Naturally Occurring Isotopes Date 10517 Topic Naturally Occurring











- Slides: 13

Naturally Occurring Isotopes Date: 10/5/17 Topic: Naturally Occurring Isotopes Learning Objective: SWBAT how elements obtain their average atomic mass by applying mathematical strategies using the element’s isotopes. Do Now: 1. In your workbook, read pages 1 -10, complete questions 1 -28.

Review Define How Isotope. do we find the neutrons, protons and electrons when the element has multiple “versions” of itself?

Hiroshima & Nagasaki Bombings

Mini-Lesson (15 - 25 min) Using your periodic table, notice how the atomic mass of all of the elements are in decimal form… The reason for this is that due to the multiple forms of elements, each of those element’s masses are “averaged”.

Examples of Isotopes Food irradiation – food is exposed to the radiation of an element (Co-60) which allows high energy particles to pass through bacteria and destroy them! Preserves Carbon foods (longer ripening) found in organic molecules such as in living organisms are found in abundance. Allowing scientists to discover how old materials are.

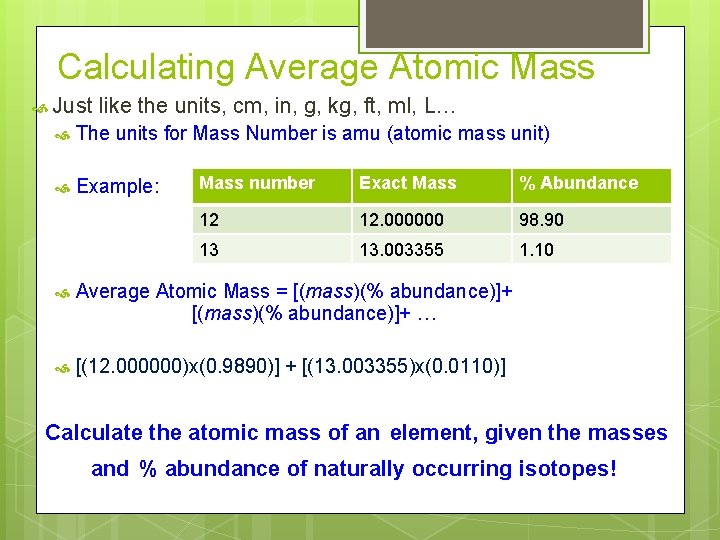
Calculating Average Atomic Mass Just like the units, cm, in, g, kg, ft, ml, L… The units for Mass Number is amu (atomic mass unit) Example: Mass number Exact Mass % Abundance 12 12. 000000 98. 90 13 13. 003355 1. 10 Average Atomic Mass = [(mass)(% abundance)]+ … [(12. 000000)x(0. 9890)] + [(13. 003355)x(0. 0110)] Calculate the atomic mass of an element, given the masses and % abundance of naturally occurring isotopes!

Steps to Calculating Atomic Mass Step 1: Determine the isotopes of the element. Step 2: Determine the relative abundance of each isotope. (Usually in %) Step 3: Multiply the atomic mass of each isotope by its proportions. Step 4: Add the results. Method One (1) Method Two (2) (23. 99 x 78. 99)+(24. 99 x 10. 00)+(25. 98 x 11. 01) = ────────────── 100 = (23. 99 x. 7899)+(24. 99 x 0. 10)+(25. 98 x 0. 1101) = 2, 430. 9099 ──── 100 = 24. 31 amu 24. 31 is closest to the 1 st isotope of Mg. = 24. 31 amu

Let’s Practice! 1. ) Find the Average Atomic Mass. Carbon

2. ) Find the Average Atomic Mass Chlorine Mass Number Exact Mass % Abundance 35 34. 968852 75. 77 37 36. 965903 24. 23

3. ) Find the Average Atomic Mass. Silicon Mass Number Exact Mass % Abundance 28 27. 976927 92. 23 29 28. 976495 4. 67 30 29. 973770 3. 10

4. ) Copper occurs naturally as Cu-63 and Cu-65. Which isotope is more abundant?

Work Period (25 - 30 min) 1. Read pages 1 -10, complete question #’s 1 -28.

Conclusion/Discussion Why are calculating average atomic weights important in the science of Chemistry?