Chapter 4 Formation of Compounds Every macroscopic change















- Slides: 45

Chapter 4 Formation of Compounds

Every macroscopic change in matter has a submicroscopic explanation……. there is a microscopic change at the atom level. Scientist’s job is to observe and explain reactions on submicroscopic level. WHAT IS A COMPOUND? Chemical combination of two or more different elements chemically joined in a fixed ratio.

IMPORTANT FAMILIAR COMPOUNDS SALT (Na. Cl) • • made up of Na and Cl Na. Cl one of the most abundant natural cpds on earth found in large solid underground deposits dissolved in oceans - essential nutrient in living things

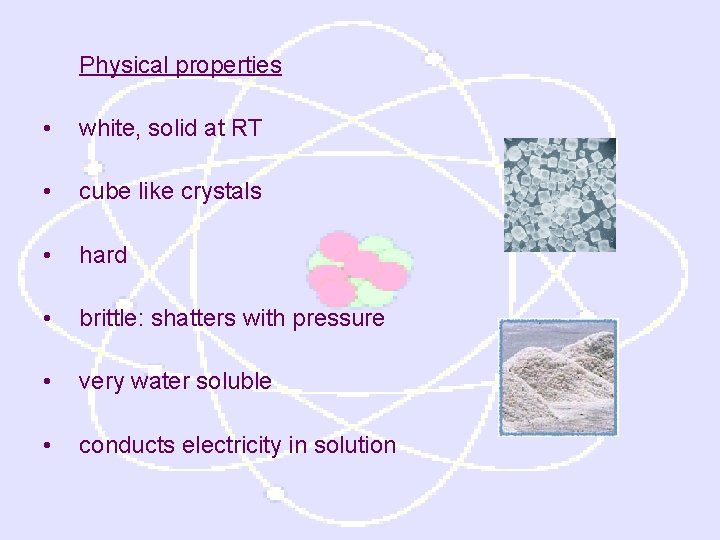
Physical properties • white, solid at RT • cube like crystals • hard • brittle: shatters with pressure • very water soluble • conducts electricity in solution

Chemical Properties • very stable crystal • not highly reactive with other substances • can last for years in hard crystal form and remain same

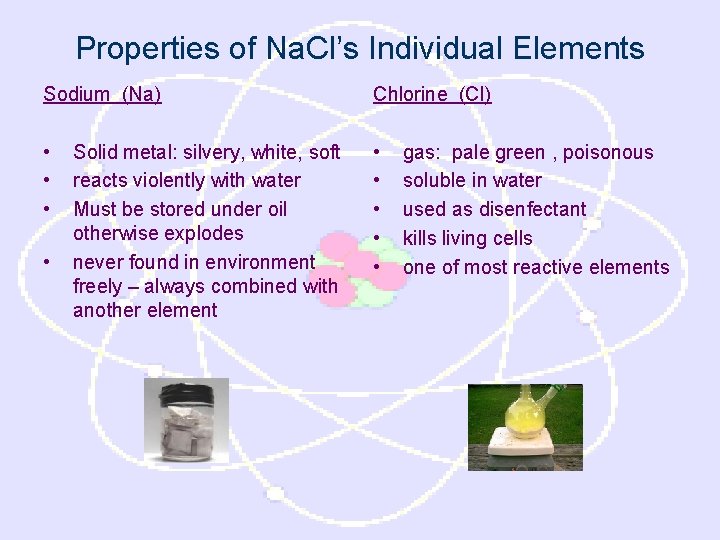
Properties of Na. Cl’s Individual Elements Sodium (Na) Chlorine (Cl) • • • Solid metal: silvery, white, soft reacts violently with water Must be stored under oil otherwise explodes never found in environment freely – always combined with another element gas: pale green , poisonous soluble in water used as disenfectant kills living cells one of most reactive elements

Do compounds have the same properties as the elements they are made of? NO Salt: solid, white, hard, stable, non metal Sodium: silver, soft, very unstable and reactive solid metal Chlorine: green, unstable, reactive gas

CARBON DIOXIDE (CO 2) Physical Properties • colorless gas • contained in small amounts in air • major compound in cellular respiration

Chemical properties • relatively stable • does not support burning (used in fire ext. ) • major product of photosynthesis • change of state: solid to gas (sublimation) ex: dry ice

Properties of CO 2 Components Carbon • non metal • fairly unreactive at RT • most abundant compound in living things • clear, grey, black in color Oxygen • non metal • colorless, odorless, tasteless gas • makes up about 21% of air • supports burning gas • slightly soluble in water (fish breathe)

WATER (H 2 O) Physical properties • occurs in all three states of matter • 70% Of earth surface and humans mass • boiling point 100 o C • freezing point 0 o C • does not conduct electricity in pure state • universal solvent

Chemical Properties • stable, not highly reactive • doesn’t break down in normal conditions • main component of all chemical reactions on earth and in body • main compound needed for life

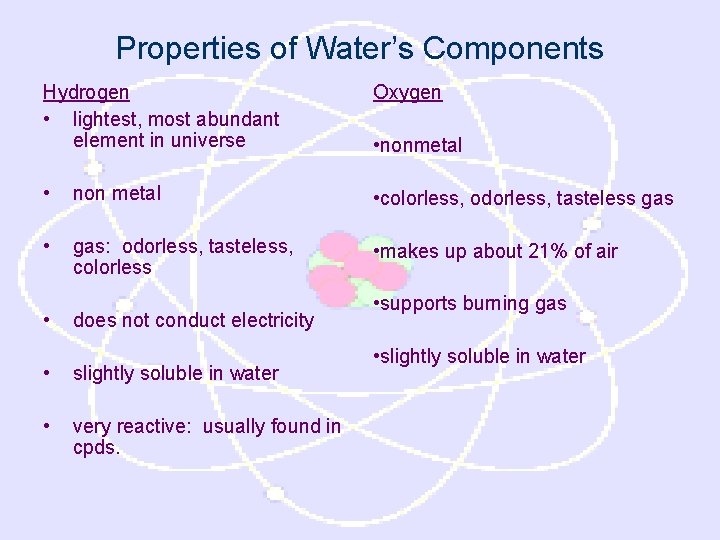
Properties of Water’s Components Hydrogen • lightest, most abundant element in universe Oxygen • non metal • colorless, odorless, tasteless gas • gas: odorless, tasteless, colorless • makes up about 21% of air • does not conduct electricity • slightly soluble in water • very reactive: usually found in cpds. • nonmetal • supports burning gas • slightly soluble in water


WE HAVE SEEN HOW COMPOUNDS CAN HAVE GREATLY DIFFERENT PROPERTIES THAN THE PROPERTIES OF ELEMENTS THAT COMPOSE THEM. IN THE NEXT SECTION WE WILL EXAMINE SUBMICROSCOPICALLY WHY THIS IS THE CASE BY EXAMINING HOW THEIR ATOMS COMBINE THE MANY DIFFERENT COMBINATIONS OF ELEMENTS DETERMINE THE CHARACTERISTICS OF THE COMPOUNDS THEY COMPRISE.

HOW ELEMENTS FORM COMPOUNDS Compounds form when electrons collide with each other. Which electrons are involved in reactions? All atoms want to be stable by completing their valence shells.

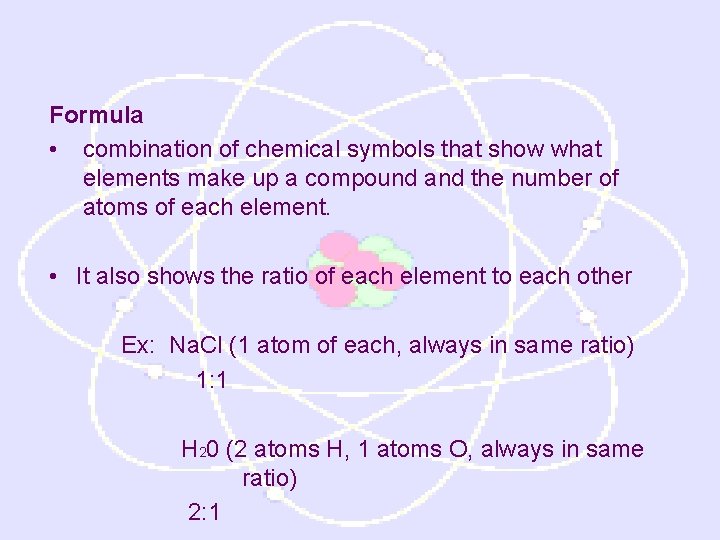
Formula • combination of chemical symbols that show what elements make up a compound and the number of atoms of each element. • It also shows the ratio of each element to each other Ex: Na. Cl (1 atom of each, always in same ratio) 1: 1 H 20 (2 atoms H, 1 atoms O, always in same ratio) 2: 1

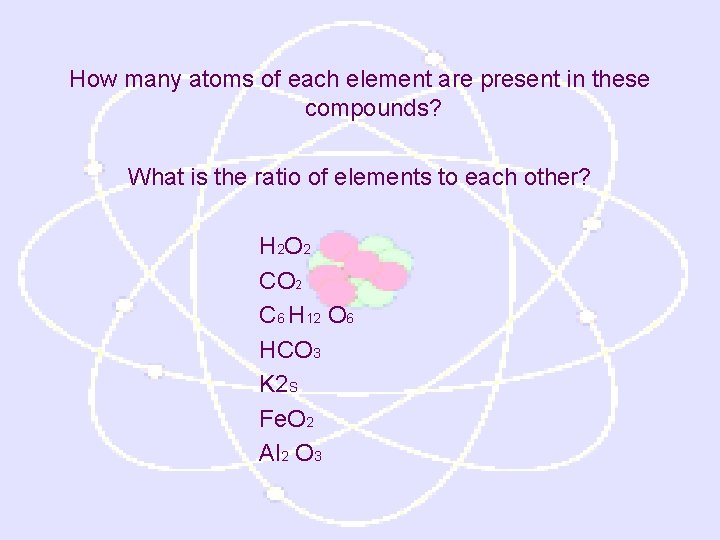
How many atoms of each element are present in these compounds? What is the ratio of elements to each other? H 2 O 2 C 6 H 12 O 6 HCO 3 K 2 S Fe. O 2 Al 2 O 3

CHEMICAL BONDS Chemical bond: process if joining atoms in a compound Electrons are subatomic particles involved in bond Goal of bond: to complete outer shell and become stable To form a compound electrons: - gain - lose - share

Bonding Basics • Atoms try to fill valence shell (orbital) to become stable • H and He: need 2 valence electrons • All other atoms: need 8 valence electrons - Metals lose electrons - Non metals gain electrons

Octet Rule • Elements gain, lose, or share electrons to reach a full octet (8 valence electrons in the outer shell) • H and He (2 valence electrons) Noble gas configuration • The state of an atom achieved by having the same valence electron configuration as the noble gases • Most stable electron configuration • All atoms try to achieve this configuration in forming compounds

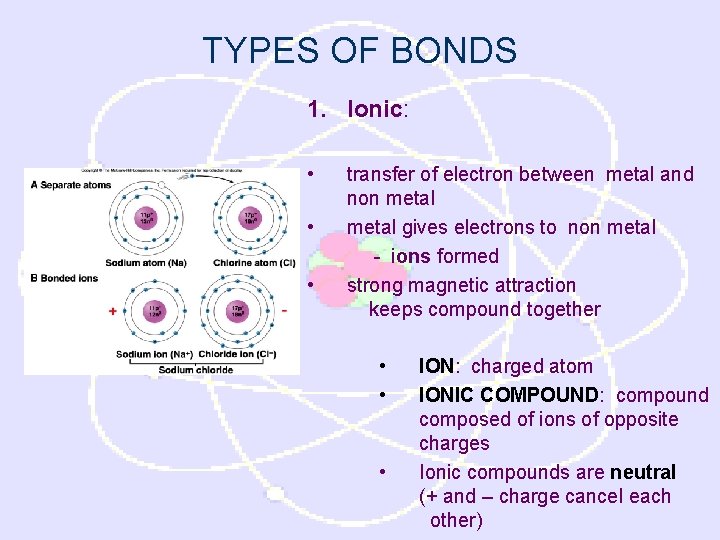
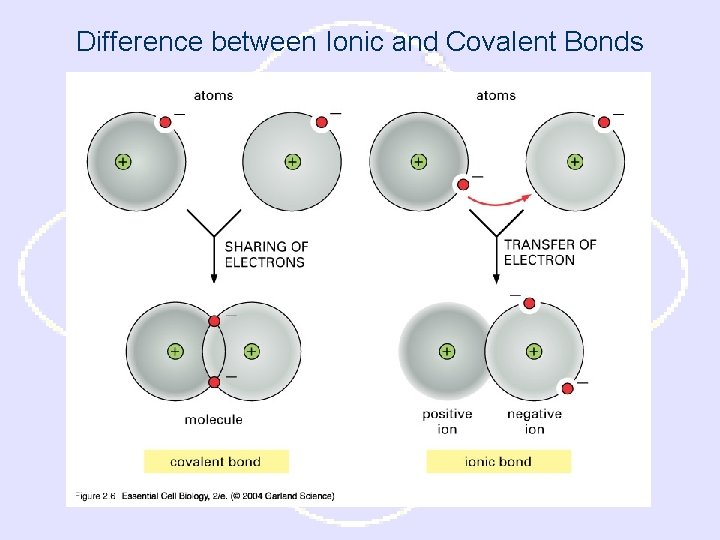
TYPES OF BONDS 1. Ionic: • • • transfer of electron between metal and non metal gives electrons to non metal - ions formed strong magnetic attraction keeps compound together • • • ION: charged atom IONIC COMPOUND: compound composed of ions of opposite charges Ionic compounds are neutral (+ and – charge cancel each other)

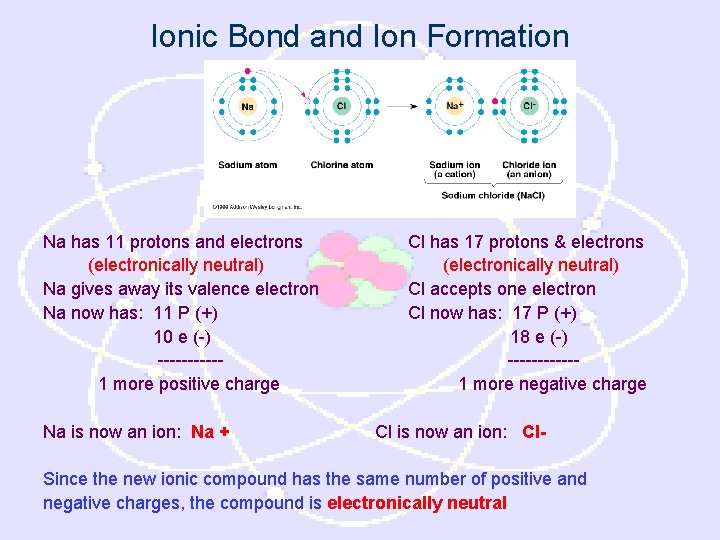
Ionic Bond and Ion Formation Na has 11 protons and electrons (electronically neutral) Na gives away its valence electron Na now has: 11 P (+) 10 e (-) -----1 more positive charge Na is now an ion: Na + Cl has 17 protons & electrons (electronically neutral) Cl accepts one electron Cl now has: 17 P (+) 18 e (-) ------1 more negative charge Cl is now an ion: Cl- Since the new ionic compound has the same number of positive and negative charges, the compound is electronically neutral


Drawing Ionic Compounds Steps 1. Find number of valence electrons for each atom 2. (use periodic table) 2. Draw Lewis dot diagram for each atom (use X’s and O’s) 3. Determine which atoms lose or gain electrons 4. Draw Lewis diagram of compound 5. Check octet rule for each atom in compound

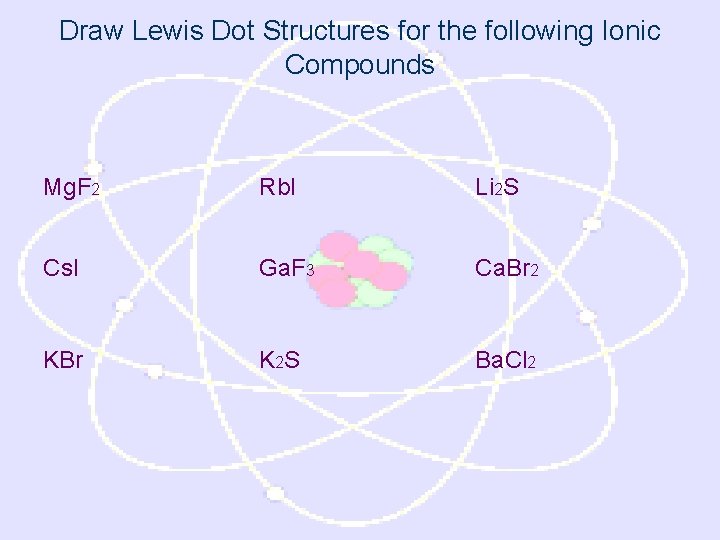
Draw Lewis Dot Structures for the following Ionic Compounds Mg. F 2 Rb. I Li 2 S Cs. I Ga. F 3 Ca. Br 2 KBr K 2 S Ba. Cl 2

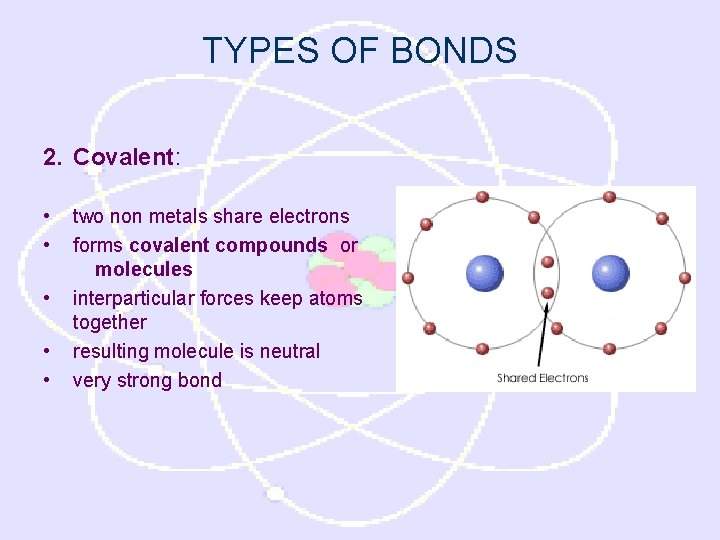
TYPES OF BONDS 2. Covalent: • • • two non metals share electrons forms covalent compounds or molecules interparticular forces keep atoms together resulting molecule is neutral very strong bond

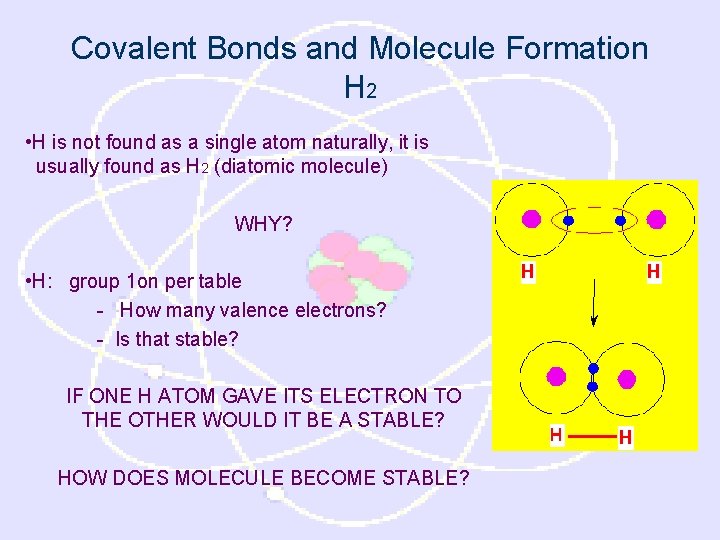
Covalent Bonds and Molecule Formation H 2 • H is not found as a single atom naturally, it is usually found as H 2 (diatomic molecule) WHY? • H: group 1 on per table - How many valence electrons? - Is that stable? IF ONE H ATOM GAVE ITS ELECTRON TO THE OTHER WOULD IT BE A STABLE? HOW DOES MOLECULE BECOME STABLE?




Difference between Ionic and Covalent Bonds

Multiple Covalent Bonds MULTIPLE COVALENT BONDS bonds with more than one shared pair of electrons between two individual atoms

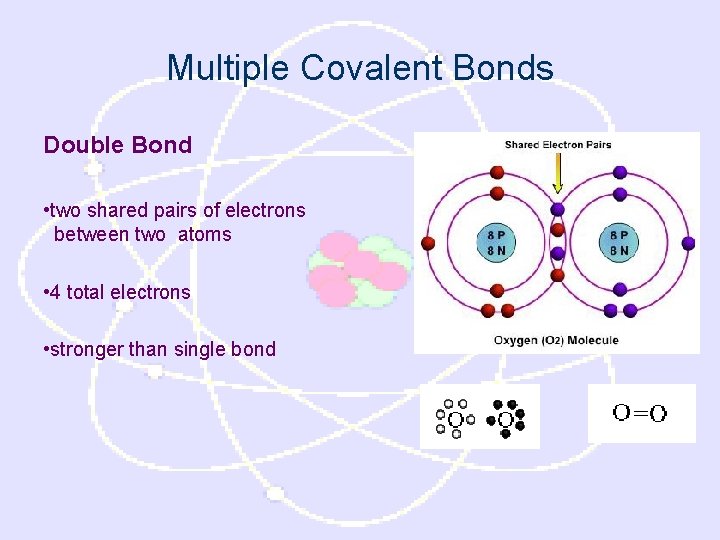
Multiple Covalent Bonds Double Bond • two shared pairs of electrons between two atoms • 4 total electrons • stronger than single bond


Drawing Covalent Compounds Steps 1. Determine number of valence electrons 2. Write the symbols to show neighboring atoms 3. Use electron pairs to form bonds 4. 4. Complete octets for each atom

Make Lewis dot structures for the following compounds: O 2 H 2 Se H 2 S HCN

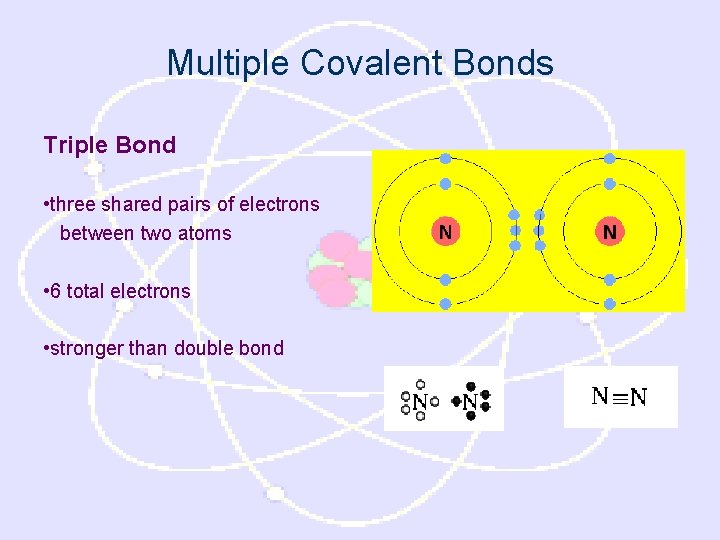
Multiple Covalent Bonds Triple Bond • three shared pairs of electrons between two atoms • 6 total electrons • stronger than double bond


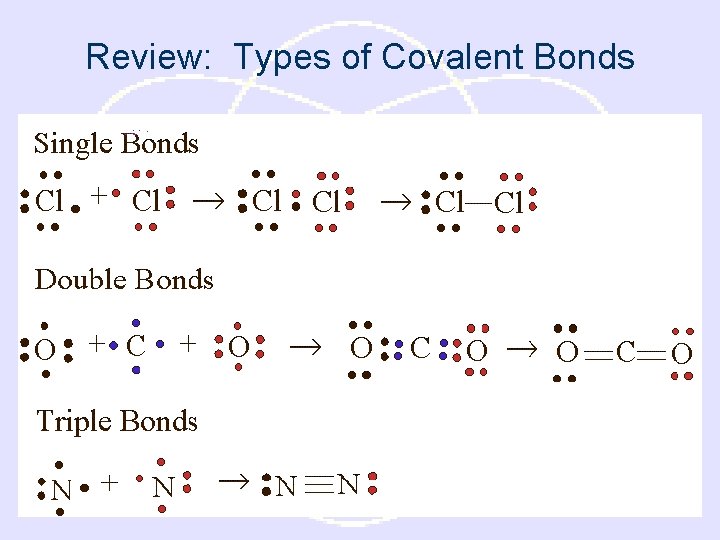
Review: Types of Covalent Bonds

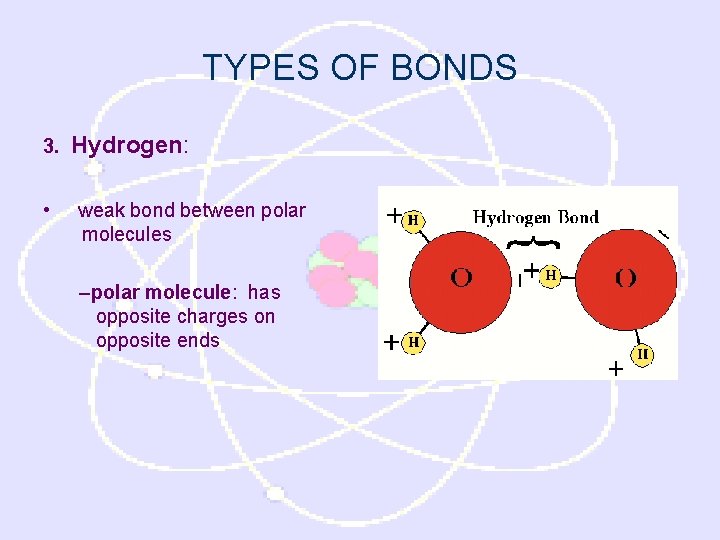
TYPES OF BONDS 3. • Hydrogen: weak bond between polar molecules –polar molecule: has opposite charges on opposite ends


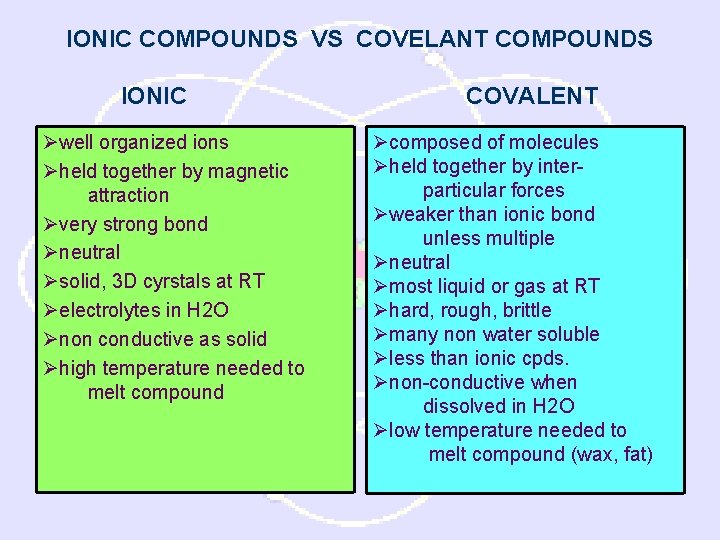
IONIC COMPOUNDS VS COVELANT COMPOUNDS IONIC Øwell organized ions Øheld together by magnetic attraction Øvery strong bond Øneutral Øsolid, 3 D cyrstals at RT Øelectrolytes in H 2 O Ønon conductive as solid Øhigh temperature needed to melt compound COVALENT Øcomposed of molecules Øheld together by interparticular forces Øweaker than ionic bond unless multiple Øneutral Ømost liquid or gas at RT Øhard, rough, brittle Ømany non water soluble Øless than ionic cpds. Ønon-conductive when dissolved in H 2 O Ølow temperature needed to melt compound (wax, fat)


Study for the test !