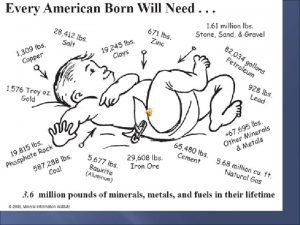
Chapter 4 Minerals Mineral a naturally occurring inorganic





















- Slides: 26

Chapter 4 Minerals

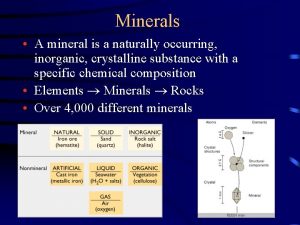
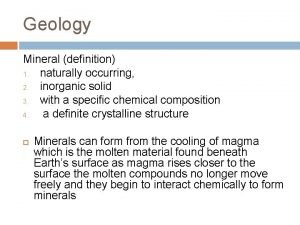
Mineral: a naturally occurring, inorganic solid with a specific chemical composition and a definite crystalline structure. -Inorganic: Are not alive, nor were ever alive during any part of their existence. -Solids: Definite shape and definite volume Some minerals are elements like sulfur or copper but most are compounds like quartz.

Crystal: A solid in which the atoms are arranged in repeating patterns. -The longer the crystal has to grow, the larger it can get. -The deeper in the ground the magma is (warmer), therefore the slower the cooling rate and the larger the crystal.

Magma: Is molten rock beneath the Earth’s surface

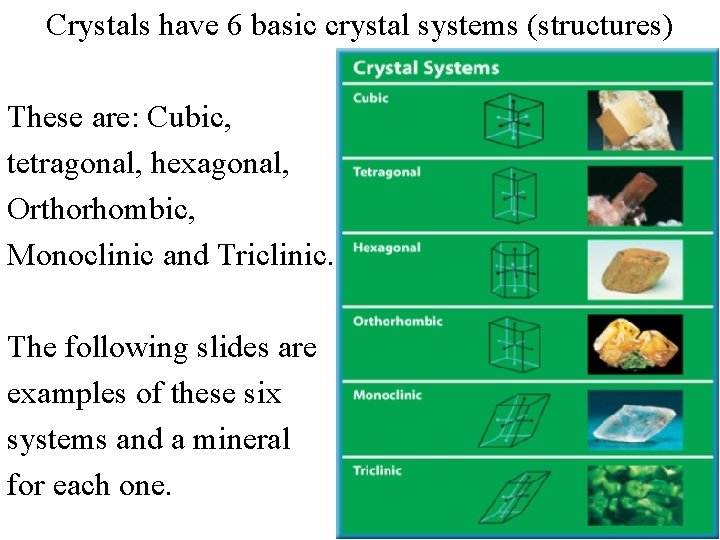
Crystals have 6 basic crystal systems (structures) These are: Cubic, tetragonal, hexagonal, Orthorhombic, Monoclinic and Triclinic. The following slides are examples of these six systems and a mineral for each one.

This is an example of a cubic crystal such as pyrite.

This is an example of Wulfenite with a tetragonal shape. The Wulfenite is the yellow mineral.

Here is Pyromorphite and the hexagonal shaped crystal.

Here is Topaz with the Orthorhombic crystal shape.

This is gypsum with the Monoclinic shape.

Last but not least is Feldspar and it’s triclinic shape.

The two main ways in which minerals form. 1) From the cooling of magma 2) From a mineral saturated solution

There are 3000 minerals. There are four groups I want you to know. The Native elements, Silicates, Carbonates, and Oxides.

The native elements: are simply composed of one element such as Copper and Sulfur and are not compounds.

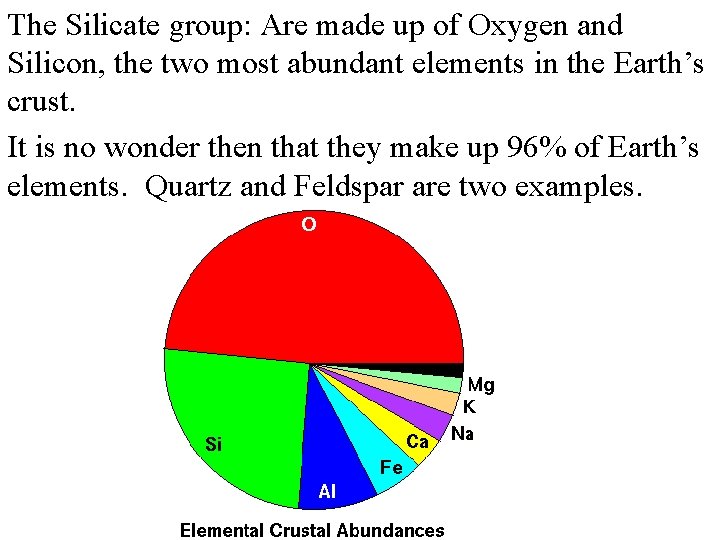
The Silicate group: Are made up of Oxygen and Silicon, the two most abundant elements in the Earth’s crust. It is no wonder then that they make up 96% of Earth’s elements. Quartz and Feldspar are two examples.

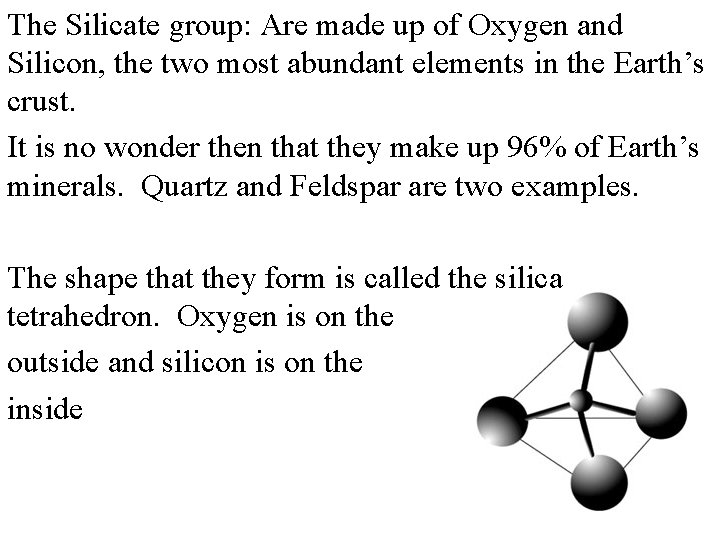
The Silicate group: Are made up of Oxygen and Silicon, the two most abundant elements in the Earth’s crust. It is no wonder then that they make up 96% of Earth’s minerals. Quartz and Feldspar are two examples. The shape that they form is called the silica tetrahedron. Oxygen is on the outside and silicon is on the inside

The next group is called the Carbonates are minerals composed of one or more metallic elements with the carbonate compound CO 3 Examples are calcite and dolomite often found in limestone or marble. These are basically the only important non silicate rock forming minerals.

Oxides are compounds of oxygen and a metal. Examples are hematite (red streak) and magnetite.

Identifying Minerals Color: The least useful because many minerals have more than one color and many minerals share the same color.

Luster: The way that a mineral reflects light. Your choices are metallic or nonmetallic. Texture: How a mineral feels to the touch. Talc feels greasy and olivine feels granular or “grainy. ”

Streak: The powdered and non-weathered color of the mineral. Hardness: A minerals resistance to being scratched. Moh’s hardness scale 1 -10 One (Talc) Is the softest and 10 (Diamond) is the hardest.

Cleavage Vs Fracture Cleavage: When a mineral splits relatively easily along one or more flat planes and has either layers or multiple flat surfaces or a geometric shape.

Fracture: When a mineral breaks unevenly due to tightly bonded atoms and have with rough or jagged edges.
Specific gravity: The ratio of weight of a substance to the weight of an equal volume of water at 4 degrees Celsius. For your lab simply put whether it feels light, medium or heavy for its size.

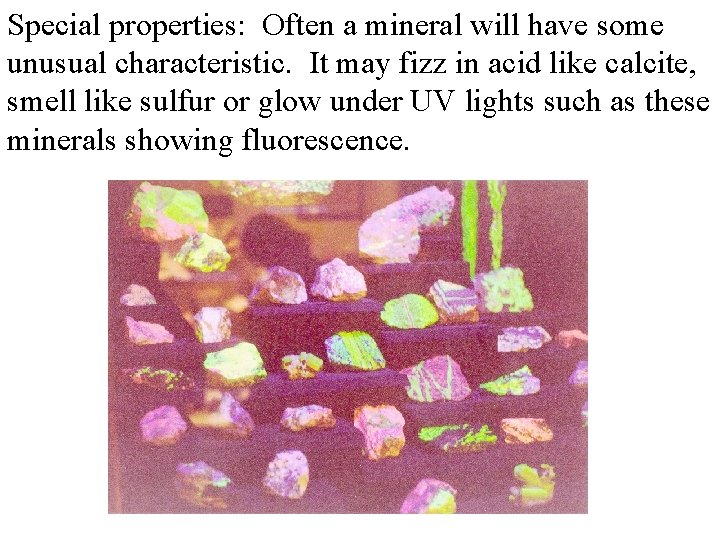
Special properties: Often a mineral will have some unusual characteristic. It may fizz in acid like calcite, smell like sulfur or glow under UV lights such as these minerals showing fluorescence.

Chapter 4 Quiz 1. Describe why sugar is not a mineral. 2. List the two main ways that minerals can form. 3. What is the name of the atomic structure of the silicates and what is the composition of quartz? 4. Why is color the least useful property for identifying minerals? 5. How do you test a minerals hardness? Bonus: How can you tell the difference between the Calcite and Halite?
 Naturally occurring inorganic solid material
Naturally occurring inorganic solid material Minerals def
Minerals def Largest naturally occurring element
Largest naturally occurring element Biofuels pros and cons
Biofuels pros and cons Naturally occurring areas of hydrothermal resources
Naturally occurring areas of hydrothermal resources Largest naturally occurring element
Largest naturally occurring element Naturally occuring fatty acids
Naturally occuring fatty acids Naturally occurring areas of hydrothermal resources
Naturally occurring areas of hydrothermal resources Reverse typing
Reverse typing Is a naturally occurring association among specific things
Is a naturally occurring association among specific things A narrow channel or slab of a mineral
A narrow channel or slab of a mineral Inorganic mineral definition
Inorganic mineral definition Minerals are inorganic elements that the body
Minerals are inorganic elements that the body Iron deficiency anemia smear
Iron deficiency anemia smear Find the probability of z occurring in the indicated region
Find the probability of z occurring in the indicated region What process is occurring
What process is occurring Find the probability of z occurring in the indicated region
Find the probability of z occurring in the indicated region Importance of pharmaceutical inorganic chemistry
Importance of pharmaceutical inorganic chemistry Strip mining vs open pit mining
Strip mining vs open pit mining Chapter 8 vitamins and minerals
Chapter 8 vitamins and minerals Chapter 2 minerals
Chapter 2 minerals Smear layer in endodontics ppt
Smear layer in endodontics ppt Charring test of organic and inorganic compounds
Charring test of organic and inorganic compounds Inorganic plant
Inorganic plant Inorganic vs organic chemistry
Inorganic vs organic chemistry Organic vs inorganic chemistry
Organic vs inorganic chemistry Inorganic vs organic
Inorganic vs organic