CHEMICAL PEELS IN ACTIVE ACNE AND ACNE SCARS




























- Slides: 39

CHEMICAL PEELS IN ACTIVE ACNE AND ACNE SCARS BY , DR. OMIDVAND • CLINICS IN DERMATOLOGY (2017)35, 179– 182 • GEORGIOS KONTOCHRISTOPOULOS, MD, PHD, EFTYCHIA PLATSIDAKI, MD • DEPARTMENT OF DERMATOLOGY AND VENEREOLOGY, ANDREAS SYGROS SKIN HOSPITAL, ATHENS, GREEC

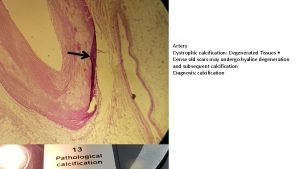
INTRODUCTION üACNE HAS A PREVALENCE OF OVER 90% IN THE ADOLESCENT COMMUNITY. üCONTRIBUTE TO THE DEVELOPMENT OF ACNE: v. FOLLICULAR HYPER KERATINIZATION v. INCREASED SEBUM PRODUCTION v. PROLIFERATION OF PROPIONI BACTERIUM ACNES WITHIN THE FOLLICLE v RELEASE OF INFLAMMATORY MEDIATORS INTO THE SKIN üPOSSIBLE OUTCOMES OF INFLAMMATORY ACNE LESIONS ARE ACNE SCARS. üMINOR ACNE SCARRING üSIGNIFICANT SCARRING UP TO 95% OF PATIENTS. 22%.

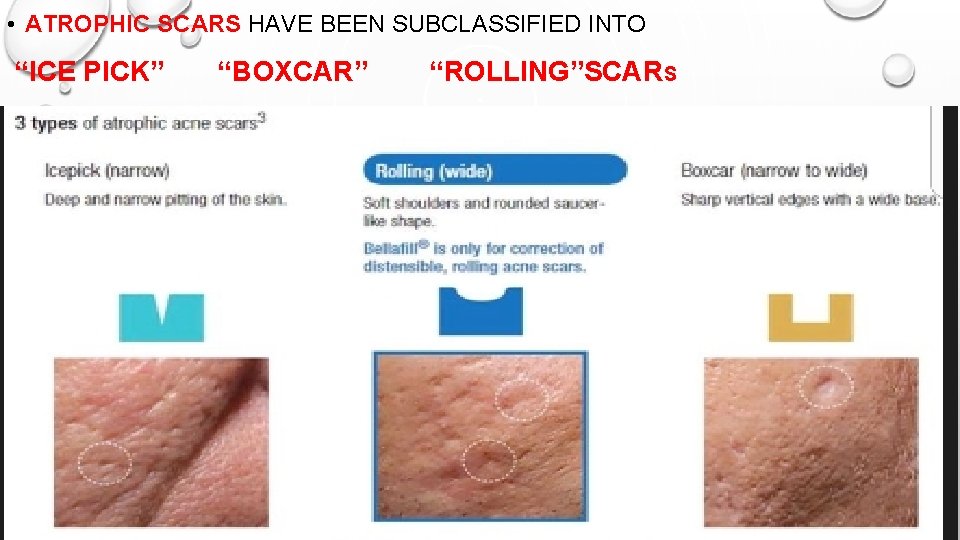
• ATROPHIC SCARS HAVE BEEN SUBCLASSIFIED INTO “ICE PICK” “BOXCAR” “ROLLING”SCARS

• APPROPRIATE, AND ADEQUATE TREATMENT OF ACNE IS IMPORTANT TO MINIMIZE INFLAMMATION AND PREVENT ACNE SCAR FORMATION. CHEMICAL PEELING ACNE SCARS. MANAGEMENT OF BOTH ACTIVE ACNE AND q. WHICH PEELING AGENT AND WHAT CONCENTRATION? CHEMICAL PEELS CONTROLLED CHEMICAL INDUCED INJURY TO THE SKIN DESTROY THE EPIDERMIS (SUPERFICIAL PEELING) AND PART OF THE DERMIS (MEDIUM OR DEEP PEELING) SKIN REGENERATION, AND REMODEL TISSUES.

THE MOST COMMONLY USED PEELING AGENTS ARE: 1. SALICYLIC ACID (SA) 2. GLYCOLIC ACID (GA) 3. PYRUVIC ACID (PA) 4. LACTIC ACID (LA) 5. MANDELIC ACID (MA) 6. JESSNER SOLUTION (JS) 7. TRICHLOROACETIC ACID (TCA) 8. PHENOL


• WHEN COMBINATION PEELS ARE USED: q BETTER CLINICAL RESULTS q REDUCED RISK OF COMPLICATIONS • IN ACTIVE ACNE SUPPLEMENT CLASSIC TREATMENT : ü PROMOTE THE EFFECT OF TOPICAL AGENTS ü PROVIDE MAINTENANCE THERAPY • IN ACNE SCARS, THEY CAN BE USED IN COMBINATION WITH OTHER RESURFACING PROCEDURES.

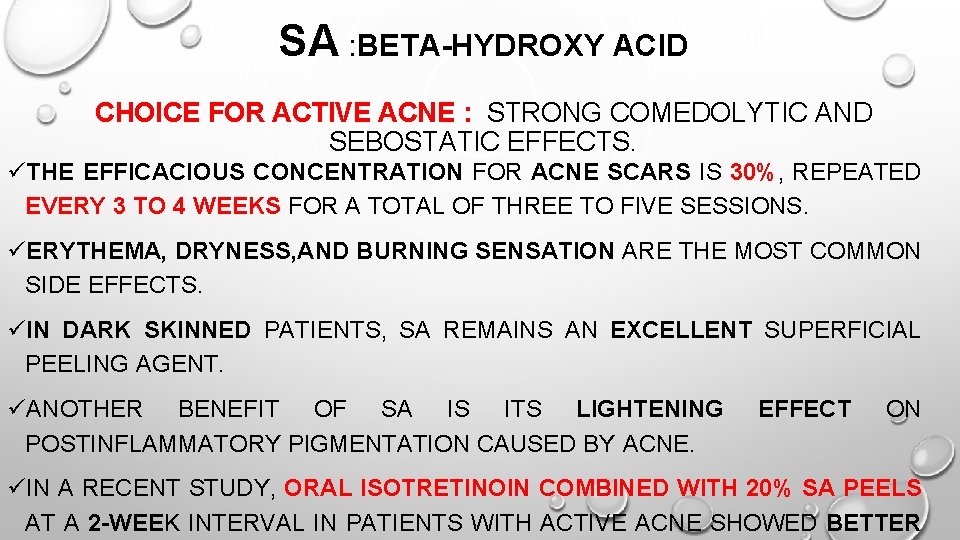
SA : BETA-HYDROXY ACID CHOICE FOR ACTIVE ACNE : STRONG COMEDOLYTIC AND SEBOSTATIC EFFECTS. üTHE EFFICACIOUS CONCENTRATION FOR ACNE SCARS IS 30%, REPEATED EVERY 3 TO 4 WEEKS FOR A TOTAL OF THREE TO FIVE SESSIONS. üERYTHEMA, DRYNESS, AND BURNING SENSATION ARE THE MOST COMMON SIDE EFFECTS. üIN DARK SKINNED PATIENTS, SA REMAINS AN EXCELLENT SUPERFICIAL PEELING AGENT. üANOTHER BENEFIT OF SA IS ITS LIGHTENING POSTINFLAMMATORY PIGMENTATION CAUSED BY ACNE. EFFECT ON üIN A RECENT STUDY, ORAL ISOTRETINOIN COMBINED WITH 20% SA PEELS AT A 2 -WEEK INTERVAL IN PATIENTS WITH ACTIVE ACNE SHOWED BETTER

GA : ALPHA-HYDROXY ACID • EFFECTIVE IN IMPROVING MILD, MODERATE, AND SEVERE NODULAR ACNE. • CONCENTRATION OF 10% TO 30% FOR 3 TO 5 MINUTES AT 2 -WEEK INTERVALS [SUPERFICIAL SCARRING] q. SIDE EFFECTS: TEMPORARY HYPERPIGMENTATION OR IRRITATION, ARE NOT SIGNIFICANT. q. GENERAL CONTRAINDICATIONS INCLUDE : 1. CONTACT DERMATITIS 2. PREGNANCY 3. GLYCOLATE HYPERSENSITIVITY.

q. GA PEELS WERE COMPARED WITH SALICYLIC–MANDELIC ACID COMBINATION PEELS (SMPS) IN ACTIVE ACNE VULGARIS AND POST ACNE SCARRING. BOTH AGENTS WERE EFFECTIVE AND SAFE, WITH SMPS DEMONSTRATING HIGHER EFFICACY. q. COMBINATION OF MICRONEEDLING AND GA PEELS SIGNIFICANTLY IMPROVED ACNE SCARS. q. IN A COMPARATIVE STUDY, IN WHICH 70% GA AND JS WAS USED IN PATIENTS WITH ACNE, ü MORE EXFOLIATION WAS SEEN IN THE JS GROUP üALTHOUGH ACNE IMPROVED IN BOTH GROUPS TO THE SAME EXTENT.

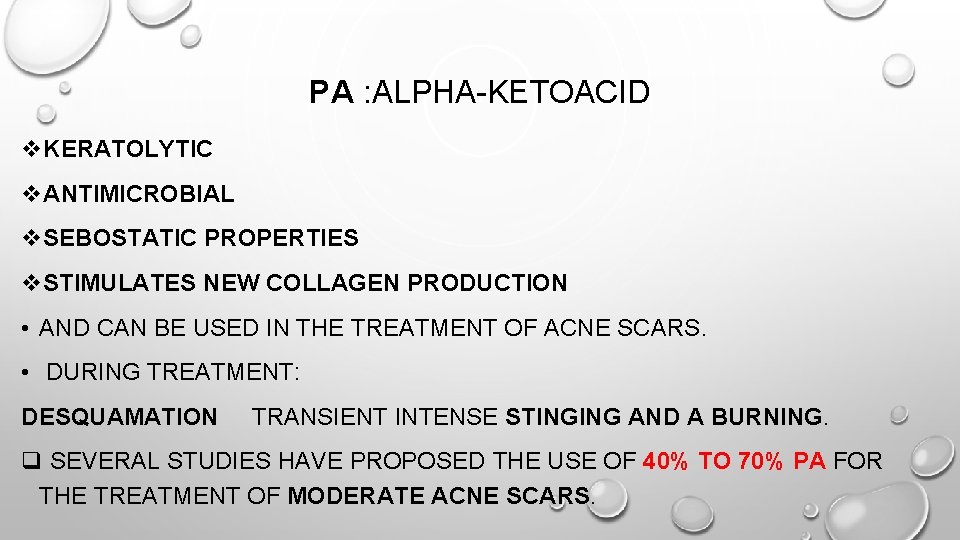
PA : ALPHA-KETOACID v. KERATOLYTIC v. ANTIMICROBIAL v. SEBOSTATIC PROPERTIES v. STIMULATES NEW COLLAGEN PRODUCTION • AND CAN BE USED IN THE TREATMENT OF ACNE SCARS. • DURING TREATMENT: DESQUAMATION TRANSIENT INTENSE STINGING AND A BURNING. q SEVERAL STUDIES HAVE PROPOSED THE USE OF 40% TO 70% PA FOR THE TREATMENT OF MODERATE ACNE SCARS.

LA : A WEAK ALPHA-HYDROXY ACID q. REDUCES THE THICKNESS OF THE STRATUM CORNEUM q. SKIN LIGHTENING q. MOISTURIZING EFFECTS ü IN A PILOT STUDY BY SACHDEVA, THE USE OF FULL-STRENGTH, 92%-PURE LA PEEL TO IMPROVE SUPERFICIAL ACNE SCARRING RESULTED IN A DEFINITE IMPROVEMENT IN THE TEXTURE, PIGMENTATION, AND APPEARANCE OF THE TREATED SKIN, WITH LIGHTENING OF SCARS.

MA : ALPHA-HYDROXY ACID • BECAUSE IT IS A LARGE MOLECULE, SKIN PENETRATION IS SLOW; • SAFE SUPERFICIAL PEELING AGENT. • IT HAS BEEN USED AT 20% TO 50% CONCENTRATIONS FOR SKIN REJUVENATION AND LIGHTENING. q. SMP, A COMBINATION OF 20% SALICYLIC ACID AND 10% MANDELIC ACID, HAS BEEN PROVEN EFFECTIVE IN TREATING ACNE VULGARIS AND POSTACNE SCARRING

• JS CONSISTS OF 14% SA, 14% RESORCINOL OR CITRIC ACID, AND 14% LA IN 95% ETHANOL AND IS USED AS A SUPERFICIAL PEELING AGENT. q IN A RECENT STUDY, MARKED DIMINUTION OF ACNE SCARS WAS SEEN IN PATIENTS TREATED WITH 20% TCA AND JS VERSUS 20% TCA ALONE. üCONTRAINDICATED : ACTIVE INFLAMMATION, DERMATITIS, OR INFECTION.

• TCA IS THE GOLD STANDARD IN TREATMENTS WITH CHEMICAL PEELS AND CAN BE USED AS A SUPERFICIAL, MEDIUM-DEPTH, OR DEEP PEEL( DEPENDING ON THE CONCENTRATION) üIT IS NOT INDICATED IN THOSE WITH DARK SKIN (HIGH RISK OF HYPERPIGMENTATION) üLOW COST AND THAT PENETRATION CAN BE EASILY EVALUATED BY THE COLOR OF FROST. q. IN ONE STUDY, THE COMBINATION OF NON ABLATIVE FRACTIONAL LASER AND 20% TCA PEEL HAD BETTER RESULTS THAN EACH INDIVIDUAL MODALITY IN THE TREATMENT OF ATROPHIC ACNE SCARS. q. ANOTHER GROUP FOUND THAT DEEP PEELING, USING PHENOL AND 20% TCA

• CHEMICAL RECONSTRUCTION OF SKIN SCARS (CROSS) METHOD üPARTICULARLY USEFUL FOR TREATING “ICE PICK”SCARRING. üUSE OF HIGH CONCENTRATIONS TCA (50– 100%) APPLIED WITH WOODEN APPLICATORS TO ISOLATED ATROPHIC ACNE SCARS FOR A FEW SECONDS UNTIL A WHITE FROSTING APPEARS WITHIN THE SCAR. üTHE PROCEDURE CAN BE REPEATED AT 4 -WEEK INTERVALS UP TO A TOTAL OF THREE TREATMENTS. ü HIGH EFFICACY AND REDUCTION OF THE RISK OF HYPOPIGMENTATION.

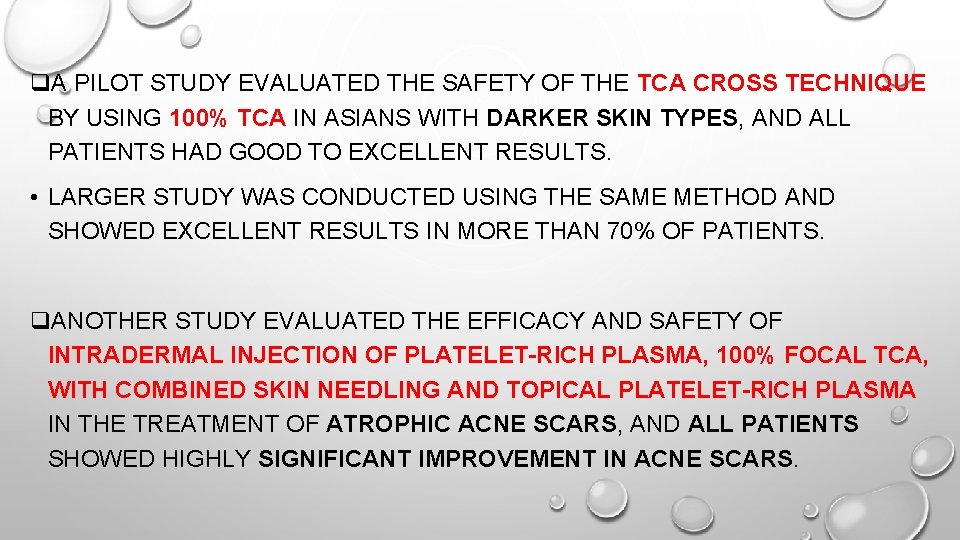
q. A PILOT STUDY EVALUATED THE SAFETY OF THE TCA CROSS TECHNIQUE BY USING 100% TCA IN ASIANS WITH DARKER SKIN TYPES, AND ALL PATIENTS HAD GOOD TO EXCELLENT RESULTS. • LARGER STUDY WAS CONDUCTED USING THE SAME METHOD AND SHOWED EXCELLENT RESULTS IN MORE THAN 70% OF PATIENTS. q. ANOTHER STUDY EVALUATED THE EFFICACY AND SAFETY OF INTRADERMAL INJECTION OF PLATELET-RICH PLASMA, 100% FOCAL TCA, WITH COMBINED SKIN NEEDLING AND TOPICAL PLATELET-RICH PLASMA IN THE TREATMENT OF ATROPHIC ACNE SCARS, AND ALL PATIENTS SHOWED HIGHLY SIGNIFICANT IMPROVEMENT IN ACNE SCARS.

• PHENOL IS A DEEP CHEMICAL PEELING AGENT üEPIDERMOLYSIS üDERMAL ELASTOLYSIS üNEOCOLLAGENESIS • CAUTION IS NECESSARY DUE TO SYSTEMIC ABSORPTION THAT MAY CAUSE CARDIOTOXICITY, NEPHROTOXICITY, AND RESPIRATORY DEPRESSION. • A PHENOL PEEL IS VERY EFFECTIVE IN TREATING ACNE SCARS


§ SUPERFICIAL PEELS, INCLUDING 30% SA, 20 -70% GA, 92% LA, JS, AND 1025% TCA; ü (SUPERFICIAL PEELS ARE USED IN COMEDONAL AND PAPULOPUSTULAR ACNE) ü NOT INDICATED FOR NODULOCYSTIC ACNE. § MEDIUM-DEPTH PEELS, INCLUDING 40 -60% PA, 25 -50% TCA; § COMBINED PEELS, SUCH AS 25% SA + 25 -30% TCA, JS + 35% TCA, SMP, ARE RECOMMENDED FOR TREATING SUPERFICIAL ACNE SCARS. § DEEP PEELS : TCA AT CONCENTRATIONS OVER 40%, THE TCA CROSS

CHEMICAL PEELS AND ISOTRETINOIN • THE EUROPEAN EVIDENCE BASED (S 3) GUIDELINES FOR THE TREATMENT OF ACNE RECOMMEND AVOIDANCE OF ACNE SCAR REPAIR PROCEDURES WITHIN 6 MONTHS AFTER ISOTRETINOIN TREATMENT. • A POSSIBLE EXPLANATION IS INCREASED PENETRATION OF THE CHEMICAL AGENT, INDUCTION OF ANGIOGENESIS, AND SYNTHESIS OF COLLAGENASE INHIBITORS. • SMALL STUDIES THAT SUGGEST THAT ISOTRETINOIN IS NOT AN ABSOLUTE CONTRAINDICATION FOR TREATMENT WITH CHEMICAL PEELS. • WE RECOMMEND AVOIDING CHEMICAL PEELS FOR AT LEAST 6 MONTHS AFTER CESSATION OF ISOTRETINOIN TREATMENT.

HORMONAL THERAPIES FOR ACNE CLINICS IN DERMATOLOGY (2017)35, 168– 17 BRITTANY BARROS, MD, DIANE THIBOUTOT, MD DEPARTMENT OF DERMATOLOGY, COLLEGE OF MEDICINE, THE PENNSYLVANIA STATE UNIVERSITY, HERSHEY, PA, USA

HORMONAL CONTRIBUTIONS TO ACNE THE VARIOUS HORMONES POSSIBLY IMPLICATED IN ACNE. q. ANDROGENS(TESTOSTERONE, DHT, DHEAS): INCREASE THE SIZE AND SECRETION OF SEBACEOUS GLANDS. SOURCES ADRENAL GLAND, OVARY, OR TESTES. AND PRODUCED LOCALLY WITHIN THE SEBACEOUS GLAND; FOR EXAMPLE, T DHT BY THE TYPE 1 5 -ALPHA-REDUCTASE OF THE SEBACEOUS GLAND. q. ESTROGENS COUNTER THE ACTION OF ANDROGENS BY THREE POTENTIAL MECHANISMS: DIRECT OPPOSITION LOCALLY, INHIBITION OF ANDROGEN PRODUCTION IN THE

q. GROWTH HORMONE AND GROWTH FACTORS (IGF-1) ARE SECRETED IN HIGH LEVELS DURING PUBERTY. IN SOME TISSUES (SEBACEOUS GLANDS) THE ACTION OF IGF-1 IS INFLUENCED BY ANDROGENS. q. MELANOCORTINS (MSH, ACTH) REGULATE SEBUM PRODUCTION. üHISTORICAL CLUES( WOMAN’S ACNE IS HORMONALLY RELATED ): v. ACNE WORSENS BEFORE MENSTRUATION v. PERSISTENCE OF ACNE DESPITE CONVENTIONAL THERAPY (TOPICAL RETINOID, TOPICAL BENZOYL PEROXIDE, AND TOPICAL OR SYSTEMIC ANTIBIOTIC)

INDICATIONS TO PURSUE AN ENDOCRINE WORK-UP q. ACNE OF SUDDEN ONSET, IN PARTICULAR IF NO PRIOR HISTORY OF ACNE q. ACNE WITH ASSOCIATED HIRSUTISM q. ACNE WITH ASSOCIATED IRREGULAR MENSTRUAL CYCLES q. ACNE WITH ASSOCIATED SIGNS OF HYPERANDROGENISM, SUCH AS CUSHINGOID FACIES, INCREASED LIBIDO, NEW ACANTHOSIS NIGRICANS, DEEPENED VOICE, INSULIN RESISTANCE, OR PATTERNED HAIR LOSS.

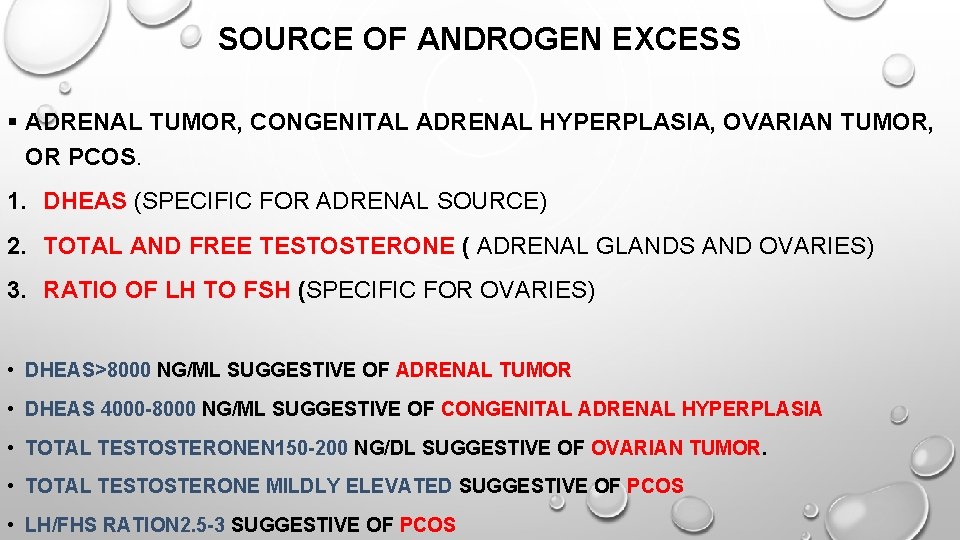
SOURCE OF ANDROGEN EXCESS § ADRENAL TUMOR, CONGENITAL ADRENAL HYPERPLASIA, OVARIAN TUMOR, OR PCOS. 1. DHEAS (SPECIFIC FOR ADRENAL SOURCE) 2. TOTAL AND FREE TESTOSTERONE ( ADRENAL GLANDS AND OVARIES) 3. RATIO OF LH TO FSH (SPECIFIC FOR OVARIES) • DHEAS>8000 NG/ML SUGGESTIVE OF ADRENAL TUMOR • DHEAS 4000 -8000 NG/ML SUGGESTIVE OF CONGENITAL ADRENAL HYPERPLASIA • TOTAL TESTOSTERONEN 150 -200 NG/DL SUGGESTIVE OF OVARIAN TUMOR. • TOTAL TESTOSTERONE MILDLY ELEVATED SUGGESTIVE OF PCOS • LH/FHS RATION 2. 5 -3 SUGGESTIVE OF PCOS

TREATMENT üANDROGEN RECEPTOR BLOCKERS SPIRONOLACTONE, CYPROTERONE ACETATE, CHLORMADINONE, FLUTAMIDE. üADRENAL ANDROGEN PRODUCTION BLOCKERS GLUCOCORTICOIDS üOVARIAN PRODUCTION BLOCKERS GNRH AGONISTS AND OCP

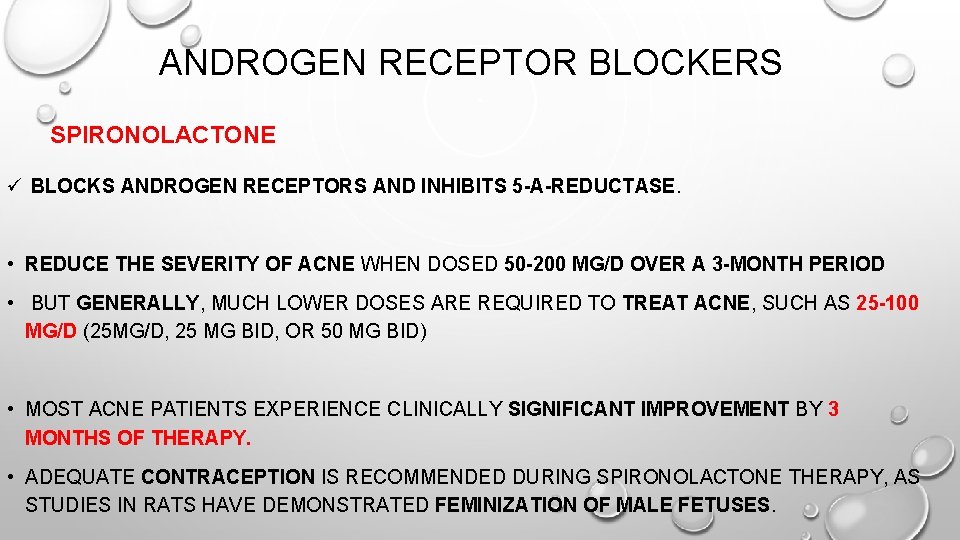
ANDROGEN RECEPTOR BLOCKERS SPIRONOLACTONE ü BLOCKS ANDROGEN RECEPTORS AND INHIBITS 5 -Α-REDUCTASE. • REDUCE THE SEVERITY OF ACNE WHEN DOSED 50 -200 MG/D OVER A 3 -MONTH PERIOD • BUT GENERALLY, MUCH LOWER DOSES ARE REQUIRED TO TREAT ACNE, SUCH AS 25 -100 MG/D (25 MG/D, 25 MG BID, OR 50 MG BID) • MOST ACNE PATIENTS EXPERIENCE CLINICALLY SIGNIFICANT IMPROVEMENT BY 3 MONTHS OF THERAPY. • ADEQUATE CONTRACEPTION IS RECOMMENDED DURING SPIRONOLACTONE THERAPY, AS STUDIES IN RATS HAVE DEMONSTRATED FEMINIZATION OF MALE FETUSES.

q. SIDE EFFECTS OF SPIRONOLACTONE § MENSTRUAL IRREGULARITIES (RESOLVED WITHIN 2 -3 MONTHS OF CONTINUED THERAPY) § NAUSEA § DIZZINESS § POLYURIA § VERTIGO § SPIRONOLACTONE WAS ASSOCIATED WITH A SMALL BUT NOT STATISTICALLY SIGNIFICANT INCREASE IN THE RISK OF BREAST AND GYNECOLOGIC CANCERS, AND THE AUTHORS CONCLUDE THAT OTHER CONFOUNDING FACTORS ARE LIKELY PRESENT.

CYPROTERONE ACETATE ü A PROGESTATIONAL ANTIANDROGEN COMBINATION ORAL CONTRACEPTIVE PILL (OCP), DIANETTE (BAYER AG, LEVERKUSEN GERMANY), (CONTAINS CYPROTERONE ACETATE 2 MG PLUS ETHINYL ESTRADIOL 35 ΜGOR 50ΜG) AS COMBINATION THERAPY WITH A METHOD NAMED HAMMERSTEIN’S REVERSE SEQUENTIAL REGIMEN, WHICH CONSISTS OF CYPROTERONE ACETATE 100 MG FOR 10 DAYS FOLLOWED BY ETHINYL ESTRADIOL 50ΜG FOR 21 DAYS. THIS REGIMEN RESULTED IN GOOD TO VERY GOOD IMPROVEMENT OF ACNE IN 90% OF STUDIED PATIENTS.

SIDE EFFECTS • HEPATOTOXICITY • HIGH DOSES AN INCREASED RISK FOR HEPATIC TUMORS üHEPATOXICITY IN HUMANS TENDS TO BE DOSE DEPENDENT AND RELATED TO THE HIGHER DOSES REQUIRED IN THE TREATMENT OF MEN WITH PROSTATE CANCER, WOMEN WITH BREAST CANCER, AND CHILDREN WITH GROWTH DISORDERS.

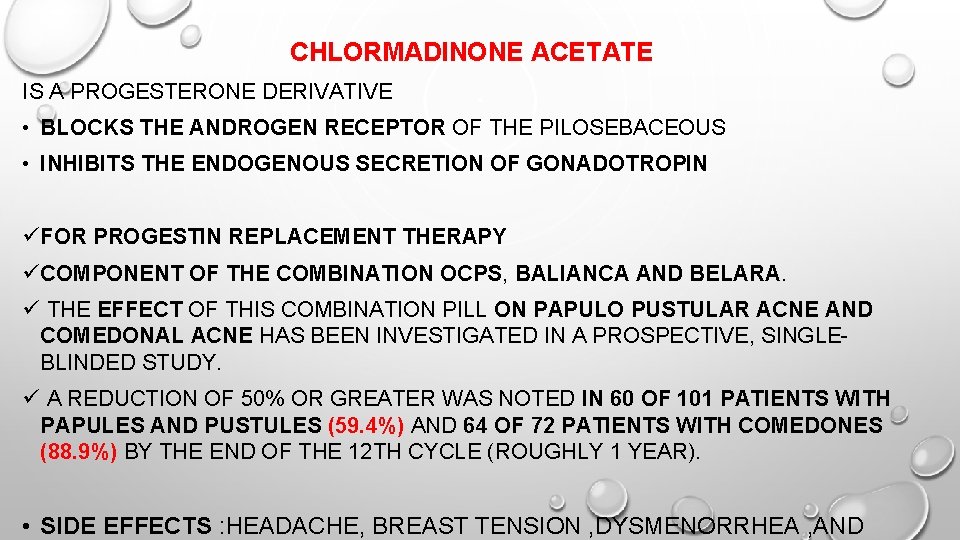
CHLORMADINONE ACETATE IS A PROGESTERONE DERIVATIVE • BLOCKS THE ANDROGEN RECEPTOR OF THE PILOSEBACEOUS • INHIBITS THE ENDOGENOUS SECRETION OF GONADOTROPIN üFOR PROGESTIN REPLACEMENT THERAPY üCOMPONENT OF THE COMBINATION OCPS, BALIANCA AND BELARA. ü THE EFFECT OF THIS COMBINATION PILL ON PAPULO PUSTULAR ACNE AND COMEDONAL ACNE HAS BEEN INVESTIGATED IN A PROSPECTIVE, SINGLEBLINDED STUDY. ü A REDUCTION OF 50% OR GREATER WAS NOTED IN 60 OF 101 PATIENTS WITH PAPULES AND PUSTULES (59. 4%) AND 64 OF 72 PATIENTS WITH COMEDONES (88. 9%) BY THE END OF THE 12 TH CYCLE (ROUGHLY 1 YEAR). • SIDE EFFECTS : HEADACHE, BREAST TENSION , DYSMENORRHEA , AND

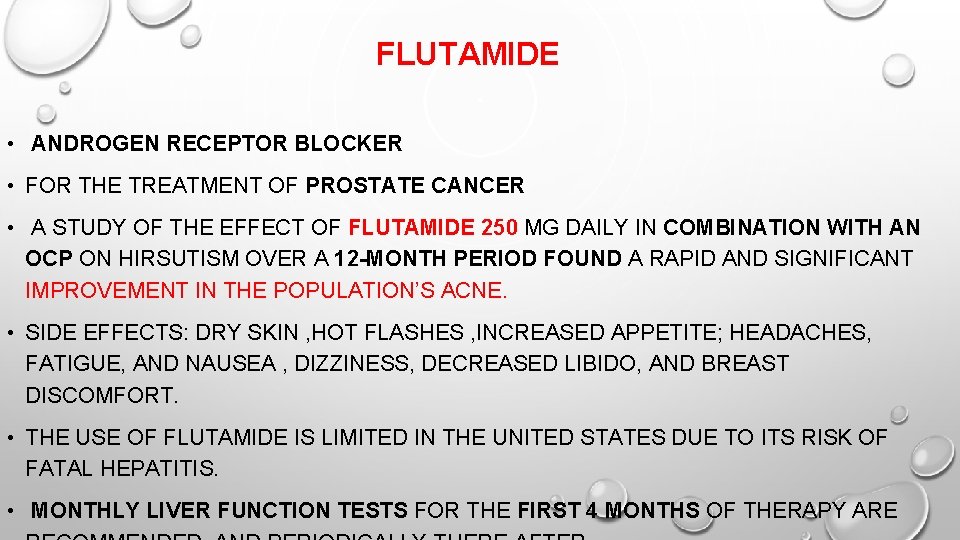
FLUTAMIDE • ANDROGEN RECEPTOR BLOCKER • FOR THE TREATMENT OF PROSTATE CANCER • A STUDY OF THE EFFECT OF FLUTAMIDE 250 MG DAILY IN COMBINATION WITH AN OCP ON HIRSUTISM OVER A 12 -MONTH PERIOD FOUND A RAPID AND SIGNIFICANT IMPROVEMENT IN THE POPULATION’S ACNE. • SIDE EFFECTS: DRY SKIN , HOT FLASHES , INCREASED APPETITE; HEADACHES, FATIGUE, AND NAUSEA , DIZZINESS, DECREASED LIBIDO, AND BREAST DISCOMFORT. • THE USE OF FLUTAMIDE IS LIMITED IN THE UNITED STATES DUE TO ITS RISK OF FATAL HEPATITIS. • MONTHLY LIVER FUNCTION TESTS FOR THE FIRST 4 MONTHS OF THERAPY ARE

ADRENAL ANDROGEN PRODUCTION BLOCKERS ü IN LOW DOSES, GLUCOCORTICOIDS SUPPRESS ADRENAL PRODUCTION OF ANDROGENS AND ARE USED IN INDIVIDUALS WITH ELEVATED SERUM DHEAS LEVELS. ü PREDNISONE 2. 5 MG OR DEXAMETHASONE 0. 25 -0. 75 MG MAY BE GIVEN EVERY DAY OR EVERY OTHER DAY, AT BEDTIME. ( MONITORED WITH SERUM DHEAS LEVEL) ü ADRENAL SUPPRESSION IS POSSIBLE, ESPECIALLY IF DEXAMETHASONE IS USED, AND CAN BE SCREENED WITH ACTH STIMULATION TEST, 2 -3 MONTHS AFTER STARTING THERAPY. ü DURATION OF THERAPY IS LIMITED BY THE LONG TERM SIDE EFFECT OF OSTEOPOROSIS, AND SHOULD THEREFORE BE LIMITED TO 6 MONTH.

OVARIAN ANDROGEN PRODUCTION BLOCKERS üGNRH AGONISTS BLOCK ANDROGEN PRODUCTION BY THE OVARY üGNRH AGONISTS INCLUDE BUSERELIN, NAFAELIN, AND LEUPROLIDE, WHICH ARE AVAILABLE IN NASAL SPRAY AND INJECTABLE (BUT NOT ORAL) FORMULATIONS ü USED TO TREAT ACNE IN THE PRESENCE OR ABSENCE OF ENDOCRINE ABNORMALITIES. q IN A SMALL PROSPECTIVE STUDY OF SIX EU MENORRHEIC WOMEN WITHOUT ADRENAL OR OVARIAN DYSFUNCTION WITH ACNE, BUSERELIN 1200ΜG NASAL SPRAY DAILY FOR 6 MONTHS RESULTED IN CLINICALLY AND STATISTICALLY SIGNIFICANT IMPROVEMENT AT 3 MONTHS OF THERAPY, AND THE IMPROVEMENT WAS MAINTAINED 6 MONTHS AFTER DISCONTINUATION. • SIDE EFFECTS MAY LIMIT THERAPY AND MIMIC MENOPAUSE WITH LOW ESTROGEN, HEADACHES, AND BONE LOSS.

• OCPS, WITH A COMBINATION OF AN ESTROGEN AND PROGESTIN, ARE COMMONLY USED WORLDWIDE IN THE TREATMENT OF ACNE. üESTROGENS(ETHINYL ESTRADIOL) INCREASE THE BINDING OF FREE TESTOSTERONE AND DECREASE ANDROGEN LEVELS VIA INHIBITION OF OVULATION. üPROGESTINS HAVE ANTI-ANDROGENIC PROPERTIES. SECOND-GENERATION PROGESTINS WITH LOW ANDROGENIC ACTIVITY INCLUDE ETHYNODIOL DIACETATE, NORETHINDRONE, AND LEVONORGESTREL THIRD-GENERATION PROGESTINS HAVE THE LOWEST OF ALL ANDROGENIX ACTIVITY INCLUDE BORGESTIMATE, DESOGESTREL, AND GESTODENE

• DROSPIRENONE IS DERIVED FROM SPIRONOLACTONE v. ANTI ANDROGENIC AND ANTI MINERALOCORTICOID v CAN BE USED TO IMPROVE ACNE AND HIRSUTISM. q. IN A DOUBLE-BLINDED, PLACEBO-CONTROLLED MULTICENTER STUDY ACROSS THE UNITED STATES INVESTIGATING THE SAFETY AND EFFICACY OF THE NEWER LOWDOSE COMBINATION OCP, 3 MG DROSPIRENONE /20ΜGEE 24/4(IE, 24 DAYS OF ACTIVE DRUG AND 4 DAYS OF INERT TABLETS PER CYCLE) WAS USED IN THE TREATMENT OF MODERATE ACNE VULGARIS (MINIMUM OF 40 ACNE LESIONS) IN REPRODUCTIVE AGED WOMEN (14 -45 YEARS OLD) BY THE THIRD CYCLE OF THERAPY, STATISTICALLY AND CLINICALLY SIGNIFICANT IMPROVEMENT IN ACNE COUNTS WERE OBSERVED AND MAINTAINED THROUGH

• NO COMPARATIVE STUDIES EXIST COMPARING VARIOUS FORMULATIONS OF OCPS AND THE TREATMENT OF ACNE. • SIDE EFFECTS OF OCPS: MOOD CHANGES, DECREASED LIBIDO, HEADACHE, BREAST TENDERNESS, AND IRREGULAR MENSTRUAL BLEEDING. INCREASE THE RISK FOR THROMBOEMBOLIC EVENTS, • ORAL CONTRACEPTIVES CONTAINING ESTROGEN SHOULD BE AVOIDED IN THE FOLLOWING SITUATIONS: ü CURRENT OR HISTORY OF DVT ü CURRENT OR HISTORY OF PULMONARY EMBOLISM ü HISTORY OF CVA ü CORONARY ARTERY OR ISCHEMIC HEART DISEASE ü HISTORY OF ESTROGEN-DEPENDENT CANCER (INCLUDING HISTORY OF BREAST CANCER) ü CURRENT SMOKER

ORAL CONTRACEPTIVES AND PRACTICAL TIPS üCONCOMITANT ANTIBIOTICS, ONLY RIFAMPIN HAS PHARMACOKINETIC EVIDENCE OF REDUCING STEROID LEVELS IN WOMEN TAKING COMBINATION ORAL CONTRACEPTIVES. üANT IINFECTIVE AGENTS THAT ARE SAFE FOR CONCOMITANT USE, INCLUDE AMPICILLIN, DOXYCYCLINE , FLUCONAZOLE, METRONIDAZOLE, MICONAZOLE, QUINOLONE ANTIMICROBIALS, AND TETRACYCLINE. • IF HORMONAL TESTING IS PLANNED, ORAL CONTRACEPTION SHOULD BE
 Plexr gone wrong
Plexr gone wrong Scars in society art
Scars in society art How to make fake injuries
How to make fake injuries Richters hernia
Richters hernia How do scars form
How do scars form Glacial scars continental drift
Glacial scars continental drift What is mid term break about
What is mid term break about Dense old scars
Dense old scars L
L Acnevulgaristreatments.com
Acnevulgaristreatments.com Asley acne expert
Asley acne expert Acne vulgaris histology
Acne vulgaris histology Peptome
Peptome L
L Sinonim drainase
Sinonim drainase Retinody
Retinody Dolore alle ovaie in menopausa
Dolore alle ovaie in menopausa Acne
Acne Sudorazioni notturne
Sudorazioni notturne Acne conglobata huidziekten
Acne conglobata huidziekten Acne estivale
Acne estivale Flonase acne
Flonase acne Billy burns acne
Billy burns acne Epiduo cmi
Epiduo cmi Active high and active low
Active high and active low Membrane structures that function in active transport
Membrane structures that function in active transport Chapter 7 review chemical formulas and chemical compounds
Chapter 7 review chemical formulas and chemical compounds Love formula
Love formula Primary active transport vs secondary active transport
Primary active transport vs secondary active transport Chemical engineering keywords
Chemical engineering keywords Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Section 1 chemical changes
Section 1 chemical changes Are kc and kp equal
Are kc and kp equal Physical change
Physical change Compare and contrast mechanical and chemical weathering
Compare and contrast mechanical and chemical weathering Causes of weathering
Causes of weathering Active and passive listening
Active and passive listening Revision passive voice
Revision passive voice Passive voice exercises 11th grade
Passive voice exercises 11th grade