Chemical Reactions Five Types of Chemical Reactions 1

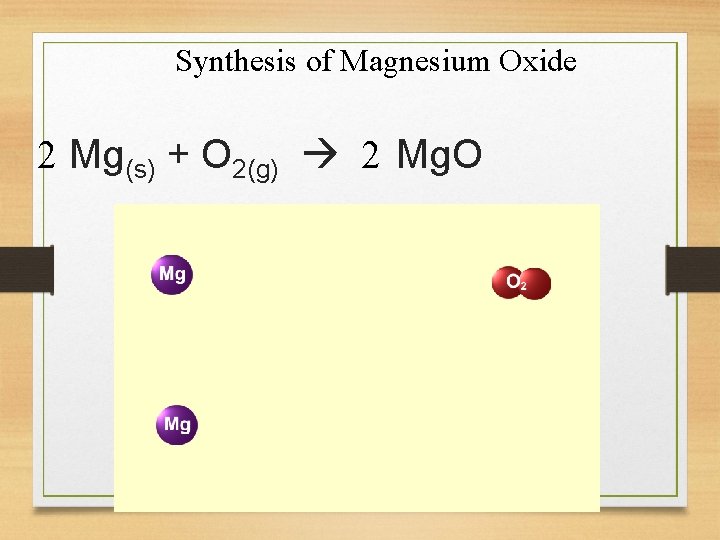




- Slides: 21

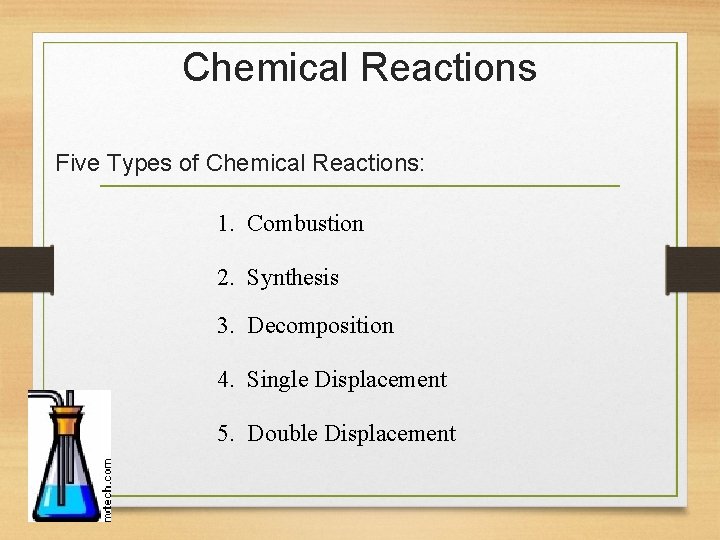
Chemical Reactions Five Types of Chemical Reactions: 1. Combustion 2. Synthesis 3. Decomposition 4. Single Displacement 5. Double Displacement

Evidence that a chemical reaction has taken place? • Colour / Odour Change l Formation l Difficult of a gas or solid to reverse l Release/Absorption of Energy (heat)

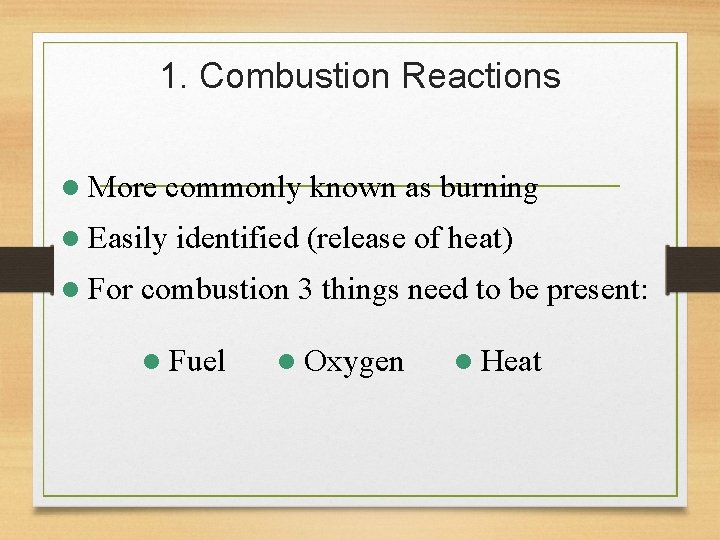
1. Combustion Reactions l More commonly known as burning l Easily l For identified (release of heat) combustion 3 things need to be present: l Fuel l Oxygen l Heat

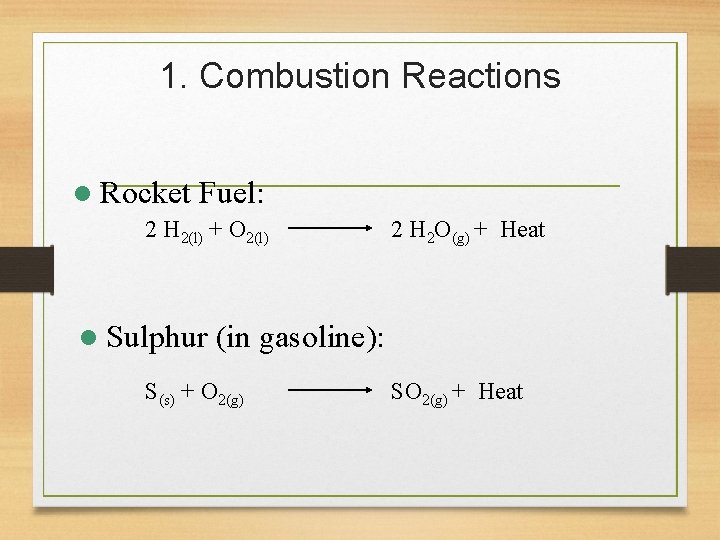
1. Combustion Reactions l Rocket Fuel: 2 H 2(l) + O 2(l) l Sulphur 2 H 2 O(g) + Heat (in gasoline): S(s) + O 2(g) SO 2(g) + Heat

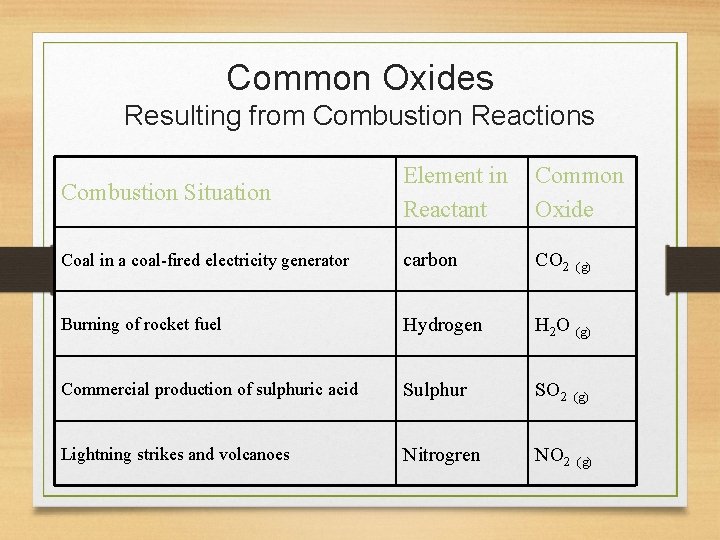
Common Oxides Resulting from Combustion Reactions Combustion Situation Element in Reactant Common Oxide Coal in a coal-fired electricity generator carbon CO 2 Burning of rocket fuel Hydrogen H 2 O (g) Commercial production of sulphuric acid Sulphur SO 2 (g) Lightning strikes and volcanoes Nitrogren NO 2 (g)

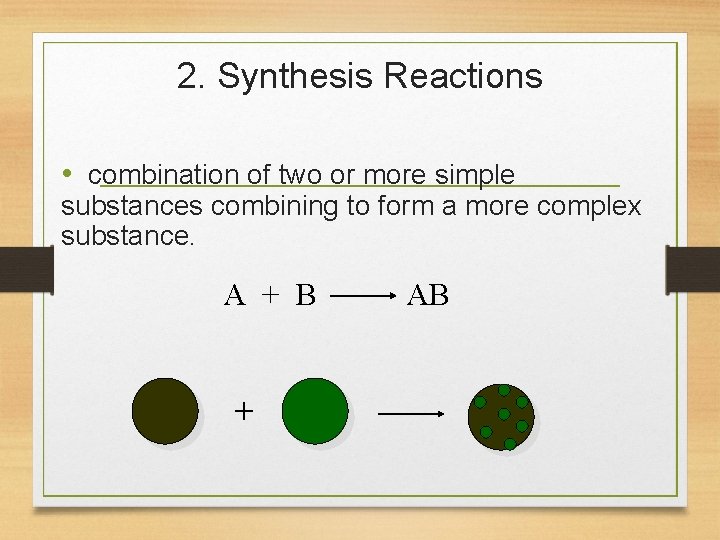
2. Synthesis Reactions • combination of two or more simple substances combining to form a more complex substance. A + B + AB
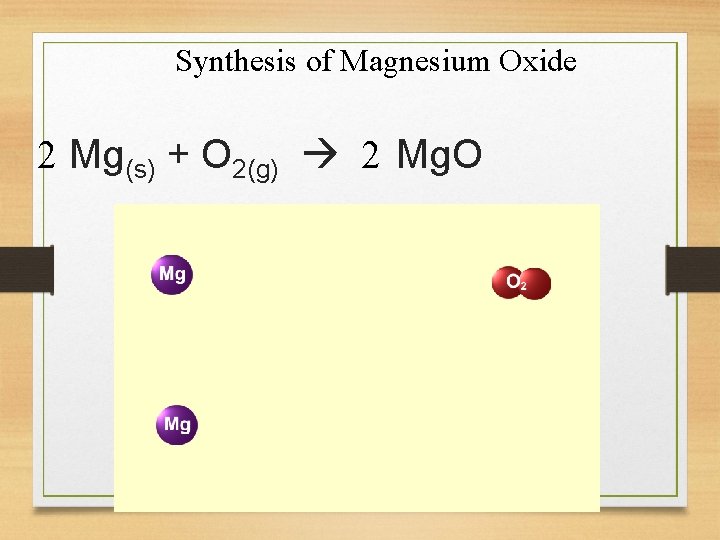
Synthesis of Magnesium Oxide 2 Mg(s) + O 2(g) 2 Mg. O

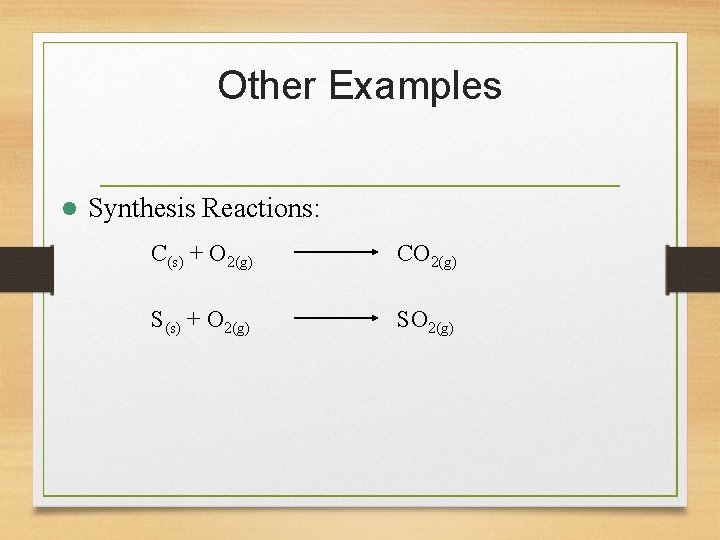
Other Examples l Synthesis Reactions: C(s) + O 2(g) CO 2(g) S(s) + O 2(g) SO 2(g)

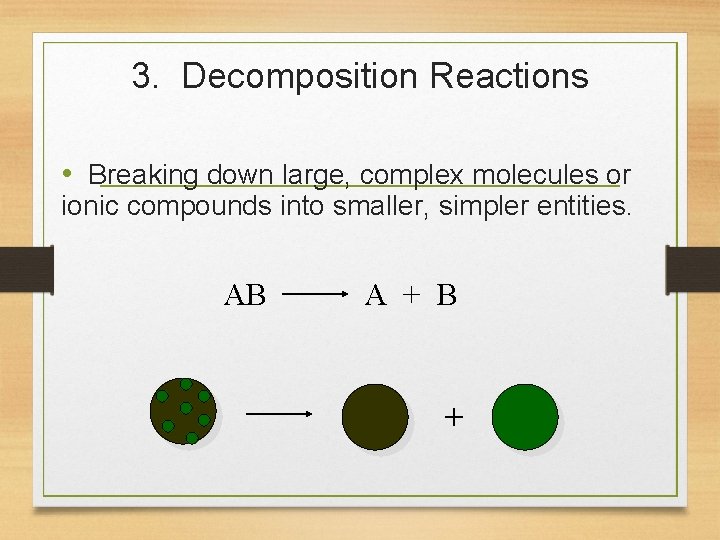
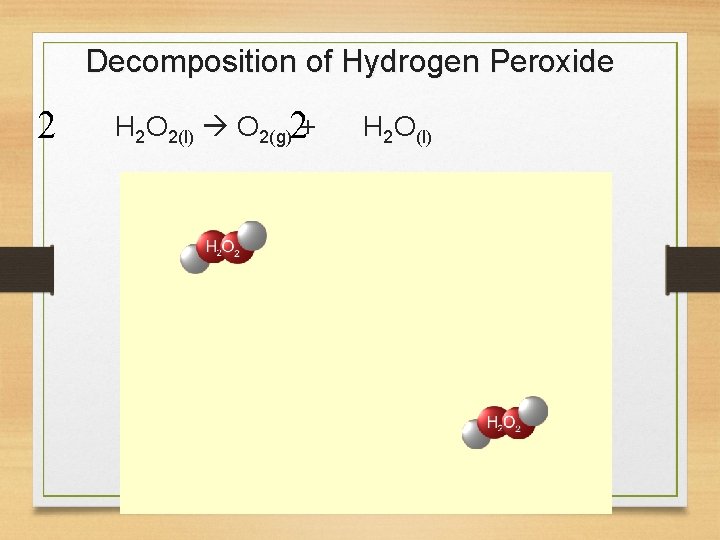
3. Decomposition Reactions • Breaking down large, complex molecules or ionic compounds into smaller, simpler entities. AB A + B +

Decomposition of Hydrogen Peroxide 2 H 2 O 2(l) O 2(g)2+ H 2 O(l)

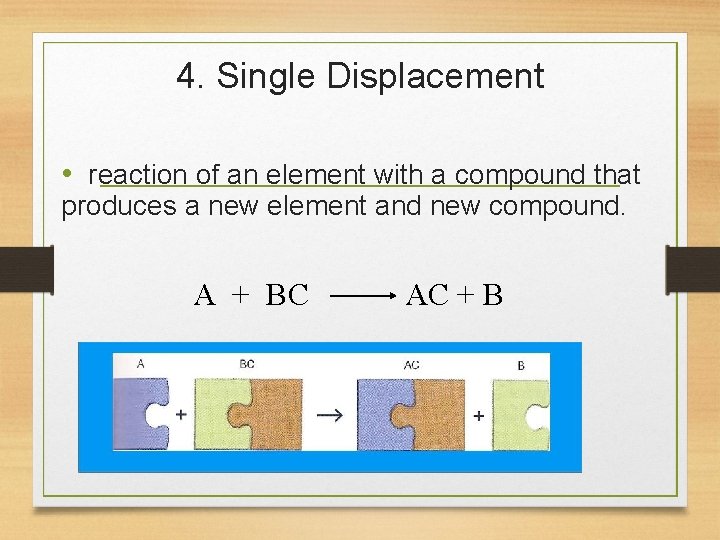
4. Single Displacement • reaction of an element with a compound that produces a new element and new compound. A + BC AC + B

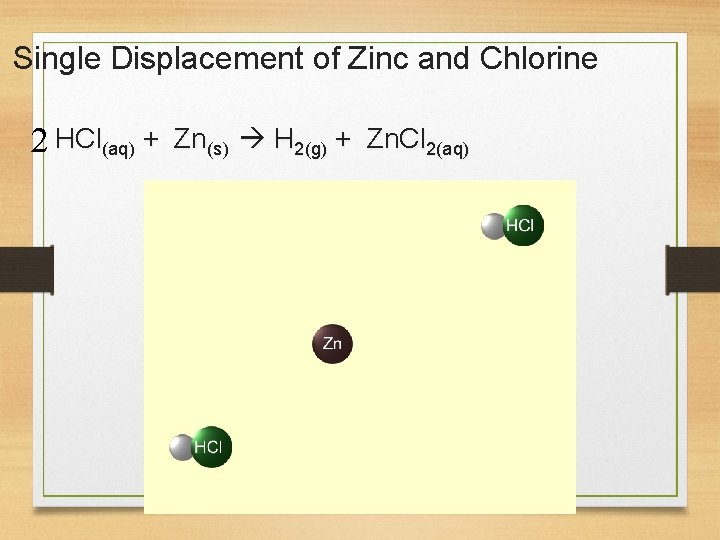
Single Displacement of Zinc and Chlorine 2 HCl(aq) + Zn(s) H 2(g) + Zn. Cl 2(aq)

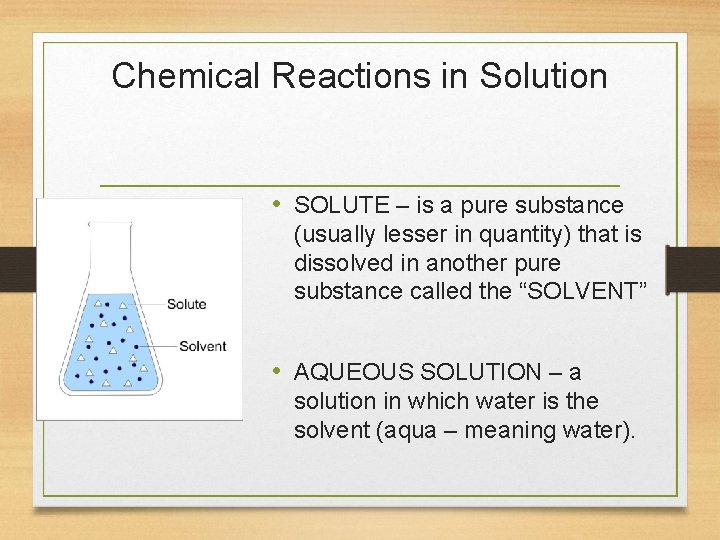
Chemical Reactions in Solution • SOLUTE – is a pure substance (usually lesser in quantity) that is dissolved in another pure substance called the “SOLVENT” • AQUEOUS SOLUTION – a solution in which water is the solvent (aqua – meaning water).

Solubility • Solutions are homogenous mixtures of solutes and solvents. • Solubility is the measure of how much of the solute can possibly dissolve in a known amount of solvent. If a substance has high solubility in water, it has a subscript of (aq). However, if it isn’t very soluble it will start to precipitate out and will have a (s) subscript for solid.

Use a Solubility Table • Is calcium hydroxide soluble? • Is sodium flouride soluble? • What about lead (II) Iodide?

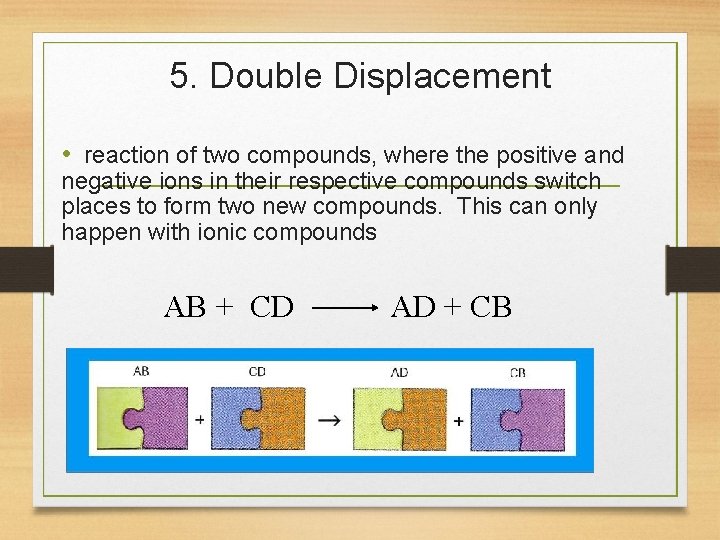
5. Double Displacement • reaction of two compounds, where the positive and negative ions in their respective compounds switch places to form two new compounds. This can only happen with ionic compounds AB + CD AD + CB

Double Displacement A double displacement reaction only occurs if one of the following three results are seen: • a precipitate is formed • a gas is produced • a change of p. H occurs (a neutralization reaction) ** If the products are both soluble then the reaction is NR (no reaction)

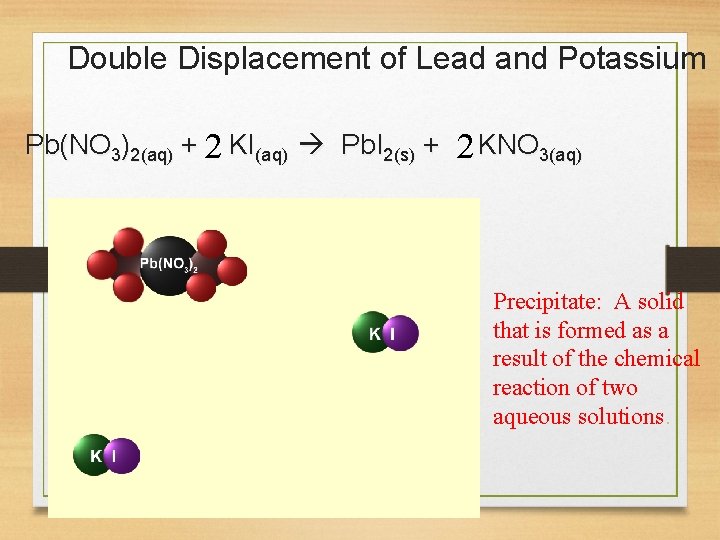
Double Displacement of Lead and Potassium Pb(NO 3)2(aq) + 2 KI(aq) Pb. I 2(s) + 2 KNO 3(aq) Precipitate: A solid that is formed as a result of the chemical reaction of two aqueous solutions.

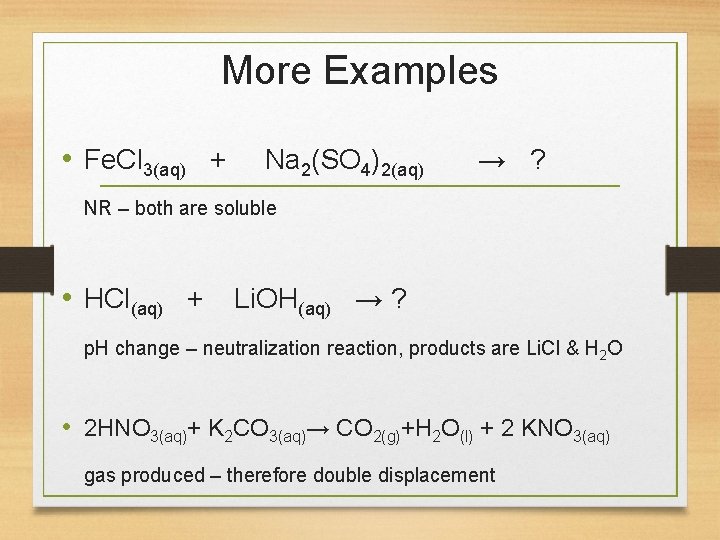
More Examples • Fe. Cl 3(aq) + Na 2(SO 4)2(aq) → ? NR – both are soluble • HCl(aq) + Li. OH(aq) → ? p. H change – neutralization reaction, products are Li. Cl & H 2 O • 2 HNO 3(aq)+ K 2 CO 3(aq)→ CO 2(g)+H 2 O(l) + 2 KNO 3(aq) gas produced – therefore double displacement

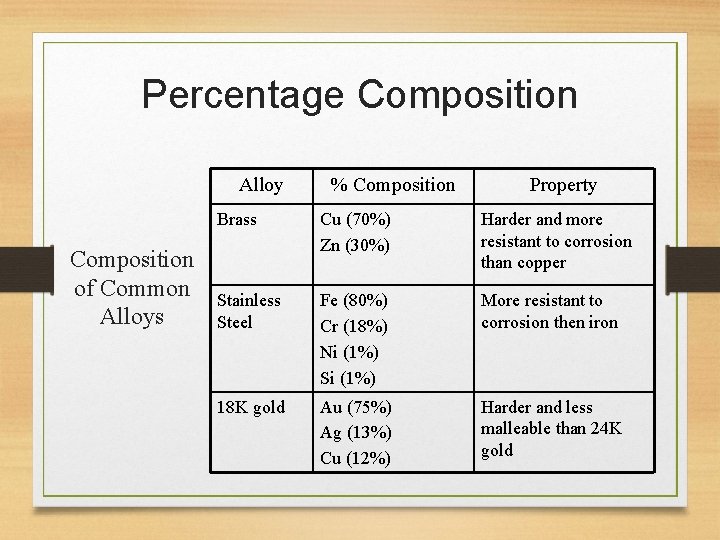
Percentage Composition Alloy Composition of Common Alloys % Composition Property Brass Cu (70%) Zn (30%) Harder and more resistant to corrosion than copper Stainless Steel Fe (80%) Cr (18%) Ni (1%) Si (1%) More resistant to corrosion then iron 18 K gold Au (75%) Ag (13%) Cu (12%) Harder and less malleable than 24 K gold

Percent Composition • Percentages are calculated by weight How much gold (in grams) is there in 10 g sample of 18 K yellow gold where the % composition is 75% Au? Answer: mass of Au = (75% / 100) x 10 g = 7. 5 g Therefore there is 7. 5 g of pure gold in a 10 g sample of 18 K yellow gold.
 Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Combustion chemical reaction
Combustion chemical reaction Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions 5 types of reactions
5 types of reactions Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Are kc and kp equal
Are kc and kp equal Types of redox reactions
Types of redox reactions How to identify types of chemical reactions
How to identify types of chemical reactions Types of reactions chemistry
Types of reactions chemistry Reaction type
Reaction type 4 types of chemical reactions
4 types of chemical reactions Four types of chemical reactions
Four types of chemical reactions What are the five chemical changes
What are the five chemical changes 5 general types of chemical reactions
5 general types of chemical reactions What are the 4 types of chemical reactions
What are the 4 types of chemical reactions Four types of chemical reactions
Four types of chemical reactions What are the 5 types of chemical reactions
What are the 5 types of chemical reactions Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Fatoumata dembele chef
Fatoumata dembele chef Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry 10 examples of redox reaction
10 examples of redox reaction







































