Chapter 24 Ocean Water Section 1 Properties of
































- Slides: 40

Chapter 24: Ocean Water { Section 1: Properties of Ocean Water Section 2: Life in the Oceans Section 3: Ocean Resources

• { • • • Salinity- measure of the mount of dissolved salts and other solids in a given liquid Thermocline- marks the distinct separation between the warm surface water and the deep, cold water Pack Ice- a floating layer of sea ice that completely covers an area of the ocean surface Density- the mass of a substance per unit volume Section 1 Key Terms

Pure liquid water is TASTELESS, ODORLESS AND COLORLESS Water in the ocean is not pure, it is a complex mixture of CHEMICALS that sustains a variety of PLANT AND ANIMAL life

3 main gases are dissolved in ocean water: 1. N 2 (Nitrogen) 2. O 2 (Oxygen) 3. CO 2 (Carbon Dioxide) Ocean water dissolves gases from a variety of sources such as: Dissolved Gases STREAMS and RIVERS that flow into the ocean Under ocean VOLCANIC ERUPTIONS Released by ORGANISMS living in the ocean From the atmosphere

Gases dissolve more readily in cold water; therefore, oceans in cold regions dissolve larger amounts of gases than water in TROPICAL REGIONS If temperature rises then gases will not dissolve and will be released into the ATMOSPHERE Temperature changes allow the ATMOSPHERE and OCEANS to exchange gases Temperature and Dissolved Gases

{ • The ocean contains 60 X more carbon than the atmosphere • Important to the regulation of CLIMATE The Oceans as a Carbon Sink

{ 96. 5% pure water { 3. 5% sea salts Dissolved Solids

{ Dissolved solids in the ocean are made up of 75 chemical elements with the 6 most abundant being: { 85% of the dissolved solids are made up of Halite (salt made from sodium and chlorine) Chlorine Sodium Magnesium Sulfur Calcium Potassium Most Abundant Elements

{ Most elements that form sea salts come from 3 main sources: 1. VOLCANIC ERUPTIONS 2. CHEMICAL WEATHERING OF ROCK ON LAND 3. CHEMICAL REACTIONS BETWEEN SEA WATER AND NEWLY FORMED SEA FLOOR ROCKS Sources of Dissolved Solids

Salinity- measure of the mount of dissolved salts and other solids in a given liquid Measured by # of grams of dissolved solids in 1, 000 g of ocean water Ex. 35 g of dissolved solids per 1, 000 g ocean water 35 g/1, 000 g =. 035 = 3. 5% Salinity of sample would be 3. 5 % Salinity of Ocean Water

{ • Precipitation changes salinity • When the rate of EVAPORATION is higher than the rate of PRECIPITATION, the salinity INCREASES Factors that Change Salinity

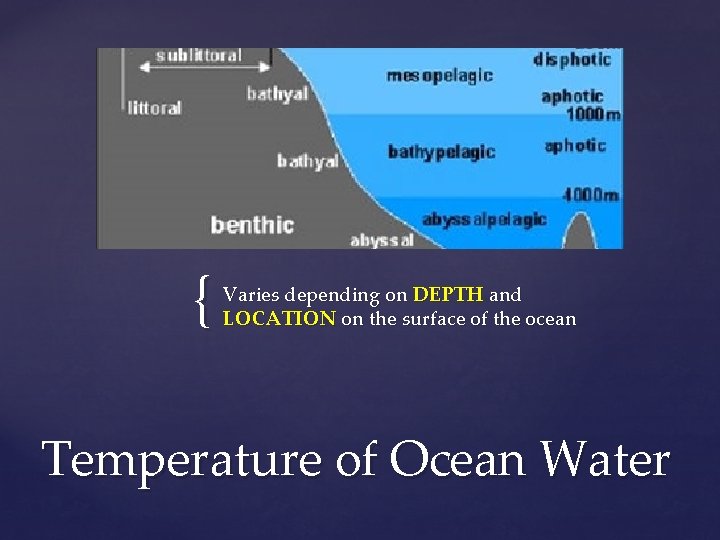
{ Varies depending on DEPTH and LOCATION on the surface of the ocean Temperature of Ocean Water

Waves and currents distribute heat DOWNWARD to 300 m thus keeping temperatures CONSTANT Water temperatures DECREASE with latitude (meaning moving North of the equator or South of the equator) Surface Water

Floating layers of sea ice that completely covers an area of ocean surface is called PACK ICE Only 5 m thick because it acts as an INSULATOR keeping water below warm Surface Water Continued…

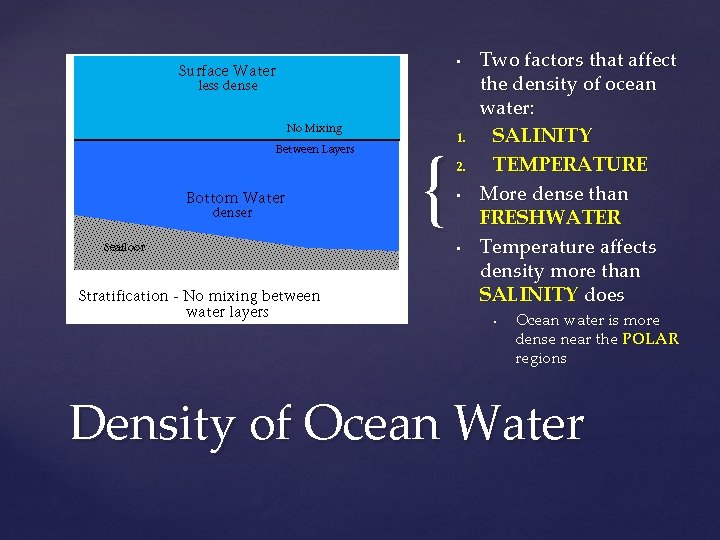
Temperature DECREASES as depth increases Thermocline: marks the distinct separation between the WARM water and COLD deep water Created because warm water is LESS dense and wont mix with the cold water Thermocline

• { 1. 2. • • Two factors that affect the density of ocean water: SALINITY TEMPERATURE More dense than FRESHWATER Temperature affects density more than SALINITY does • Ocean water is more dense near the POLAR regions Density of Ocean Water

{ Appears blue because blue wavelengths tend to be REFLECTED Color of the Ocean Water

Phytoplankton Absorb RED and BLUE light, but reflect GREEN; therefore, they can affect the color of ocean water Require NUTRIENTS to survive, so by determining the amount of phytoplankton can indicate the HEALTH of the ocean Why is Ocean Color Important?

Why would surface water in the North Sea be more likely to contain a high percentage of dissolved gases than surface water in the Caribbean Sea would? North Sea waters are colder. Cold waters hold more dissolved gases. Therefore…North Sea would contain a higher percentage. If global temperatures increase, how would this change affect the ability of the ocean to absorb CO 2? Oceans would warm. Warmer waters cannot hold as much CO 2, thus more CO 2 would accumulate in the atmosphere. If an area of the ocean has a large decrease in phytoplankton, how would this change affect other ocean organisms? Phytoplankton are at the bottom of the food chain; therefore, marine animals that eat phytoplankton will decrease and so would marine animals that eat those animals. Critical Thinking

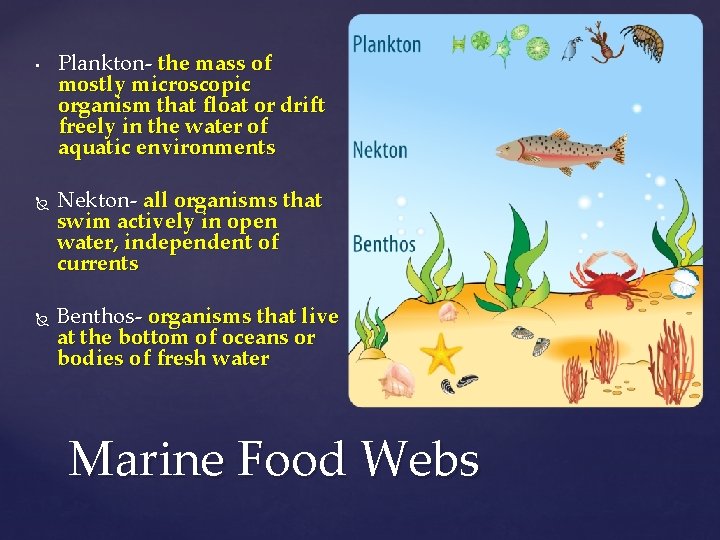
• • { • • • Section 2 Key Terms • Upwelling- the movement of deep, cold and nutrientrich water to the surface Plankton- the mass of mostly microscopic organism that float or drift freely in the water of aquatic environments Nekton- all organisms that swim actively in open water, independent of currents Benthos- organisms that live at the bottom of oceans or bodies of fresh water Benthic zone- the bottom region of oceans and bodies of fresh water Pelagic zone- the region of an ocean or body of fresh water above the benthic zone

{ 1. 2. Sunlight 2. Essential Nutrients Marine organisms depend on 2 major factors:

{ • • Marine organisms help to maintain the CHEMICAL balance of the oceans REMOVE nutrients and gases and return other nutrients and gases Ocean Chemistry and Marine Life

Elements are consumed by organisms then released back into the ocean when the organisms DIE, SINK TO LOWER DEPTHS AND DECAY Deep water is a STORAGE AREA for nutrients Nutrients return to the surface by upwelling, which is the MOVEMENT OF DEEP, COLD AND NUTRIENT-RICH WATER TO THE SURFACE Surface winds move water offshore, allowing deep-cold water to replace the water that moved away Upwelling Visualization Animation Link

• Plankton- the mass of mostly microscopic organism that float or drift freely in the water of aquatic environments Nekton- all organisms that swim actively in open water, independent of currents Benthos- organisms that live at the bottom of oceans or bodies of fresh water Marine Food Webs

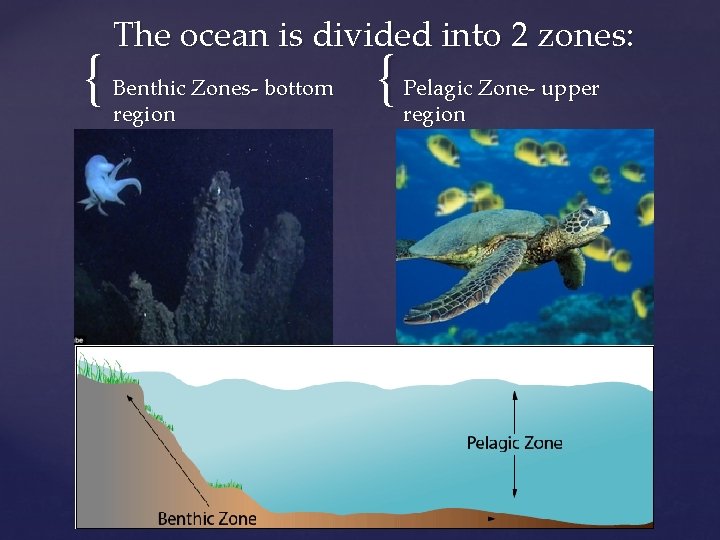
{ The ocean is divided into 2 zones: Benthic Zones- bottom region { Pelagic Zone- upper region

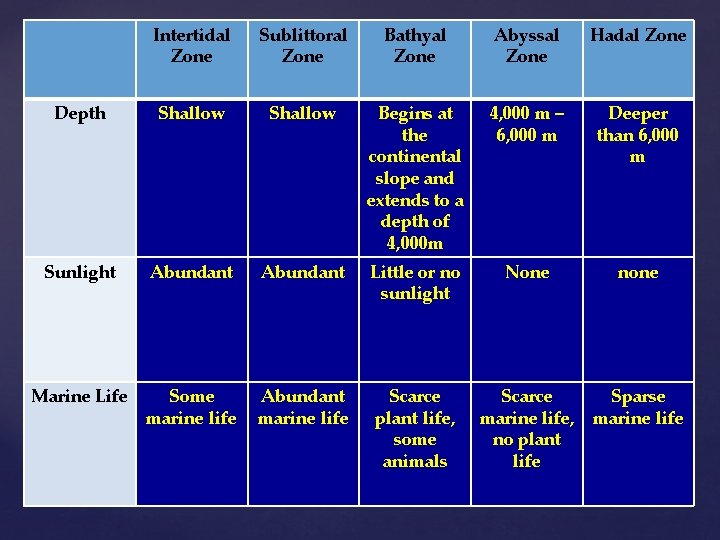
Intertidal Zone Sublittoral Zone Bathyal Zone Abyssal Zone Hadal Zone Depth Shallow Begins at the continental slope and extends to a depth of 4, 000 m 4, 000 m – 6, 000 m Deeper than 6, 000 m Sunlight Abundant Little or no sunlight None none Marine Life Some marine life Abundant marine life Scarce plant life, some animals Scarce marine life, no plant life Sparse marine life

{ Region above the benthic zone and divided into 2 parts: 1. 2. Neritic Zone Oceanic Zone Pelagic Zones

{ Neritic Zone 3 Characteristics 1. Abundant sunlight 2. Moderate temperatures 3. Relatively low water pressure Source of much of the FISH and SEAFOOD humans eat

{ Broken into 4 zones: 1. Epipelagic 2. Mesopelagic 3. Bethypelagic 4. Abyssopelagic Zones are based on depth Oceanic Zone

Explain the effects that marine organisms have on the chemistry of ocean water. They remove gases and nutrients from the ocean while returning others. Explain how plankton form the base of ocean food webs. They use sunlight to make their own food and provide a food source for those organisms that cannot make their own food. Critical Thinking

• { • Desalination- a process of removing salt from ocean water Aquaculturethe raising of aquatic plants and animals for human use or consumption Section 3 Key Terms

{ • Desalination- a process of removing salt from ocean water • Process is costly Freshwater from the Ocean

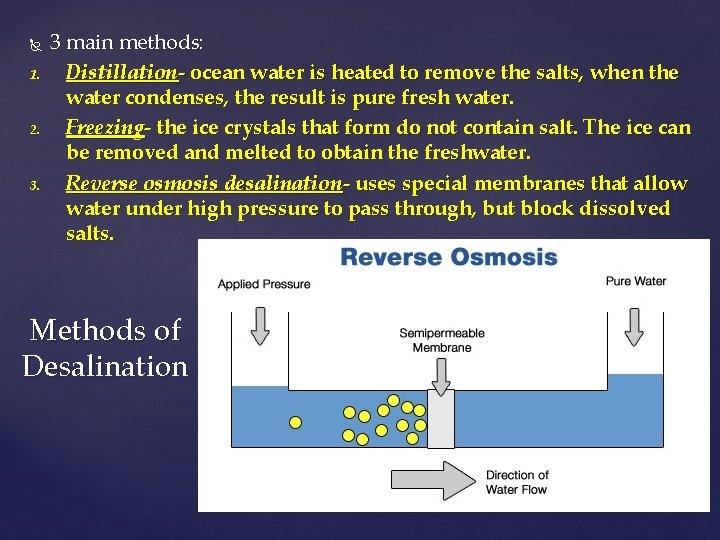
1. 2. 3 main methods: Distillation- ocean water is heated to remove the salts, when the water condenses, the result is pure fresh water. Freezing- the ice crystals that form do not contain salt. The ice can be removed and melted to obtain the freshwater. Reverse osmosis desalination- uses special membranes that allow water under high pressure to pass through, but block dissolved salts. Methods of Desalination

Most valuable resource in the ocean and located BENEATH THE SEA FLOOR ¼ of the world’s oil is now obtained from OFFSHORE wells Mineral and Energy Resources- Petroleum

1. 2. 3. 4. 5. 6. POTATO-SHAPED lumps of minerals Found on the ABYSSAL FLOORS Valuable source of: Manganese Iron Copper Nickel Cobalt Phosphates Mineral and Energy Resources- Nodules

{ Food is in the greatest demand of the ocean resources Food from the Ocean

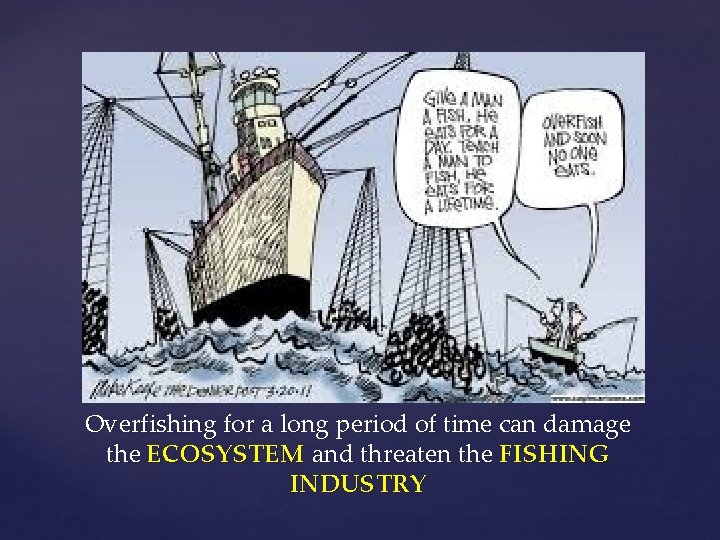
Overfishing for a long period of time can damage the ECOSYSTEM and threaten the FISHING INDUSTRY

Aquaculture- the raising of aquatic plants and animals for human use or consumption 1 major problem for aquaculturalists is that ocean farms are susceptible to pollution and may themselves be a local source of pollution Aquaculture

Concentrations of pollutants are so high that the fish have become UNSAFE for humans to eat

Describe how the mining of nodules may create problems between countries. Normally found in deep waters outside of national boundaries- problems determining who has the rights to them. How does pollution of the ocean affect the fishing industry? Destroy fish populations. How could humans be affected if microscopic marine organisms absorbed small amounts of mercury? Becomes concentrated in the bodies of the organisms and then end up in human bodies when humans consume the organisms. Critical Thinking