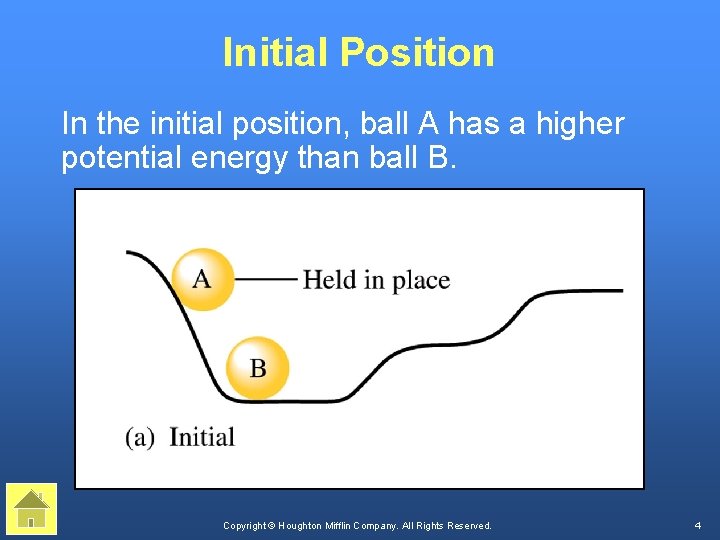
Initial Position In the initial position, ball A has a higher potential energy than ball B. Copyright © Houghton Mifflin Company. All Rights Reserved. 4

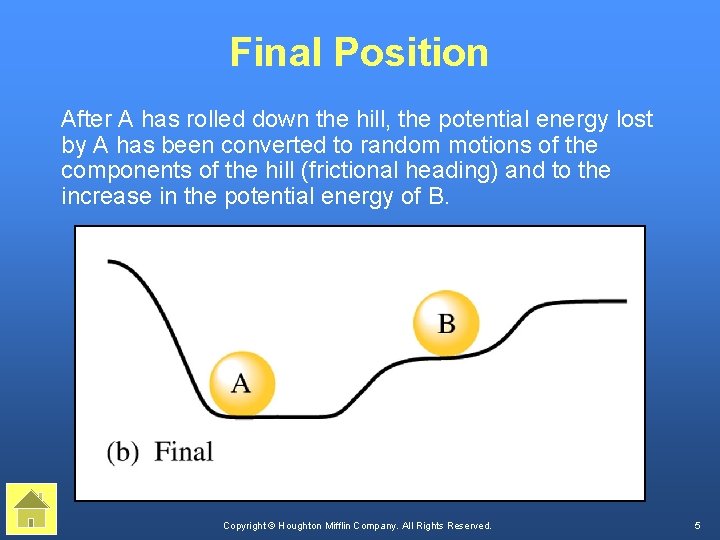
Final Position After A has rolled down the hill, the potential energy lost by A has been converted to random motions of the components of the hill (frictional heading) and to the increase in the potential energy of B. Copyright © Houghton Mifflin Company. All Rights Reserved. 5

Thermite Copyright © Houghton Mifflin Company. All Rights Reserved. 6

Ammonium Chloride and Barium Hydroxide Copyright © Houghton Mifflin Company. All Rights Reserved. 7

1 t eac R You strike an unlit match and it burns. Explain the energy transfers of this scenario using the terms exothermic, endothermic, system, surroundings, potential energy, and kinetic energy in your discussion. Sketch an energy level diagram for the process. Copyright © Houghton Mifflin Company. All Rights Reserved. 8

2 t eac R Is the freezing of water an exothermic or an endothermic process? Explain. Copyright © Houghton Mifflin Company. All Rights Reserved. 9

3 t eac R Classify each of the following processes as exothermic or endothermic. Explain. I. Your hand gets cold when you touch ice. II. The ice gets warmer when you touch it. III. Water boils in a kettle being heated on a stove. IV. Water vapor condenses on a cold pipe. V. Ice cream melts. Copyright © Houghton Mifflin Company. All Rights Reserved. 10

4 t eac R For each of the following, define a system and its surroundings and give the direction of energy transfer. I. Methane is burning in a Bunsen burner in a laboratory. II. Water drops, sitting on your skin after you’ve been swimming, evaporate. III. Two chemicals mixing in a beaker give off heat. Copyright © Houghton Mifflin Company. All Rights Reserved. 11

5 t eac R Hydrogen gas and oxygen gas react violently to form water. Which is lower in energy: a mixture of hydrogen and oxygen gases or water? Explain. Sketch an energy-level diagram for this reaction. Copyright © Houghton Mifflin Company. All Rights Reserved. 12

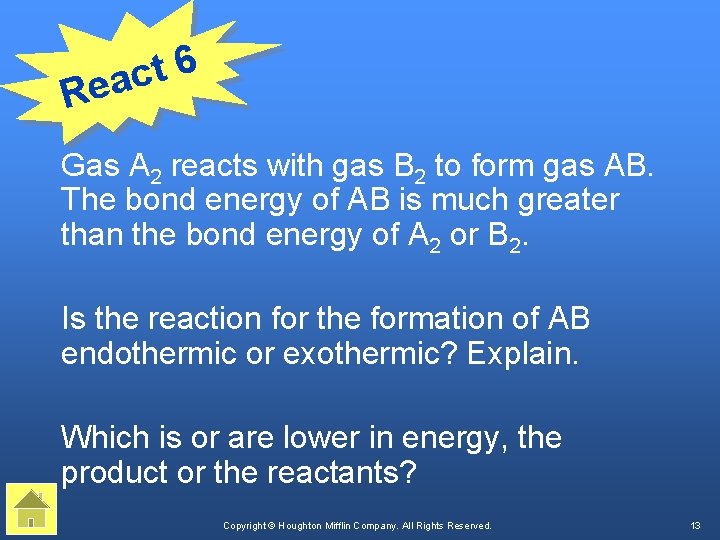
6 t eac R Gas A 2 reacts with gas B 2 to form gas AB. The bond energy of AB is much greater than the bond energy of A 2 or B 2. Is the reaction for the formation of AB endothermic or exothermic? Explain. Which is or are lower in energy, the product or the reactants? Copyright © Houghton Mifflin Company. All Rights Reserved. 13

Work vs. Energy Flow Copyright © Houghton Mifflin Company. All Rights Reserved. 14

7 t eac R Which of the following performs more work? • A gas expanding against a pressure of 2 atm from 1. 0 L to 4. 0 L • A gas expanding against a pressure of 3 atm from 1. 0 L to 3. 0 L Copyright © Houghton Mifflin Company. All Rights Reserved. 15

8 t eac R Determine the sign of E for each of the following with the listed conditions: An endothermic process that performs work. • |work| > |heat| • |work| < |heat| Work is done on a gas and the process is exothermic. • |work| > |heat| • |work| < |heat| Copyright © Houghton Mifflin Company. All Rights Reserved. 16

Enthalpy and Calorimetry Copyright © Houghton Mifflin Company. All Rights Reserved. 17

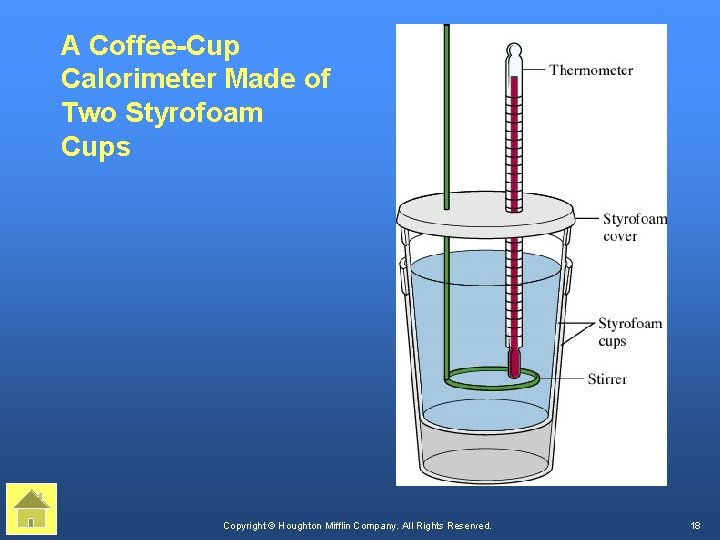
A Coffee-Cup Calorimeter Made of Two Styrofoam Cups Copyright © Houghton Mifflin Company. All Rights Reserved. 18

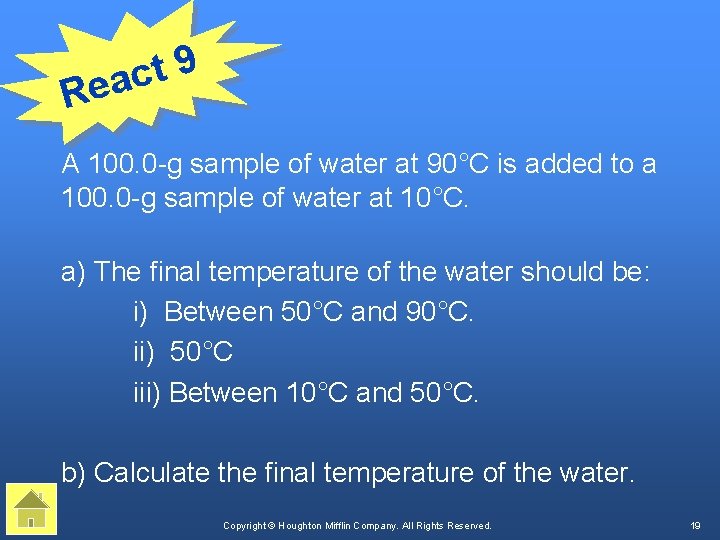
9 t eac R A 100. 0 -g sample of water at 90°C is added to a 100. 0 -g sample of water at 10°C. a) The final temperature of the water should be: i) Between 50°C and 90°C. ii) 50°C iii) Between 10°C and 50°C. b) Calculate the final temperature of the water. Copyright © Houghton Mifflin Company. All Rights Reserved. 19

Re 0 1 act A 100. 0 -g sample of water at 90°C is added to a 500. 0 -g sample of water at 10°C. a) The final temperature of the water is: i) Between 50°C and 90°C. ii) 50°C iii) Between 10°C and 50°C. b) Calculate the final temperature of the water. Copyright © Houghton Mifflin Company. All Rights Reserved. 20

1 1 t eac R You have a Styrofoam cup with 50. 0 g of water at 10 C. You add a 50. 0 -g iron ball at 90 C to the water. a) The final temperature of the water is: i) Between 50°C and 90°C. ii) 50°C iii) Between 10°C and 50°C. b) Calculate the final temperature of the water. Copyright © Houghton Mifflin Company. All Rights Reserved. 21

Hess’s Law and Standard Enthalpies of Formation Copyright © Houghton Mifflin Company. All Rights Reserved. 22

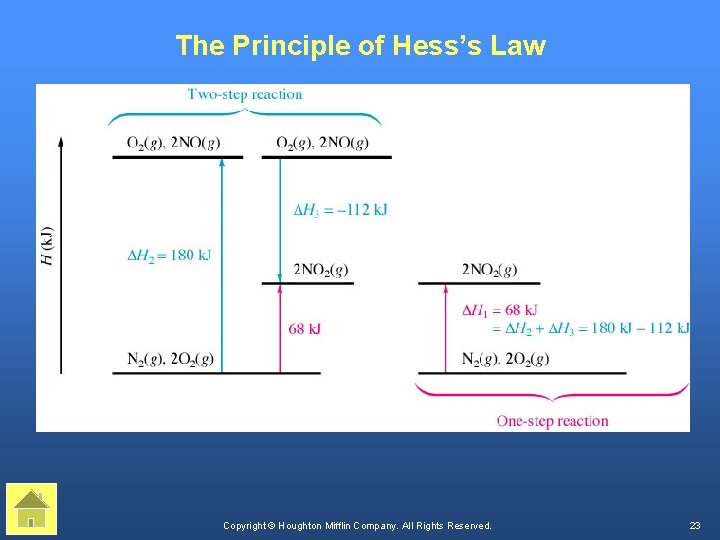
The Principle of Hess’s Law Copyright © Houghton Mifflin Company. All Rights Reserved. 23

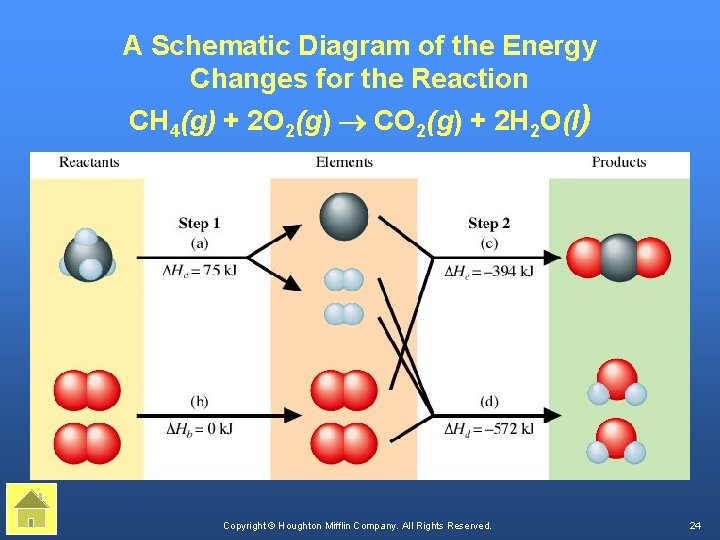
A Schematic Diagram of the Energy Changes for the Reaction CH 4(g) + 2 O 2(g) ® CO 2(g) + 2 H 2 O(l) Copyright © Houghton Mifflin Company. All Rights Reserved. 24

2 1 t eac R Using the following data, calculate the standard heat of formation of the compound ICl(g) at 25°C, and show your work: H° (k. J/mol) Cl 2(g) 2 Cl(g) 242. 3 I 2(g) 2 I(g) 151. 0 ICl(g) I(g) + Cl(g) 211. 3 I 2(s) I 2(g) 62. 8 Copyright © Houghton Mifflin Company. All Rights Reserved. 25

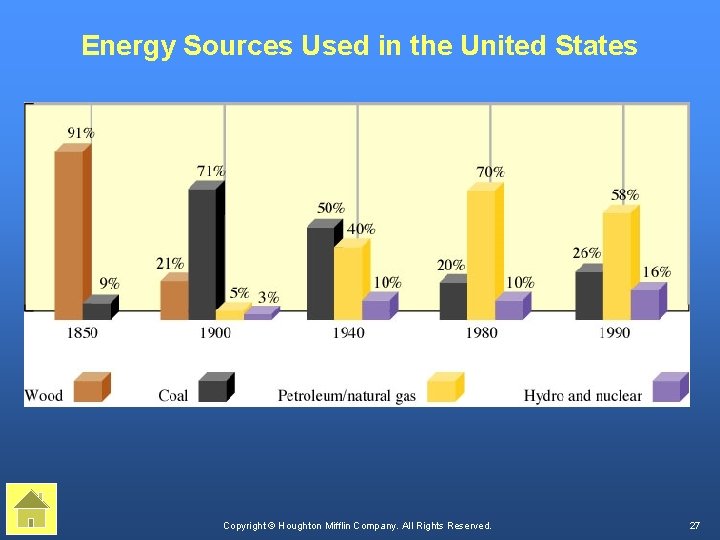
Present Sources of Energy Copyright © Houghton Mifflin Company. All Rights Reserved. 26

Energy Sources Used in the United States Copyright © Houghton Mifflin Company. All Rights Reserved. 27

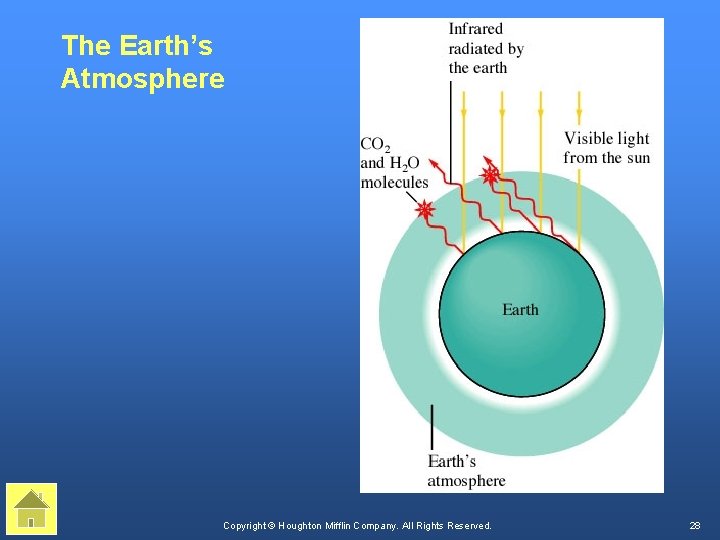
The Earth’s Atmosphere Copyright © Houghton Mifflin Company. All Rights Reserved. 28
 Ap chemistry notes zumdahl
Ap chemistry notes zumdahl C/lamda
C/lamda Zumdahl chemistry
Zumdahl chemistry Ion dipole intermolecular forces
Ion dipole intermolecular forces Electron cloud model
Electron cloud model Zumdahl chapter 4
Zumdahl chapter 4 Zumdahl chapter 12
Zumdahl chapter 12 Ib chemistry organic chemistry
Ib chemistry organic chemistry Organic vs inorganic chemistry
Organic vs inorganic chemistry Electric circuits by james nilsson and susan riedel
Electric circuits by james nilsson and susan riedel Trifles quiz
Trifles quiz Susan bordo the body and the reproduction of femininity
Susan bordo the body and the reproduction of femininity Steven khoury ministries
Steven khoury ministries Brett steven taylor
Brett steven taylor Steven copp
Steven copp Steven mair westminster
Steven mair westminster Steven benson murder
Steven benson murder Satisfaction
Satisfaction Googlenomics
Googlenomics Steven benini
Steven benini Steven izenour
Steven izenour Steven johnson menular atau tidak
Steven johnson menular atau tidak Dr steven kahn
Dr steven kahn Steven kyle cornell
Steven kyle cornell Steven walchek
Steven walchek Paul boyce md
Paul boyce md Personal na pahayag ng
Personal na pahayag ng Resectoscopie
Resectoscopie Steven quilos mortais
Steven quilos mortais