Chemical Reactions Five Types of Chemical Reactions Simplified




- Slides: 11

Chemical Reactions Five Types of Chemical Reactions Simplified for Introduction

Lesson Essential Question What are the general types of chemical reactions?

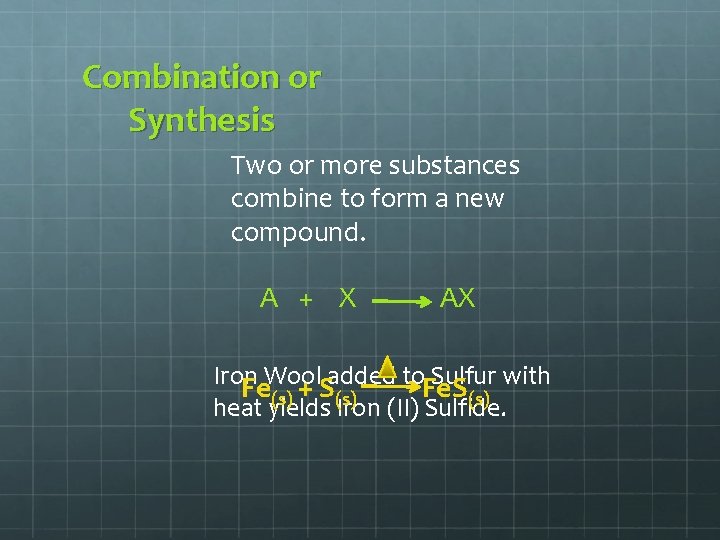
Combination or Synthesis Two or more substances combine to form a new compound. A + X AX Iron added to. Fe. S Sulfur with Fe. Wool + S (s) (s) heat yields Iron (II) Sulfide.

Iron + Sulfur

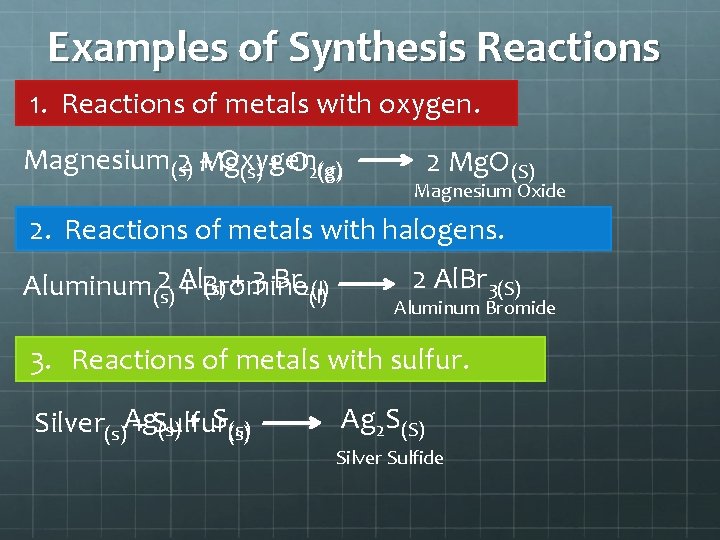
Examples of Synthesis Reactions 1. Reactions of metals with oxygen. Magnesium(s) Oxygen 2 +Mg (g) (s) + O 2(g) 2 Mg. O(S) Magnesium Oxide 2. Reactions of metals with halogens. 2 Al Aluminum(s) + Bromine (s) + 3 Br 2(l) 2 Al. Br 3(S) Aluminum Bromide 3. Reactions of metals with sulfur. Silver(s)Ag + Sulfur (s) + S(s) Ag 2 S(S) Silver Sulfide

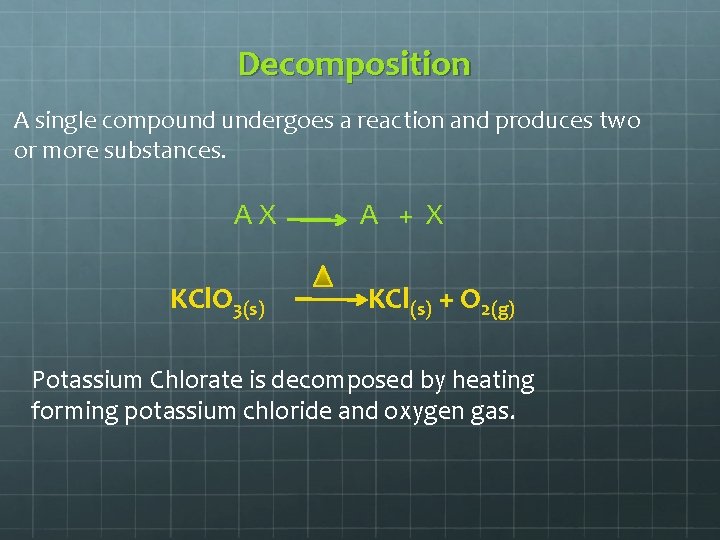
Decomposition A single compound undergoes a reaction and produces two or more substances. AX KCl. O 3(s) A + X KCl(s) + O 2(g) Potassium Chlorate is decomposed by heating forming potassium chloride and oxygen gas.

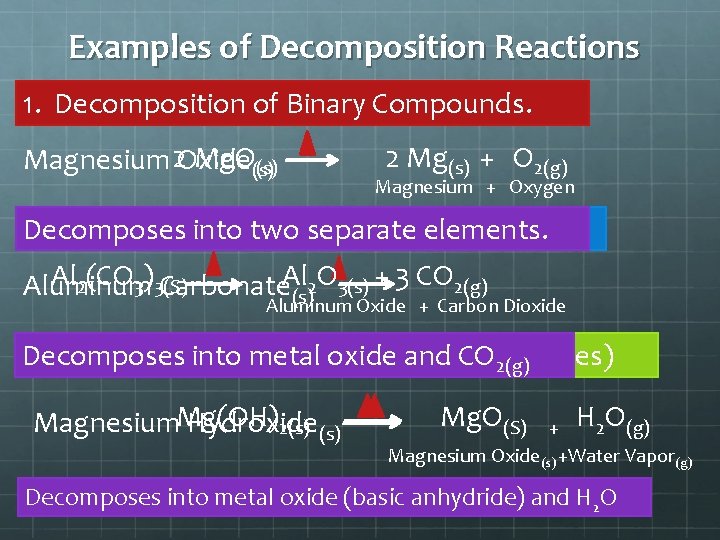
Examples of Decomposition Reactions 1. Decomposition of Binary Compounds. Mg. O(s) Magnesium 2 Oxide (s) 2 Mg(s) + O 2(g) Magnesium + Oxygen 2. Decomposition Decomposes into two of Metal separate Carbonates. elements. Al 2(CO 3)3(S) O 3(s) + 3 CO 2(g) 2 Aluminum Carbonate. Al (s) Aluminum Oxide + Carbon Dioxide Decomposes 3. Decomposition into metal of Metal oxide Hydroxides and CO 2(g)(Bases) Magnesium. Mg(OH) Hydroxide 2(s) Mg. O(S) + H 2 O(g) Magnesium Oxide(s) +Water Vapor(g) Decomposes into metal oxide (basic anhydride) and H 2 O

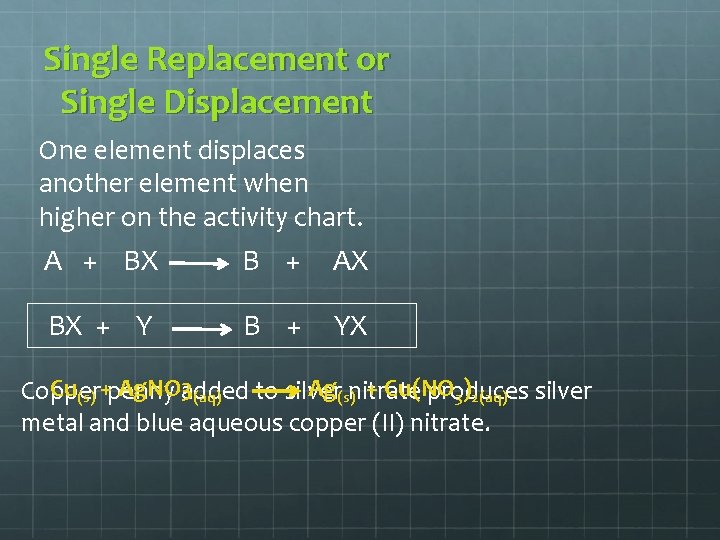
Single Replacement or Single Displacement One element displaces another element when higher on the activity chart. A + BX B + AX BX + Y B + YX Cu(s) +penny Ag. NO 3 added Ag(s)nitrate + Cu(NO Copper to silver produces silver (aq) 3)2(aq) metal and blue aqueous copper (II) nitrate.

Double Replacement or Double Displacement The ions of two compounds switch places in an aqueous solution to form two new compounds. AY + BX BY + AX One of the products is usually an insoluble gas that bubbles out of the solution, a precipitate, or a molecular compound, usually water. Lead (II) Nitrate added to Potassium Iodide produces a Lead (II) Iodide ppt. and+aqueous solution. Pb(NO KI(aq) Potassium Pb. I 2(s) Nitrate + KNO 3(aq) 3)2(aq)

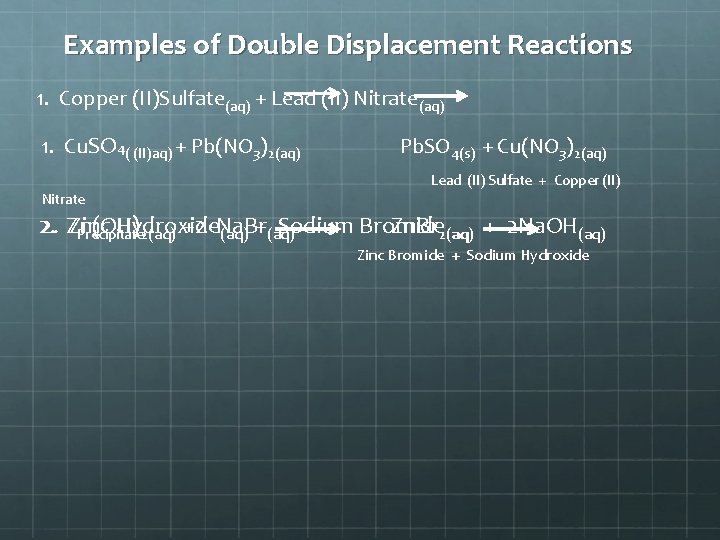
Examples of Double Displacement Reactions 1. Copper (II)Sulfate(aq) + Lead (II) Nitrate(aq) 1. Cu. SO 4( (II)aq) + Pb(NO 3)2(aq) Pb. SO 4(s) + Cu(NO 3)2(aq) Lead (II) Sulfate + Copper (II) Nitrate 2. Zn. Br 2(aq) 2. Zn(OH) Zinc Hydroxide Sodium Bromide 2(aq) +2 Na. Br Precipitate (aq) +(aq) + 2 Na. OH(aq) Zinc Bromide + Sodium Hydroxide

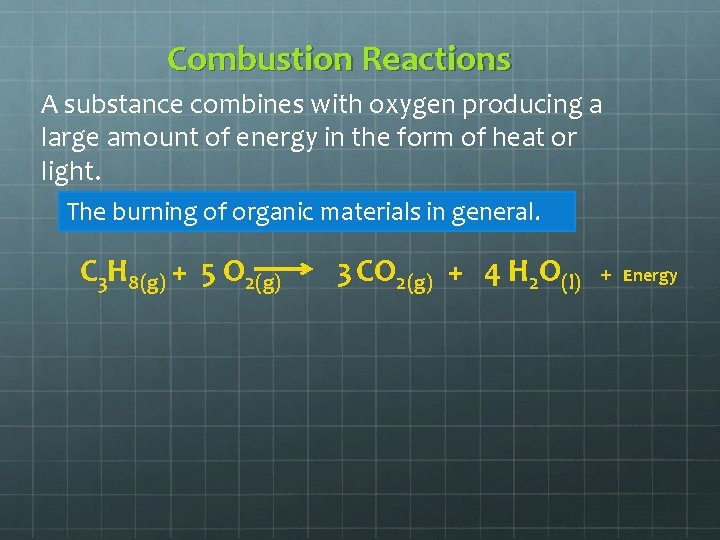
Combustion Reactions A substance combines with oxygen producing a large amount of energy in the form of heat or light. The burning of organic materials in general. C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O(l) + Energy