Chemical Reactions Classifying Reactions The five general types







- Slides: 17

Chemical Reactions

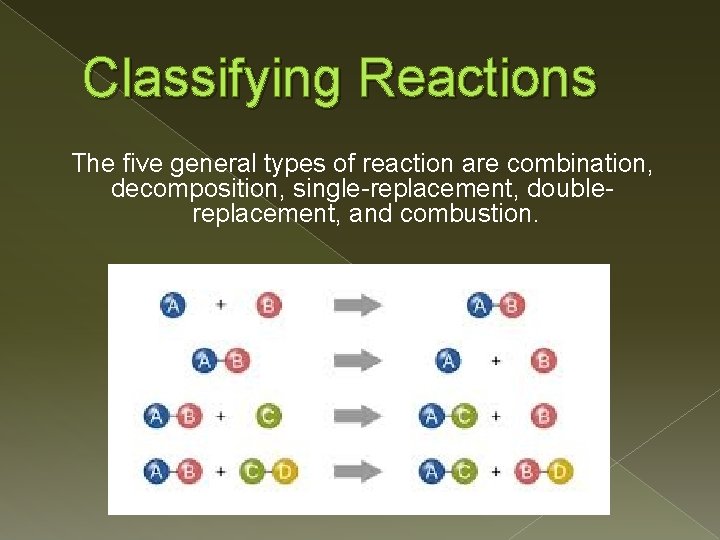
Classifying Reactions The five general types of reaction are combination, decomposition, single-replacement, doublereplacement, and combustion.

Synthesis/ Combination A combination reaction is a chemical change in which two or more substances react to form a single new substance. Magnesium + nitrogen yields magnesium nitride

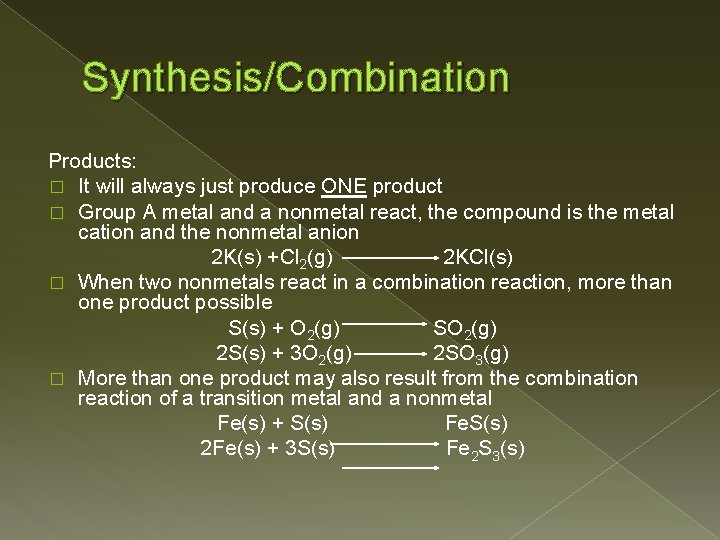
Synthesis/Combination Products: � It will always just produce ONE product � Group A metal and a nonmetal react, the compound is the metal cation and the nonmetal anion 2 K(s) +Cl 2(g) 2 KCl(s) � When two nonmetals react in a combination reaction, more than one product possible S(s) + O 2(g) SO 2(g) 2 S(s) + 3 O 2(g) 2 SO 3(g) � More than one product may also result from the combination reaction of a transition metal and a nonmetal Fe(s) + S(s) Fe. S(s) 2 Fe(s) + 3 S(s) Fe 2 S 3(s)

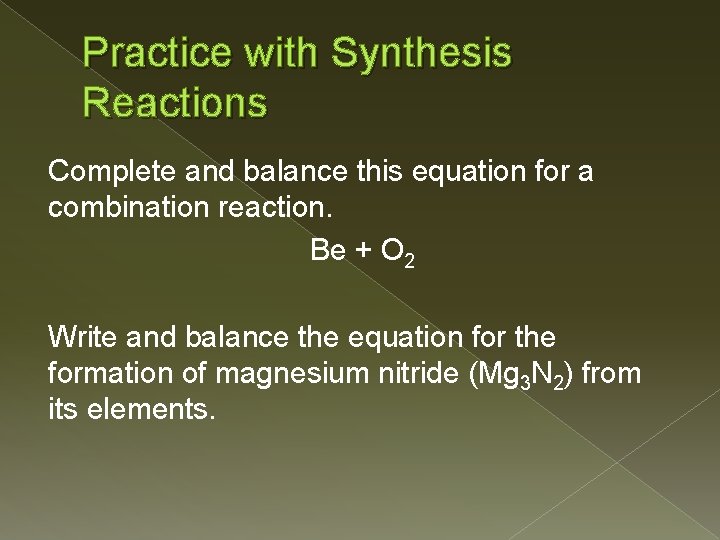
Practice with Synthesis Reactions Complete and balance this equation for a combination reaction. Be + O 2 Write and balance the equation for the formation of magnesium nitride (Mg 3 N 2) from its elements.

Decomposition A decomposition reaction is a chemical change in which a single compound breaks down into two or more simpler products. Copper chloride decomposes to copper and chloride

Decomposition Products: � The products can be any combination of elements and compounds. � Usually difficult to predict. � A simple binary compound will break down to its constituents. � Most decomposition reactions require energy in the form of light, heat or electricity 2 Hg. O(s) 2 Hg(l) + O 2(g)

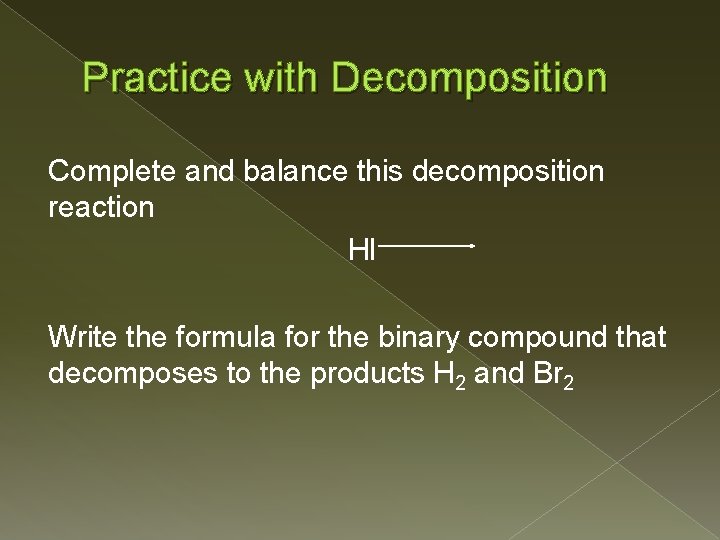
Practice with Decomposition Complete and balance this decomposition reaction HI Write the formula for the binary compound that decomposes to the products H 2 and Br 2

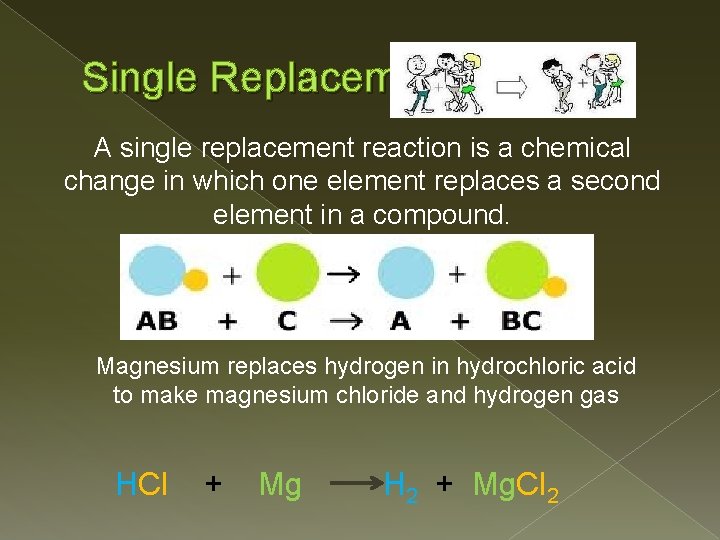
Single Replacement A single replacement reaction is a chemical change in which one element replaces a second element in a compound. Magnesium replaces hydrogen in hydrochloric acid to make magnesium chloride and hydrogen gas HCl + Mg H 2 + Mg. Cl 2

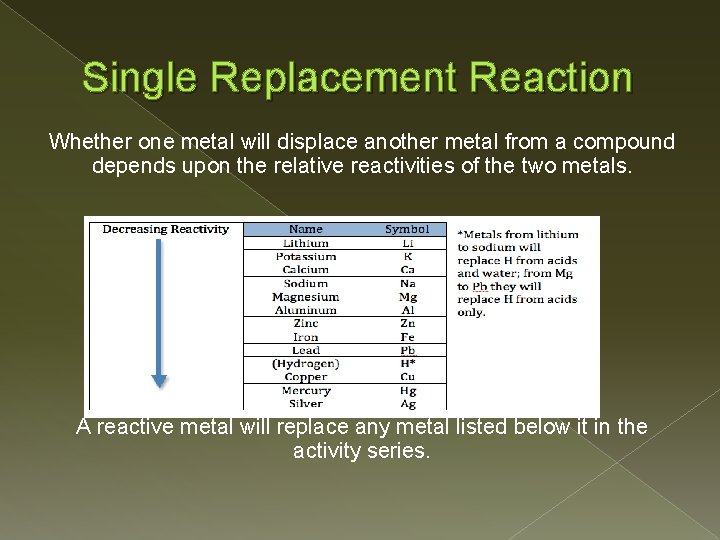
Single Replacement Reaction Whether one metal will displace another metal from a compound depends upon the relative reactivities of the two metals. A reactive metal will replace any metal listed below it in the activity series.

Single Replacement A halogen can replace another halogen from a compound. The activity of the halogens decrease as you go down. Br 2(aq) + Na. I(aq) Br 2(aq) + Na. Cl(aq) Na. Br(aq) + I 2(aq) No Reaction

Practice with Single Replacement Reactions Complete the equations for these single replacement reactions in aqueous solution. Balance each equation. Put “no reaction” if a reaction does not occur. Fe(s) + Pb(NO 3)2(aq) Cl 2(aq) + Na. I(aq) Ca(aq) + H 2 O(l)

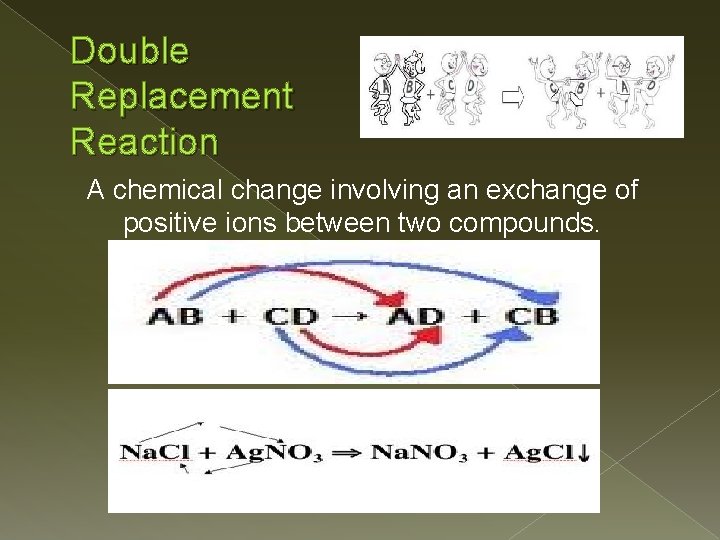
Double Replacement Reaction A chemical change involving an exchange of positive ions between two compounds.

For a Double Replacement Reaction to Take Place: � One of the products is only slightly soluble and precipitates from solution. � One of the products is a gas. product is a molecular compound such as water.

Practice with Double Replacement Write the products of these double-replacement reactions. Then balance each equation. Na. OH(aq) + Fe(NO 3)3(aq) (Iron(III)hydroxide is a precipitate) Ba(NO 3)2(aq) + H 3 PO 4(aq) (Barium phosphate is a precipitate)

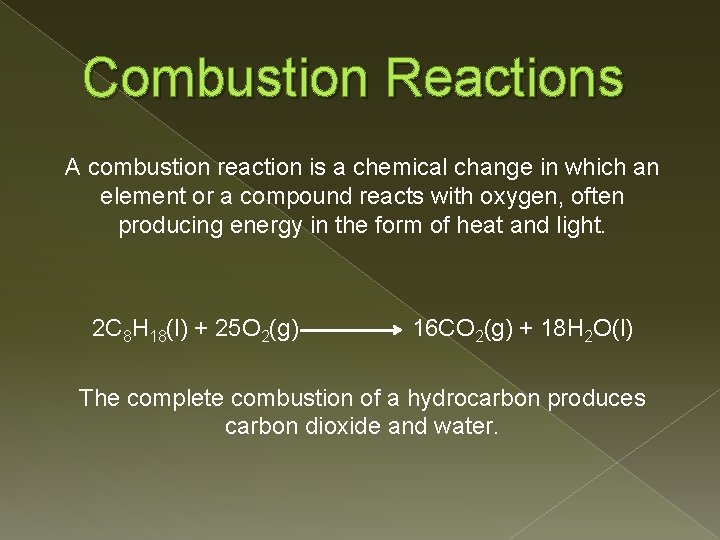
Combustion Reactions A combustion reaction is a chemical change in which an element or a compound reacts with oxygen, often producing energy in the form of heat and light. 2 C 8 H 18(l) + 25 O 2(g) 16 CO 2(g) + 18 H 2 O(l) The complete combustion of a hydrocarbon produces carbon dioxide and water.

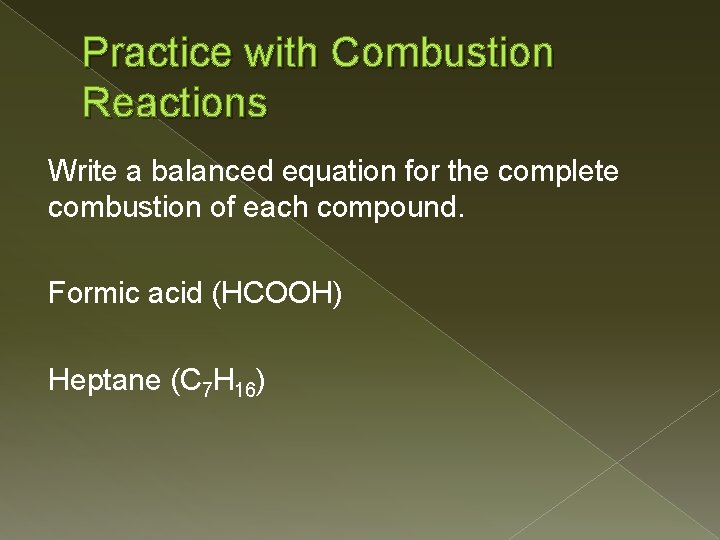
Practice with Combustion Reactions Write a balanced equation for the complete combustion of each compound. Formic acid (HCOOH) Heptane (C 7 H 16)