Chapter 9 Cellular Respiration and Fermentation Essential Knowledge



























- Slides: 70

Chapter 9: Cellular Respiration and Fermentation

Essential Knowledge � 2. a. 1 – All living systems require constant input of free energy (9. 1 -9. 5). � 2. a. 2 – Organisms capture and store free energy for use in biological processes (9. 19. 5).

Cellular Respiration - Preview � Def - The process of releasing energy/ATP from food � Food - Stored energy in chemical bonds (provides fuel) � ATP - Useable energy for cellular processes � Wastes – CO 2 and H 2 O � Mitochondrion store most of equipment needed for rxn

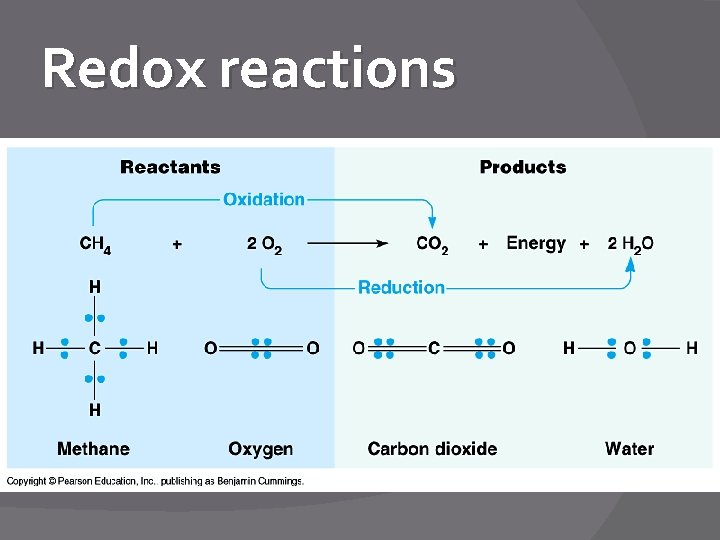
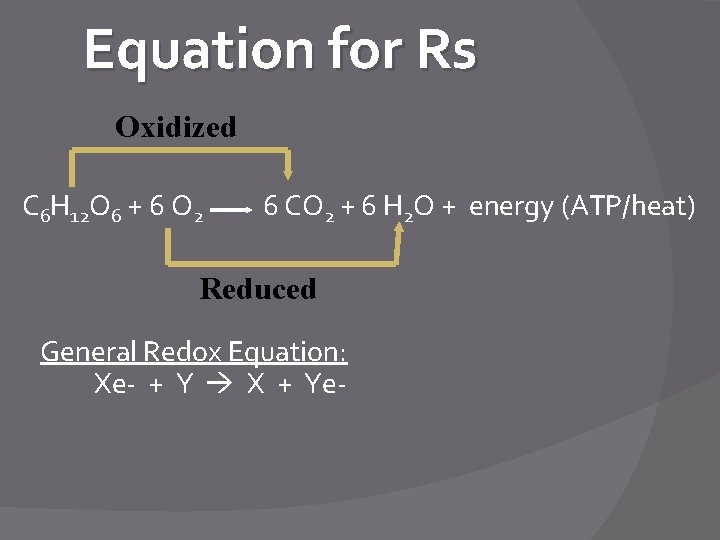
Respiration (Rs) Equation C 6 H 12 O 6 + 6 O 2 6 CO 2 + 6 H 2 O + energy (ATP or heat) � Rxn is spontaneous (-∆G) � The energy is released (exergonic) from the bonds in the org molecules �Remember: Org molecules store energy in their arrangement of atoms �Org molecules can be carbs, proteins or fats/lipids


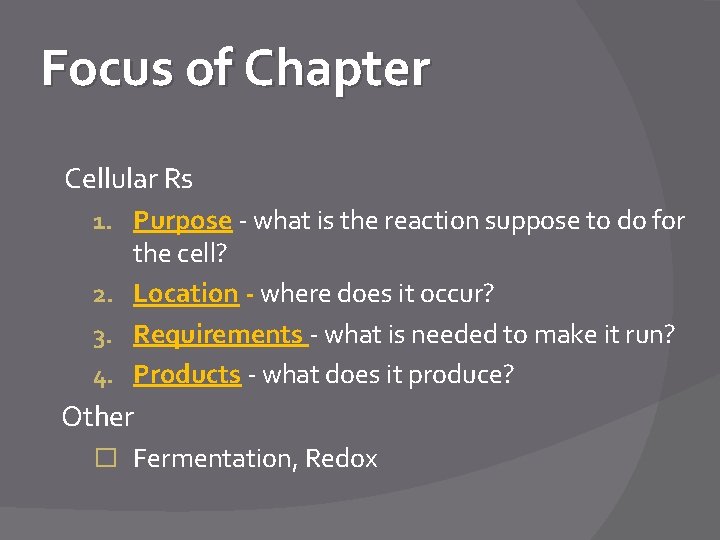
Focus of Chapter Cellular Rs 1. Purpose - what is the reaction suppose to do for the cell? 2. Location - where does it occur? 3. Requirements - what is needed to make it run? 4. Products - what does it produce? Other � Fermentation, Redox

Fuel? What is used? � Organic molecules with a large amt of hydrogen make great fuel! Why? �H becomes oxidized (only has one e-) very easily and energy is released �Remember: Carbs, fats, proteins are storage bins for e- associated with hydrogen

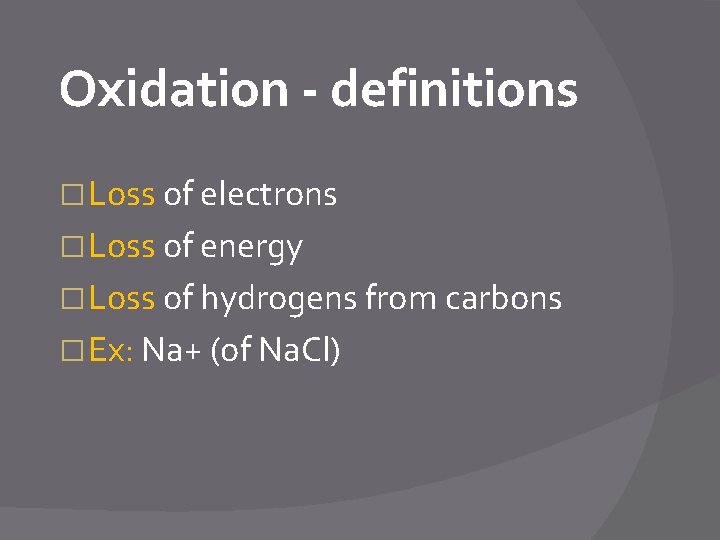
Oxidation - definitions � Loss of electrons � Loss of energy � Loss of hydrogens from carbons � Ex: Na+ (of Na. Cl)

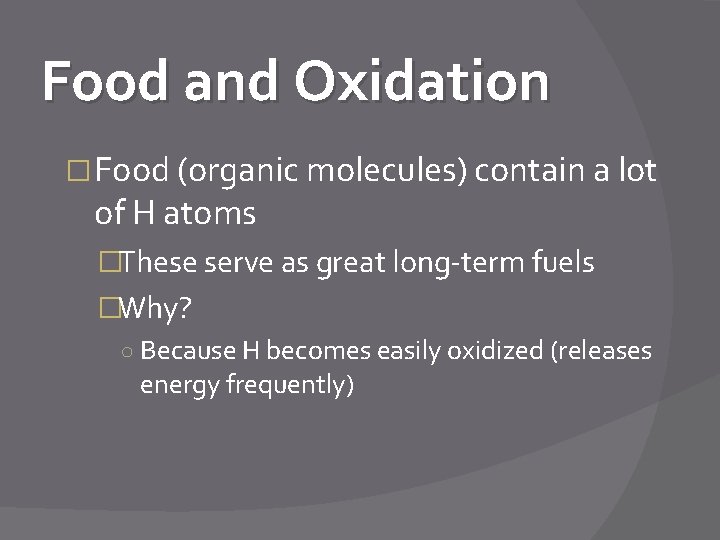
Food and Oxidation � Food (organic molecules) contain a lot of H atoms �These serve as great long-term fuels �Why? ○ Because H becomes easily oxidized (releases energy frequently)

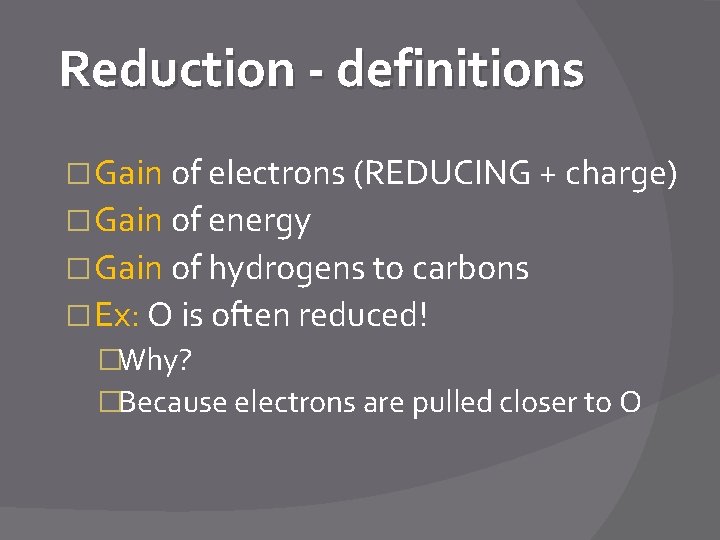
Reduction - definitions � Gain of electrons (REDUCING + charge) � Gain of energy � Gain of hydrogens to carbons � Ex: O is often reduced! �Why? �Because electrons are pulled closer to O

Redox reactions

Equation for Rs Oxidized C 6 H 12 O 6 + 6 O 2 6 CO 2 + 6 H 2 O + energy (ATP/heat) Reduced General Redox Equation: Xe- + Y X + Ye-

Redox reactions � Involves transfer of e- and energy release �Sometimes doesn’t involve complete transfer � Red and Oxd reactions are usually paired or linked together. �Why? Because e- transfer requires donor and acceptor � Many of the reactions will be done by phosphorylation � Redox video

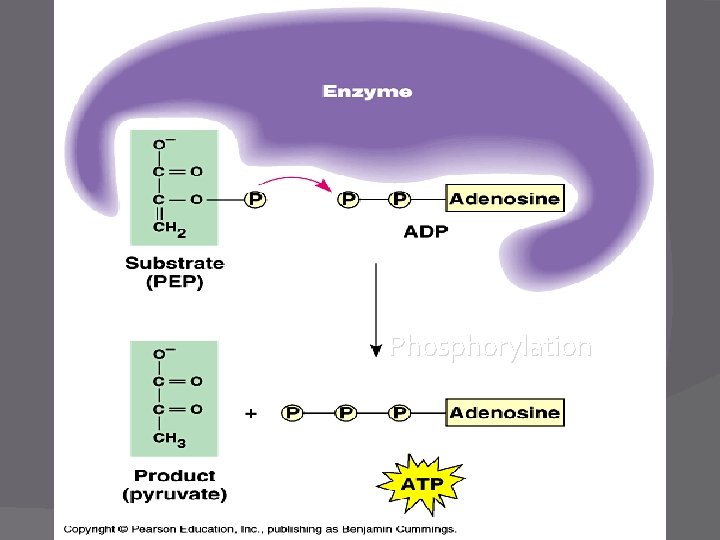
Phosphorylation � Adding a phosphate group to a molecule �Ex: ATP cycle (add P to ADP = ATP) � Two types: �Oxidative AND substrate-level � The phosphate group adds “energy” to the molecule for chemical reactions (think ATP cycle) �Endergonic rxn

Phosphorylation

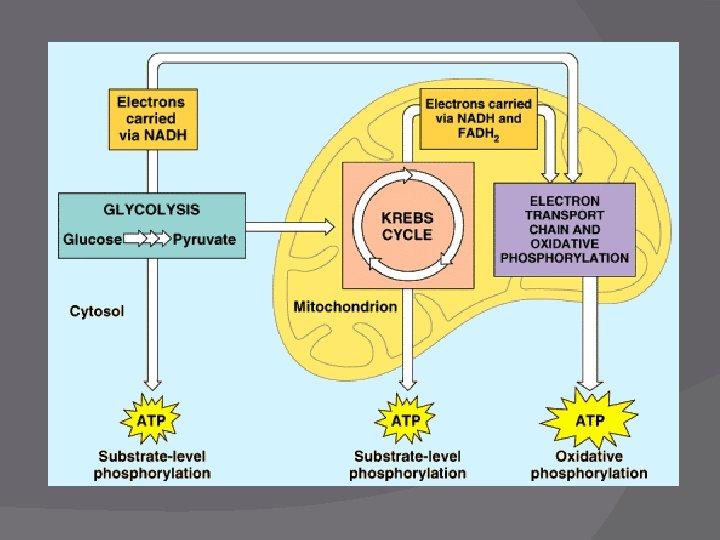
Cell Respiration – 3 parts 1. Glycolysis 2. Krebs Cycle 3. Electron Transport Chain **Use page 167 as a starting point: Cellular Respiration - A Preview


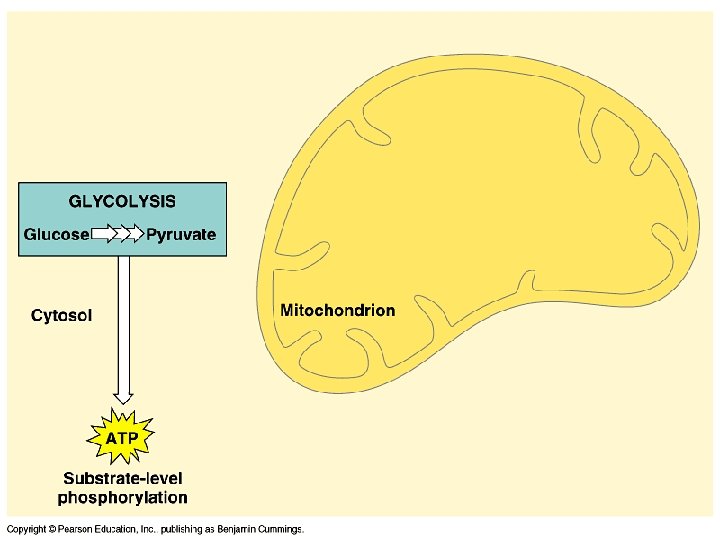
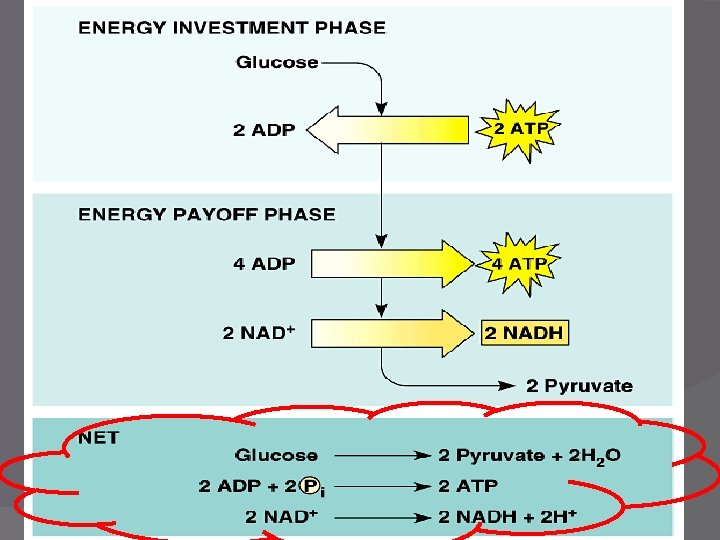
P E T S 1 Glycolysis � Glyco- glucose -lysis: to split � Formula for glucose: C 6 H 12 O 6 � Universal step in all Rs types. � Likely the earliest type of cell energy processes � Overview: �Glucose splits into 2 3 -C sugars (then oxidizes to form pyruvate)

Glycolysis � Function - To split glucose and produce NADH and ATP �ATP made by substrate-level phosphorylation ○ Enzyme transfers phosphate group from substrate/reactant to ADP to make ATP � Location – Cytoplasm of the cell


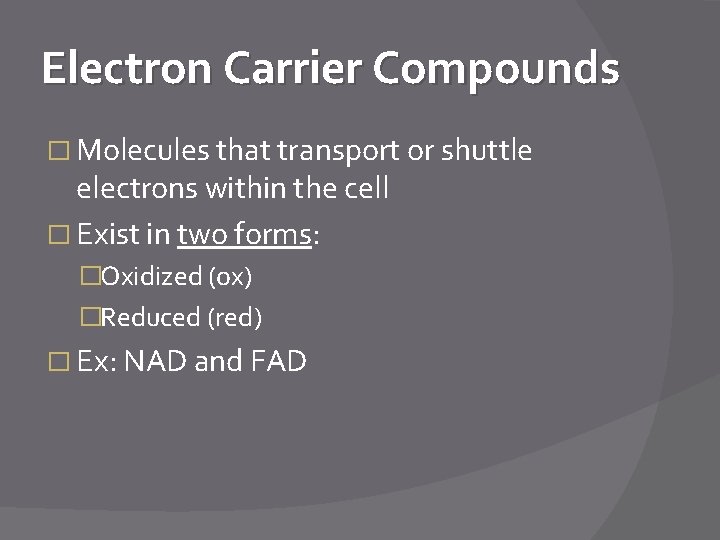
Electron Carrier Compounds � Molecules that transport or shuttle electrons within the cell � Exist in two forms: �Oxidized (ox) �Reduced (red) � Ex: NAD and FAD

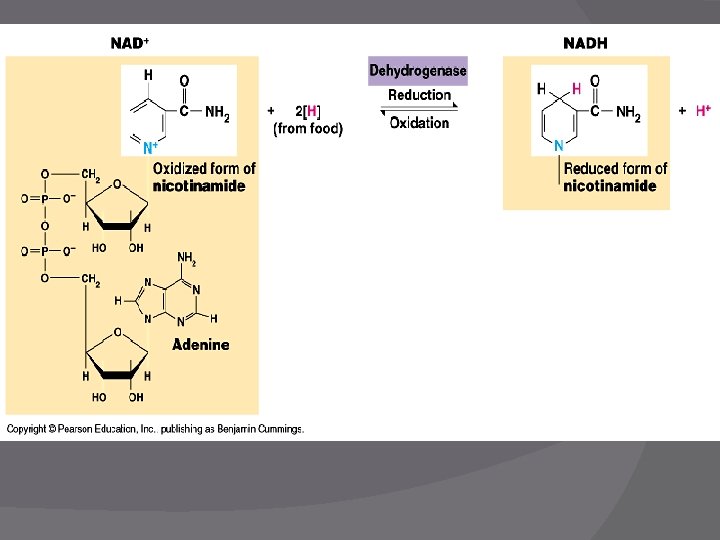
NAD � Nicotinamide Adenine Dinucleotide � NAD+ + 2 e- NADH � NAD+ = oxidized form � NADH = reduced form* *Reduced by e- from food oxidation


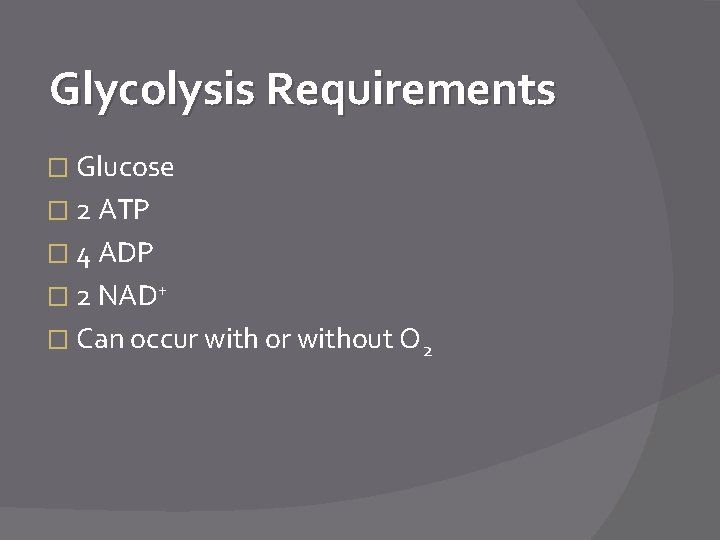
Glycolysis Requirements � Glucose � 2 ATP � 4 ADP � 2 NAD+ � Can occur with or without O 2

o tr n I s ysi l o c Glycolysis - Products � 2 Pyruvic Acids (a 3 -Carbon acid) � 2 ADP, 4 ATP, 2 NADH � NET RESULT: � 2 ATP per glucose � 2 NADH � 2 pyruvate �H 2 O Notice: No CO 2 made during this step!


P E T S 2 Krebs Cycle � Oxidizes fuel from pyruvate molecules �Remember? Pyruvate formed during glycolysis � Also called: �Citric Acid Cycle �Tricarboxylic Acid Cycle

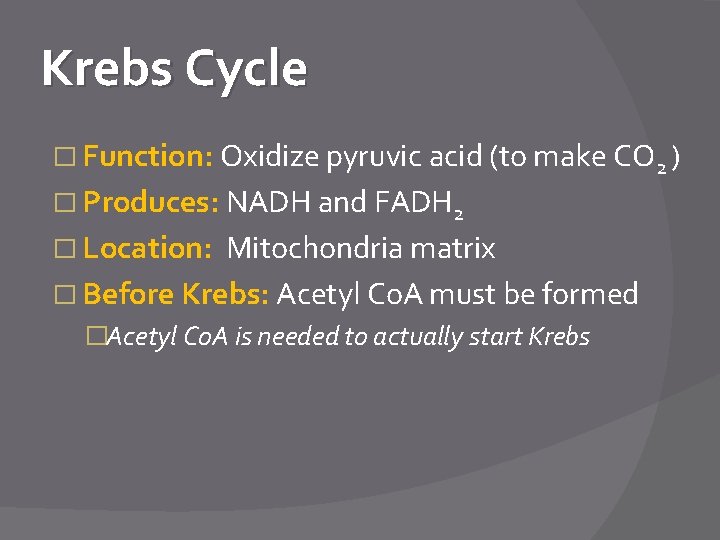
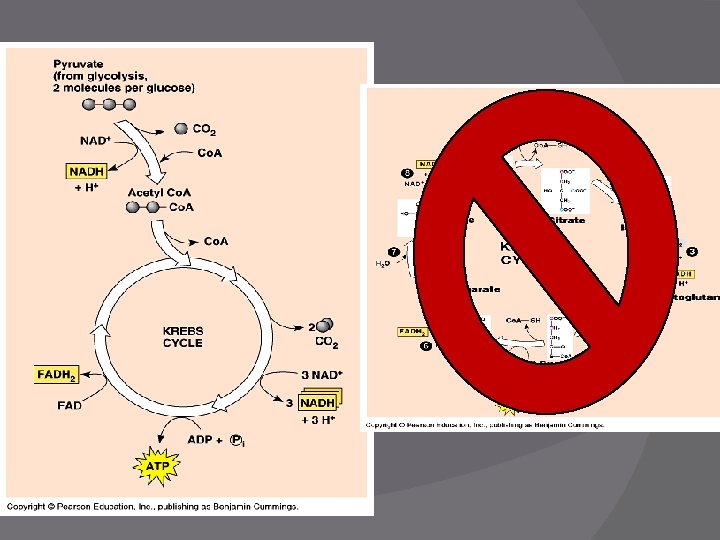
Krebs Cycle � Function: Oxidize pyruvic acid (to make CO 2 ) � Produces: NADH and FADH 2 � Location: Mitochondria matrix � Before Krebs: Acetyl Co. A must be formed �Acetyl Co. A is needed to actually start Krebs

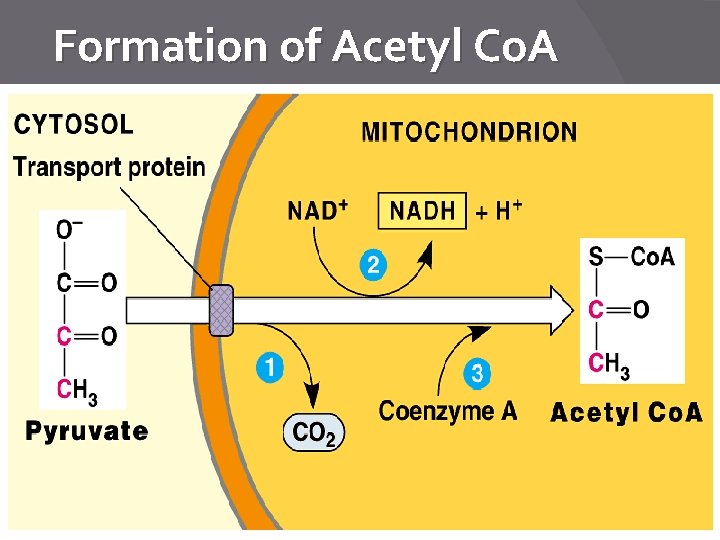
Pyruvate moved into mito? � Why? How? �Pyruvate is moved into mitochondria (from cytoplasm) �Why? This is where the 2 nd step occurs (specific enzymes are in mito) �Serves as a checkpoint �Uses active transport and transport proteins. ○ Why? Pyruvate is a charged molecule!

Formation of Acetyl Co. A

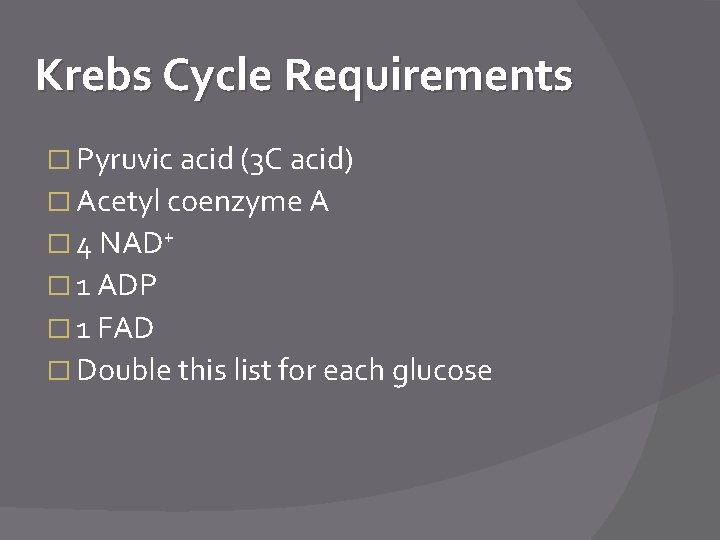
Krebs Cycle Requirements � Pyruvic acid (3 C acid) � Acetyl coenzyme A � 4 NAD+ � 1 ADP � 1 FAD � Double this list for each glucose

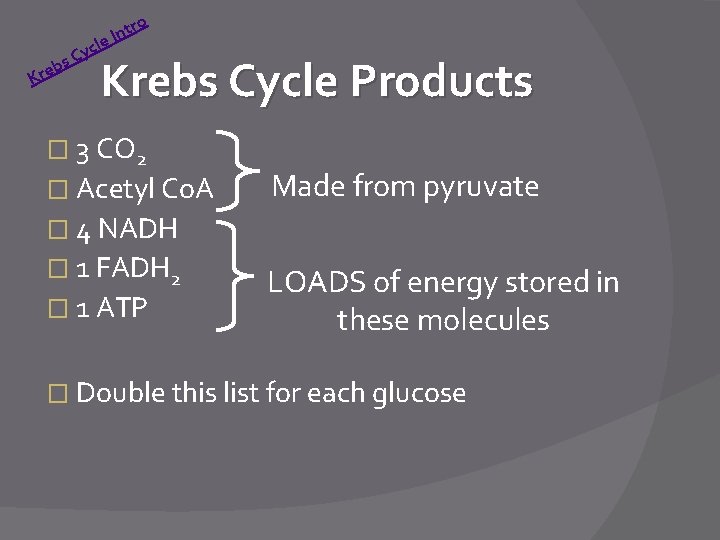
tr n I le c Cy s b Kre o Krebs Cycle Products � 3 CO 2 � Acetyl Co. A Made from pyruvate � 4 NADH � 1 FADH 2 � 1 ATP LOADS of energy stored in these molecules � Double this list for each glucose


Krebs Cycle notes � Notice: �Only 1 ATP made per cycle �Produces most of the cell's energy in the form of NADH and FADH 2 � Does NOT require O 2

Comment about ATP � The ATPs produced directly in Krebs Cycle and Glycolysis are by: �Substrate-level phosphorylation � The ADP P group is transferred from a substrate to �Making ATP

At this point… � After the Krebs and glycolysis cycles, the cell has made a total of 4 ATP. �Remember: some ATP had to be used to power the cycles. � Most energy (at this point) comes from NADH and FADH 2

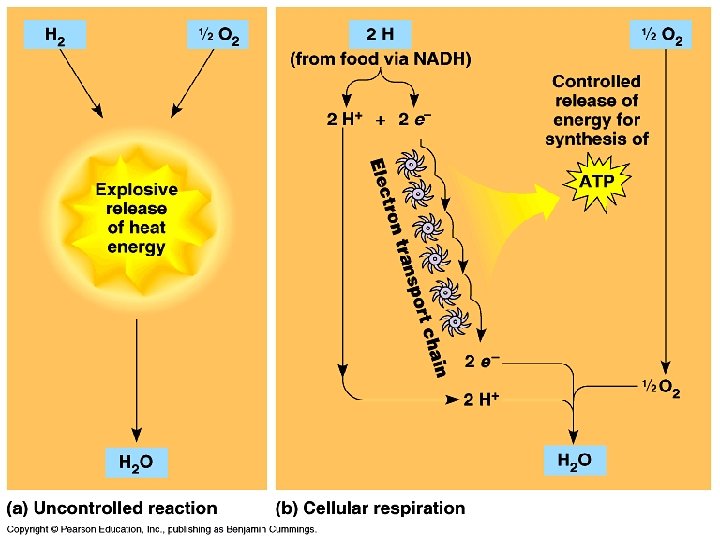
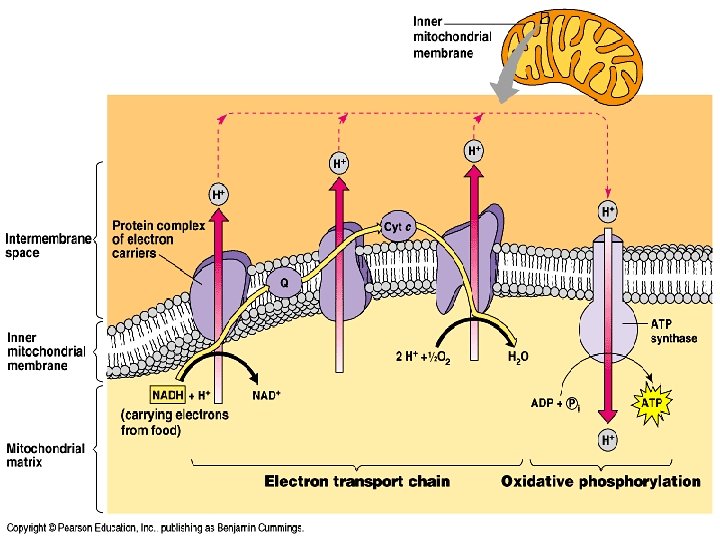
3 Electron Transport P System E T S � ETC/S or Electron Transport Chain � This is a collection of proteins that are structurally linked � Located in inner membrane of mito �Folding of mito (cristae) allows for lots of places (large surface area!) for ETC to occur

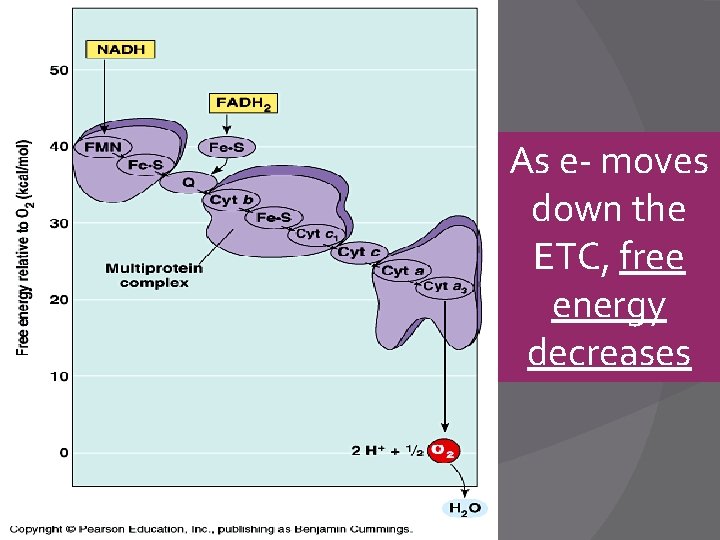
ETC/S � Uses sets of Cytochromes �Fe (Iron)-containing proteins to pass electrons � The Cytochromes alternate between Red and Ox forms and pass electrons down to O 2 �Remember: LEO, GER; LEO the lion goes GER �Losing Electrons is Oxidation; Gaining Electrons is Reduction

As e- moves down the ETC, free energy decreases

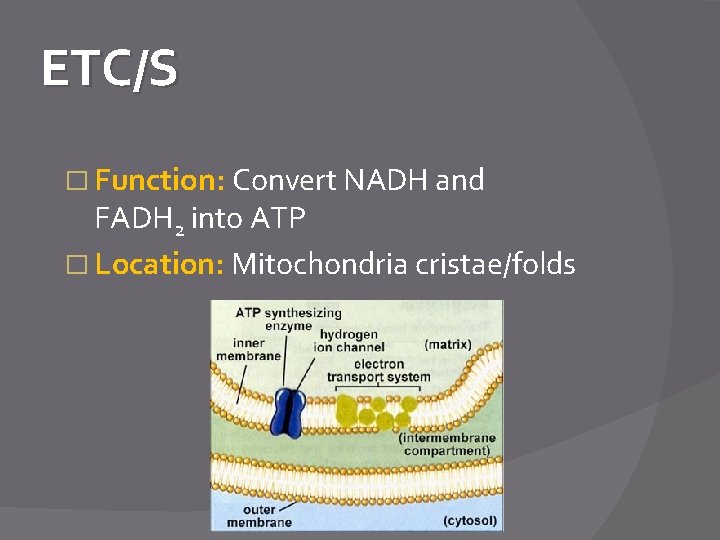
ETC/S � Function: Convert NADH and FADH 2 into ATP � Location: Mitochondria cristae/folds

ETC Requirements � NADH or FADH 2 � ADP � O 2 �We finally see the need/requirement of Oxygen

ETC Products � NAD+ and FAD � ATP (LOTS!!!) � H 2 O exp ETC lan atio n �Remember: Water was also produced during glycolysis

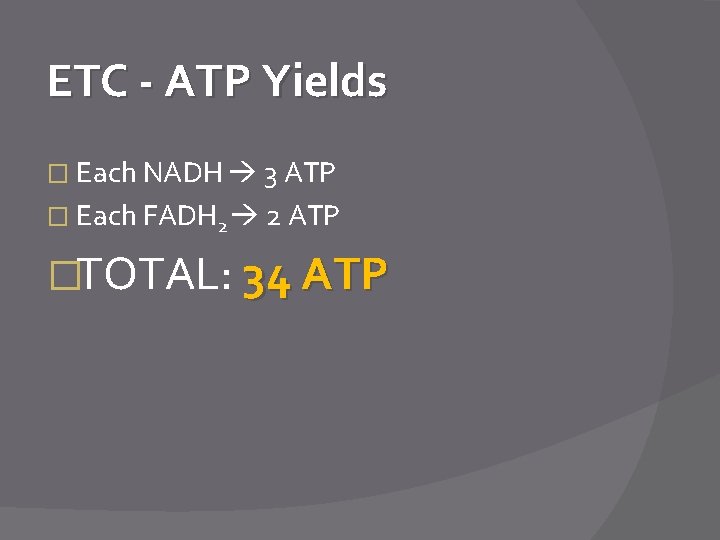
ETC - ATP Yields � Each NADH 3 ATP � Each FADH 2 2 ATP �TOTAL: 34 ATP

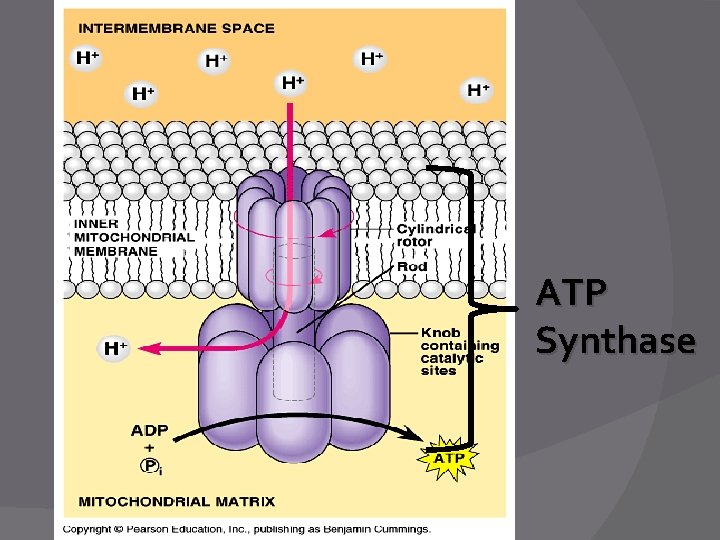
Chemiosmotic Hypothesis � ETC energy is used to move H+ (protons) across the mito/cristae membrane � ATP is generated as the H+ diffuse back into the matrix


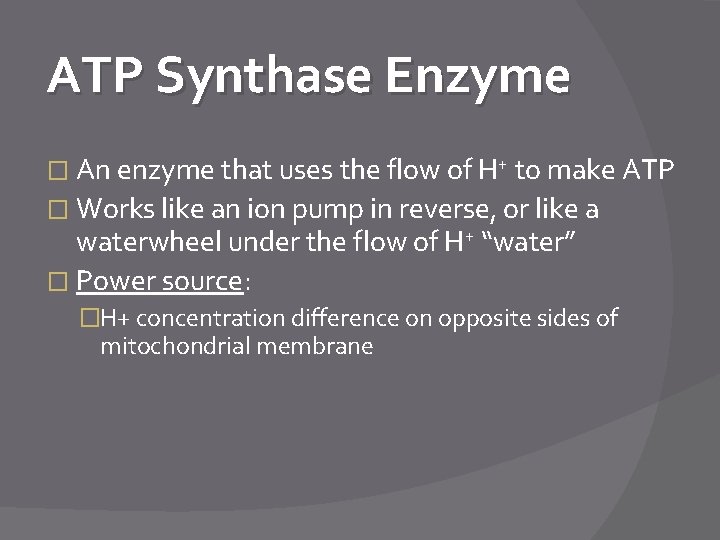
ATP Synthase Enzyme � An enzyme that uses the flow of H+ to make ATP � Works like an ion pump in reverse, or like a waterwheel under the flow of H+ “water” � Power source: �H+ concentration difference on opposite sides of mitochondrial membrane

Oxidative Phosphorylation � ATP synthase uses oxidative phosphorylation to make ATP during ETC � Uses H ions to make ATP and water (using Oxygen)

ATP Synthase

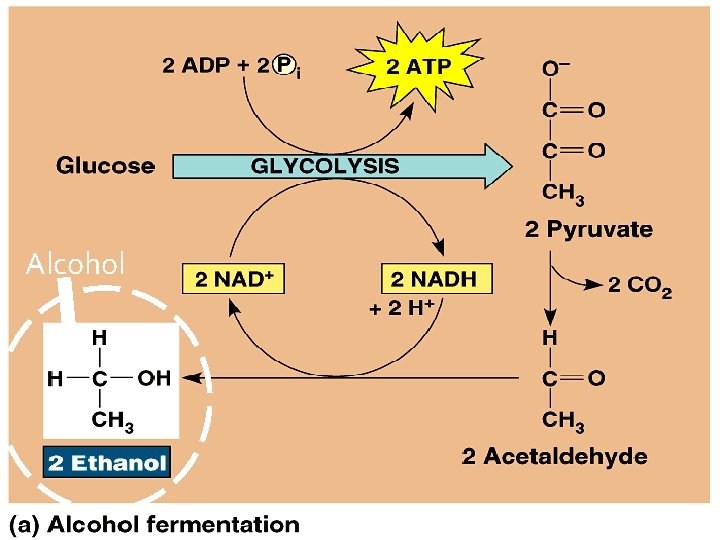
Alcoholic Fermentation � Done by yeast �A kind of fungus � Used in brewing beer, winemaking, and baking �CO 2 bubbles generated give: ○ Bread a rising effect ○ Wine/Beer the carbonated effect

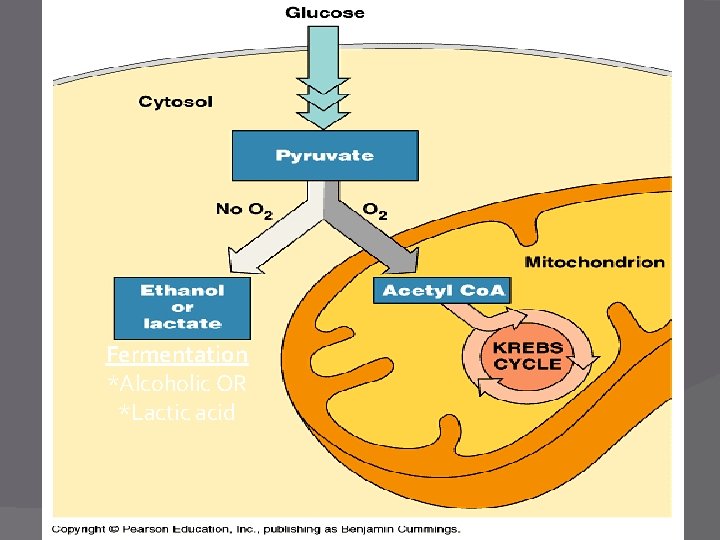
Alcoholic Fermentation � Uses only Glycolysis � An incomplete oxidation - energy is still left in the products (alcohol) � Does NOT require O 2 � Produces ATP (when O 2 is not available)

Alcohol

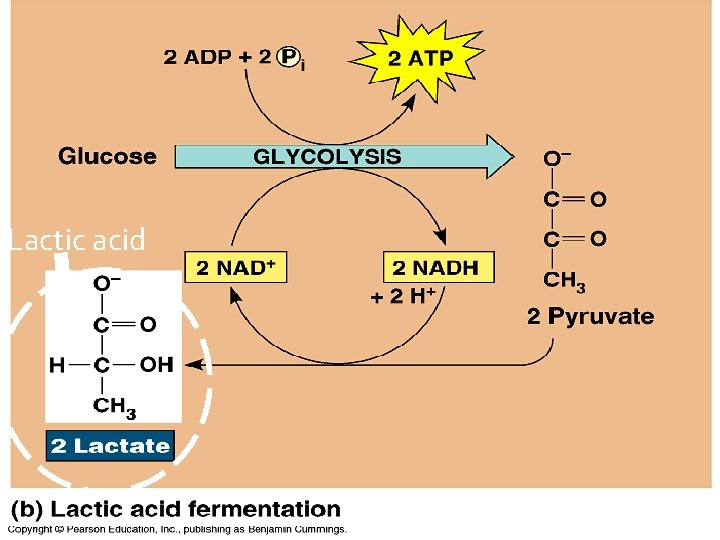
Lactic Acid Fermentation � Uses only Glycolysis � An incomplete oxidation - energy is still left in the products (lactic acid) � Does NOT require O 2 � Produces ATP (when O 2 is not available)

Lactic acid

Lactic Acid Fermentation � Done by human muscle cells under oxygen debt �Lactic Acid is a toxin and can cause soreness and stiffness in muscles �Oxygen intake can’t keep up with sugar breakdown � Used in dairy industry (yogurt/cheese) � Also used to produce methanol and acetone

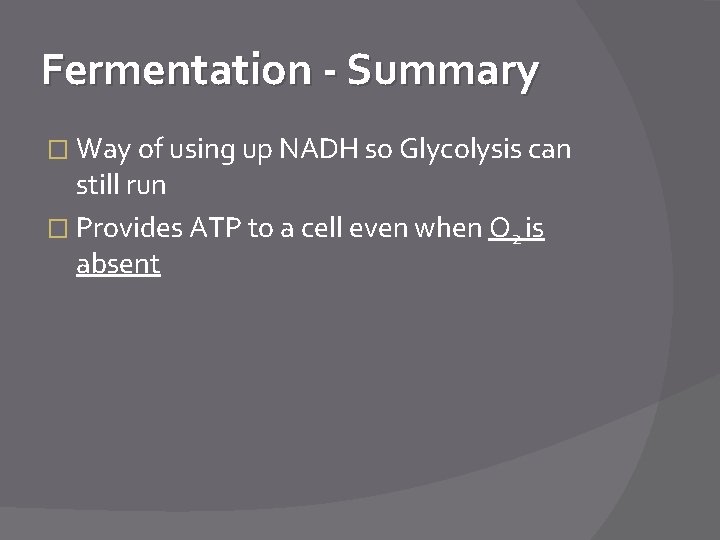
Fermentation - Summary � Way of using up NADH so Glycolysis can still run � Provides ATP to a cell even when O 2 is absent

Fermentation *Alcoholic OR *Lactic acid

Aerobic vs Anaerobic � Aerobic - Rs with O 2 � Anaerobic - Rs without O 2 � Aerobic - All three Rs steps � Anaerobic - Glycolysis only

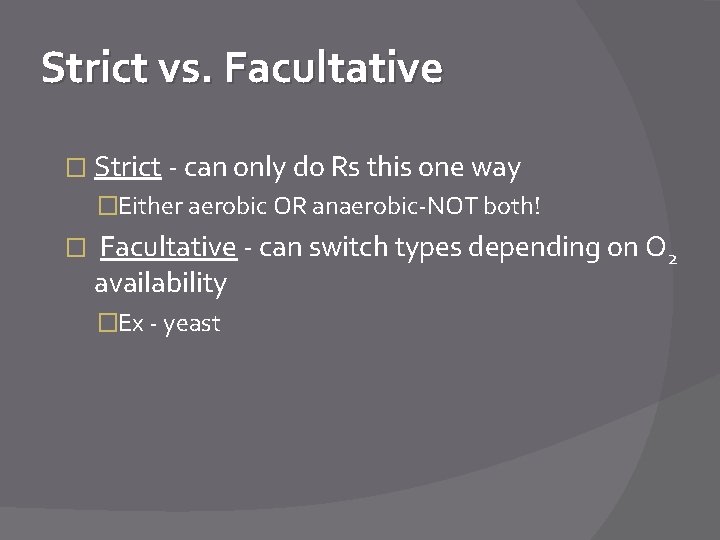
Strict vs. Facultative � Strict - can only do Rs this one way �Either aerobic OR anaerobic-NOT both! � Facultative - can switch types depending on O 2 availability �Ex - yeast

Question? ? � Since yeast can do both aerobic and anaerobic Rs, which is the better process if given a choice? �Hint: Check the ATP yields from both processes.

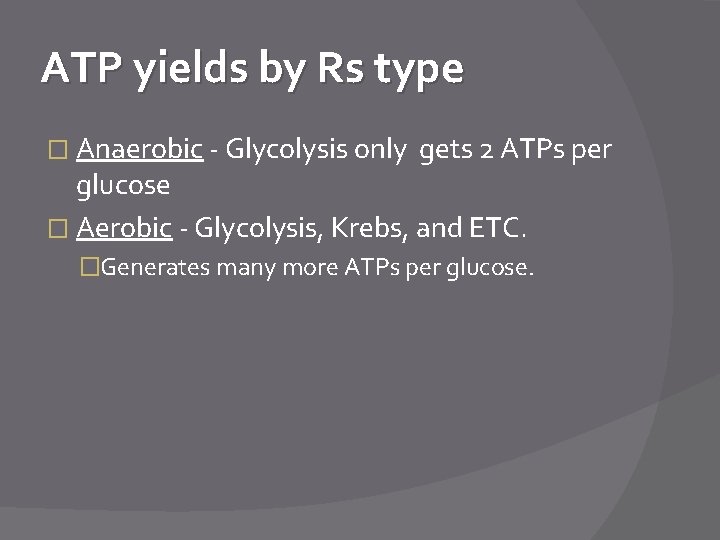
ATP yields by Rs type � Anaerobic - Glycolysis only gets 2 ATPs per glucose � Aerobic - Glycolysis, Krebs, and ETC. �Generates many more ATPs per glucose.

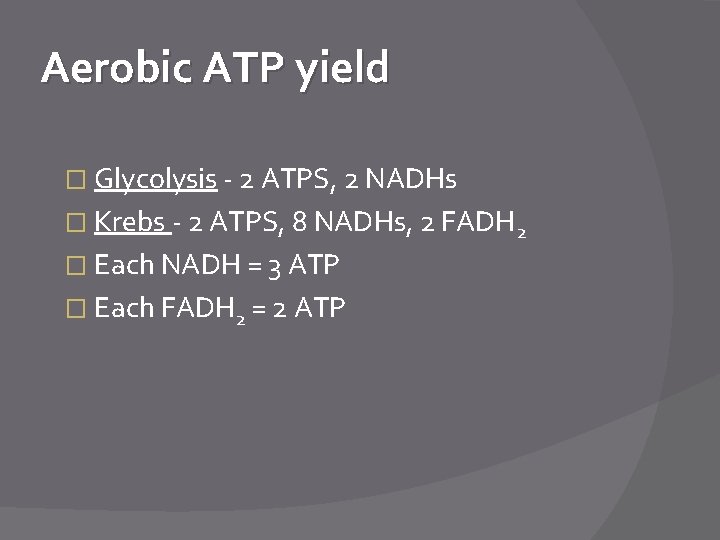
Aerobic ATP yield � Glycolysis - 2 ATPS, 2 NADHs � Krebs - 2 ATPS, 8 NADHs, 2 FADH 2 � Each NADH = 3 ATP � Each FADH 2 = 2 ATP

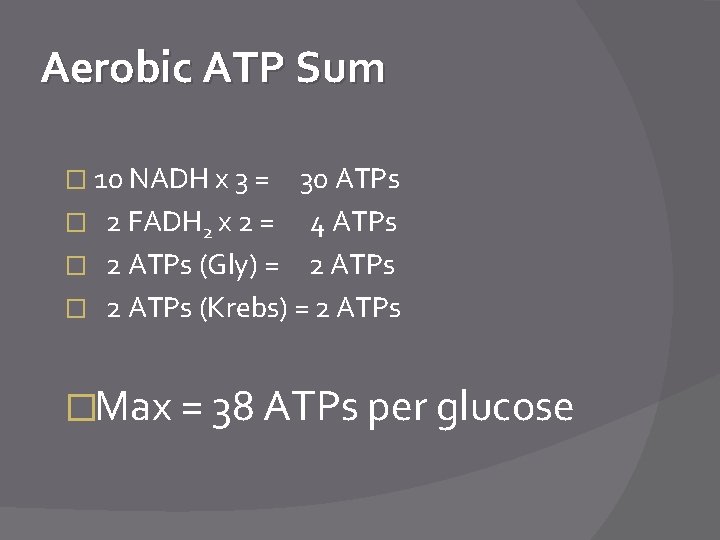
Aerobic ATP Sum � 10 NADH x 3 = 30 ATPs � 2 FADH 2 x 2 = 4 ATPs � 2 ATPs (Gly) = 2 ATPs � 2 ATPs (Krebs) = 2 ATPs �Max = 38 ATPs per glucose

However. . . � Some energy (2 ATP) is used in shuttling the NADH and pyruvate from Glycolysis into the mitochondria � Actual ATP yield ~ 36/glucose

Yeast � Would rather do aerobic Rs; �This has 18 x more energy per glucose than anaerobic � But, anaerobic will keep you alive if oxygen is not present.

Importance of Rs � Convert food to ATP �Living orgs use ATP to fuel body processes �Ex: reproduction, cell division � Provides materials for use in other cellular pathways

Other Respiration Items of Importance � Alcohol Industry - almost every society has a fermented beverage � Baking Industry - many breads use yeast to provide bubbles to raise the dough


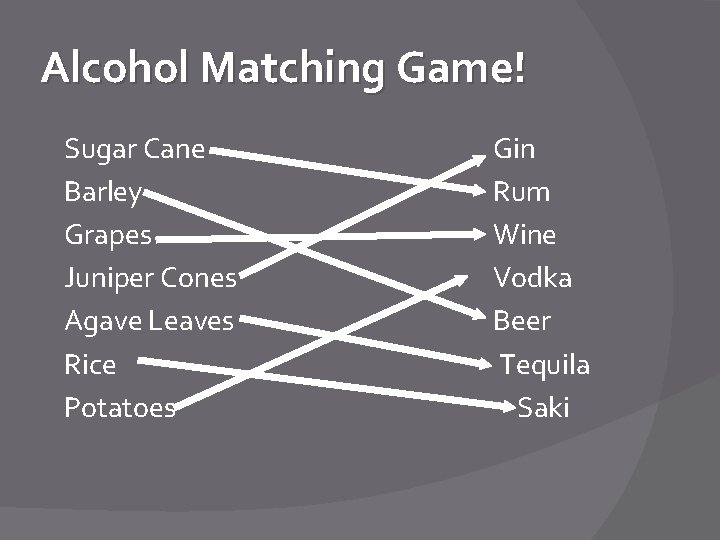
Alcohol Matching Game! Sugar Cane Barley Grapes Juniper Cones Agave Leaves Rice Potatoes Gin Rum Wine Vodka Beer Tequila Saki

Summary � � � � Identify the basic chemical equation for cellular respiration. Identify the main reaction sequences of cellular respiration. Recognize the location, function, requirements, and products, for each cellular respiration reaction. Recognize and be able to discuss the chemiosmotic model for ATP generation. Recognize the reactions and importance of fermentation. Contrast and compare aerobic and anaerobic respiration. Identify the biological and commercial importances of respiration.

Exclusion Statements � You do NOT need to memorize the steps in glycolysis and the Krebs cycle, the structures of the molecules, or the names of the enzymes that are involved. � You do NOT need to memorize the names of the specific electron carriers in the electron transport chain (ETC).