Chapter 17 Corrosion and Degradation of Materials Introduction



![Nernst Equation • • • [M 1 n+] and [M 2 n+] are the Nernst Equation • • • [M 1 n+] and [M 2 n+] are the](https://slidetodoc.com/presentation_image_h2/033a9820a32b510f2f3434d1701cb6a9/image-14.jpg)


- Slides: 20

Chapter 17 Corrosion and Degradation of Materials

Introduction • • Deterioration of a material due to chemical attack by the environment – Typically, a non-stress related failure • There are exceptions Terminology – Metals: • Corrosion – Dissolution in the surrounding medium – Primarily an electrochemical phenomenon • Oxidation – formation of oxides – A chemical reaction – Ceramics: Chemical reaction • Corrosion • Oxidation of non-oxide ceramics – Polymers: • Degradation – Chemical reaction with solvents – Decomposition due to UV radiation

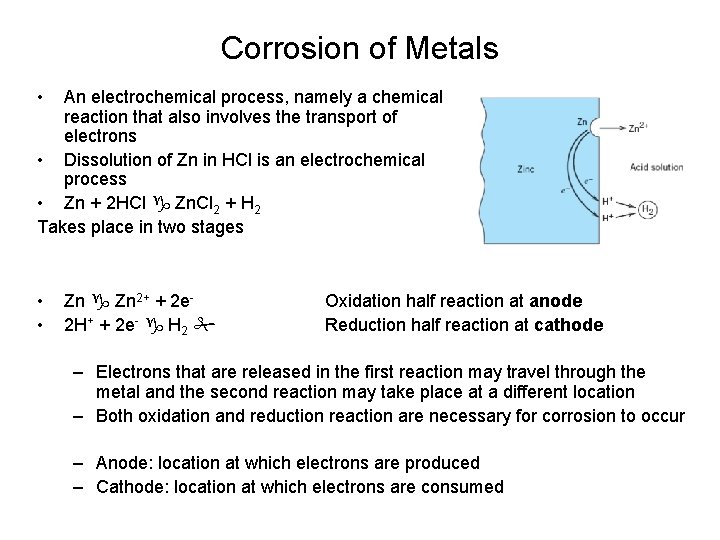
Corrosion of Metals • An electrochemical process, namely a chemical reaction that also involves the transport of electrons • Dissolution of Zn in HCl is an electrochemical process • Zn + 2 HCl Zn. Cl 2 + H 2 Takes place in two stages • • Zn 2+ + 2 e 2 H+ + 2 e- H 2 Oxidation half reaction at anode Reduction half reaction at cathode – Electrons that are released in the first reaction may travel through the metal and the second reaction may take place at a different location – Both oxidation and reduction reaction are necessary for corrosion to occur – Anode: location at which electrons are produced – Cathode: location at which electrons are consumed

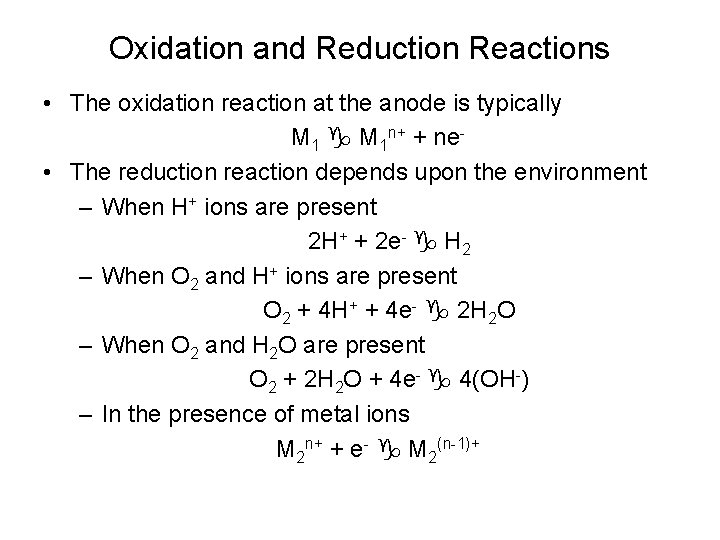
Oxidation and Reduction Reactions • The oxidation reaction at the anode is typically M 1 n+ + ne • The reduction reaction depends upon the environment – When H+ ions are present 2 H+ + 2 e- H 2 – When O 2 and H+ ions are present O 2 + 4 H+ + 4 e- 2 H 2 O – When O 2 and H 2 O are present O 2 + 2 H 2 O + 4 e- 4(OH-) – In the presence of metal ions M 2 n+ + e- M 2(n-1)+

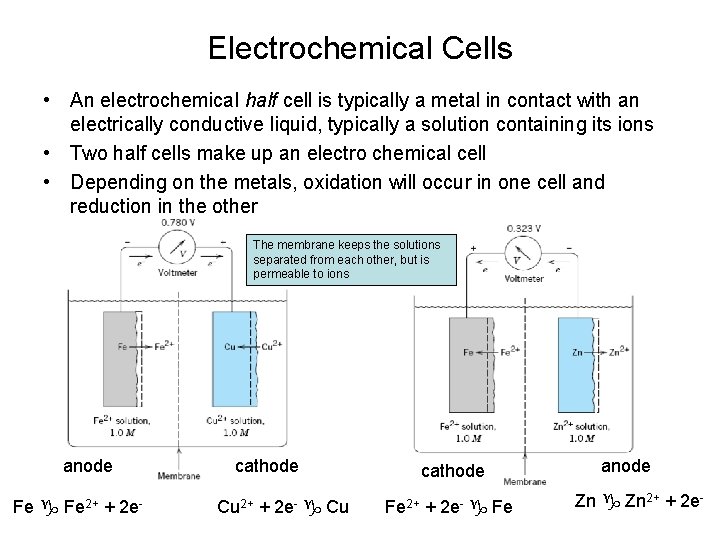
Electrochemical Cells • An electrochemical half cell is typically a metal in contact with an electrically conductive liquid, typically a solution containing its ions • Two half cells make up an electro chemical cell • Depending on the metals, oxidation will occur in one cell and reduction in the other The membrane keeps the solutions separated from each other, but is permeable to ions anode Fe 2+ + 2 e- cathode Cu 2+ + 2 e- Cu cathode Fe 2+ + 2 e- Fe anode Zn 2+ + 2 e-

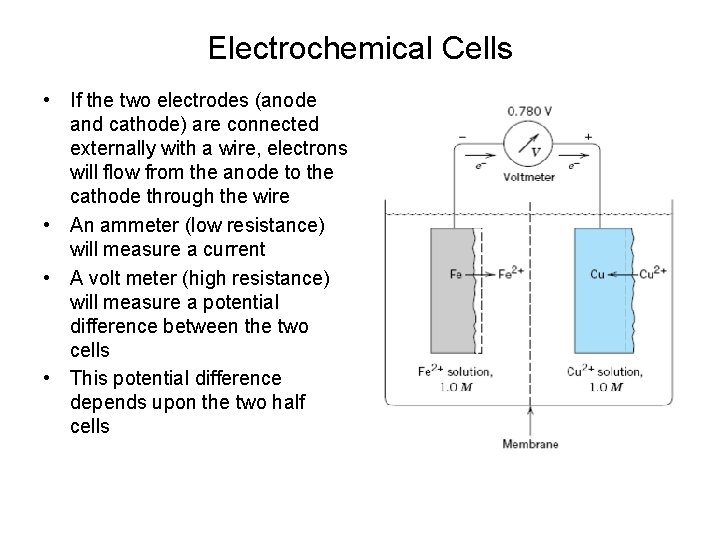
Electrochemical Cells • If the two electrodes (anode and cathode) are connected externally with a wire, electrons will flow from the anode to the cathode through the wire • An ammeter (low resistance) will measure a current • A volt meter (high resistance) will measure a potential difference between the two cells • This potential difference depends upon the two half cells

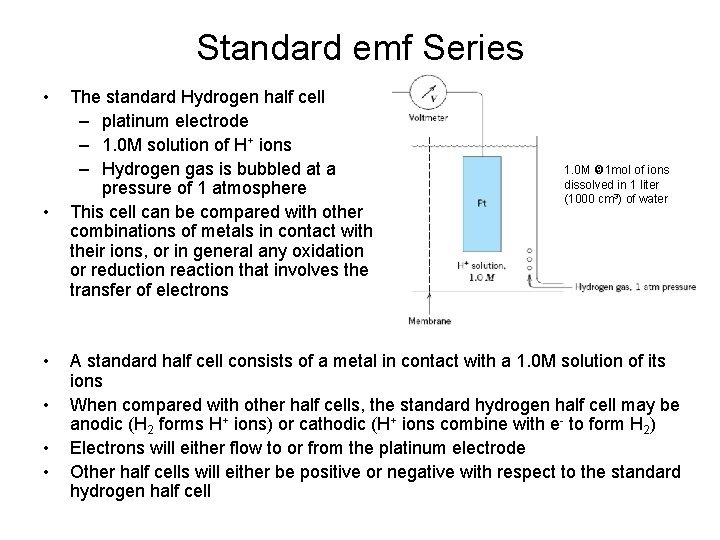
Standard emf Series • • • The standard Hydrogen half cell – platinum electrode – 1. 0 M solution of H+ ions – Hydrogen gas is bubbled at a pressure of 1 atmosphere This cell can be compared with other combinations of metals in contact with their ions, or in general any oxidation or reduction reaction that involves the transfer of electrons 1. 0 M 1 mol of ions dissolved in 1 liter (1000 cm 3) of water A standard half cell consists of a metal in contact with a 1. 0 M solution of its ions When compared with other half cells, the standard hydrogen half cell may be anodic (H 2 forms H+ ions) or cathodic (H+ ions combine with e- to form H 2) Electrons will either flow to or from the platinum electrode Other half cells will either be positive or negative with respect to the standard hydrogen half cell

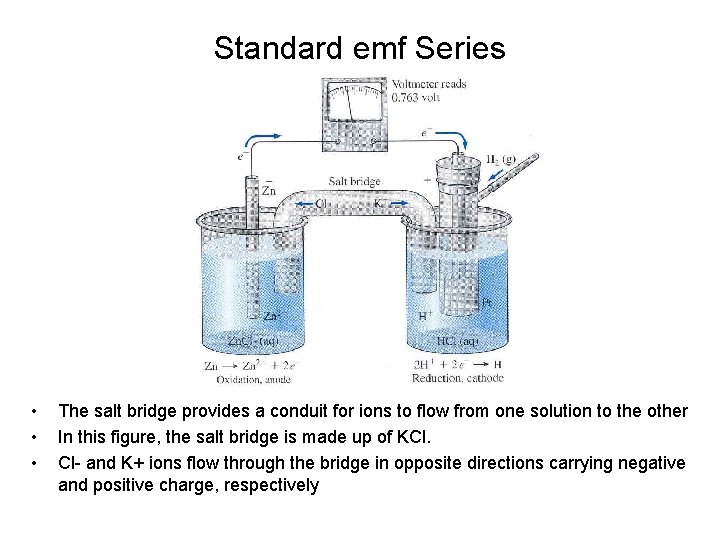
Standard emf Series • • • The salt bridge provides a conduit for ions to flow from one solution to the other In this figure, the salt bridge is made up of KCl. Cl- and K+ ions flow through the bridge in opposite directions carrying negative and positive charge, respectively

Standard emf Series

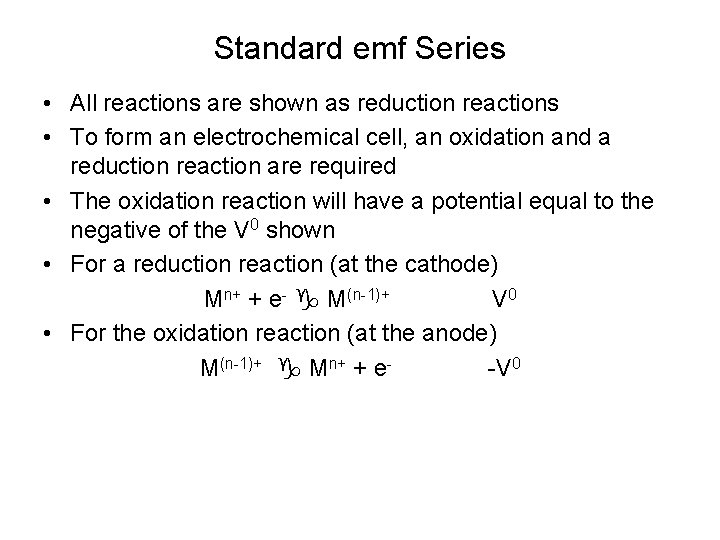
Standard emf Series • All reactions are shown as reduction reactions • To form an electrochemical cell, an oxidation and a reduction reaction are required • The oxidation reaction will have a potential equal to the negative of the V 0 shown • For a reduction reaction (at the cathode) Mn+ + e- M(n-1)+ V 0 • For the oxidation reaction (at the anode) M(n-1)+ Mn+ + e-V 0

Electrochemical Cells • If any two half cells are combined to form an electrochemical cell – The half cell with the more positive V 0 will be the cathode • Reduction occurs at this electrode – The half cell with the more negative V 0 will be the anode • Oxidation occurs at this electrode – The potential difference that is measured between the two electrodes is ∆V = V 0 cathode – V 0 anode

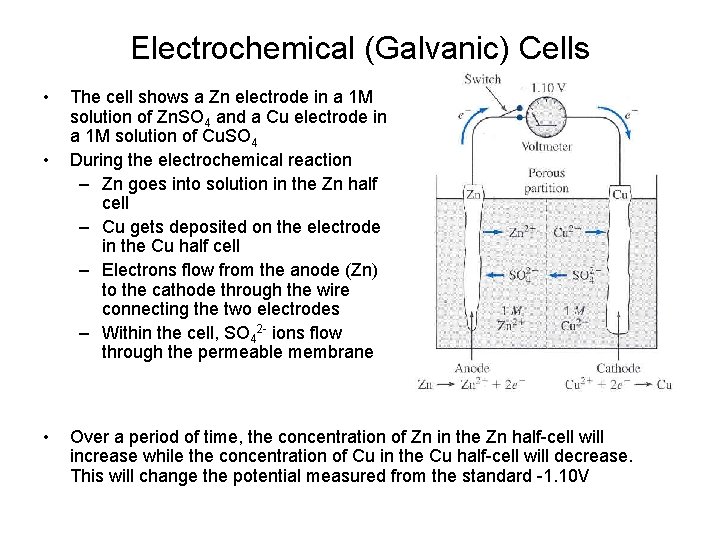
Electrochemical (Galvanic) Cells • • • The cell shows a Zn electrode in a 1 M solution of Zn. SO 4 and a Cu electrode in a 1 M solution of Cu. SO 4 During the electrochemical reaction – Zn goes into solution in the Zn half cell – Cu gets deposited on the electrode in the Cu half cell – Electrons flow from the anode (Zn) to the cathode through the wire connecting the two electrodes – Within the cell, SO 42 - ions flow through the permeable membrane Over a period of time, the concentration of Zn in the Zn half-cell will increase while the concentration of Cu in the Cu half-cell will decrease. This will change the potential measured from the standard -1. 10 V

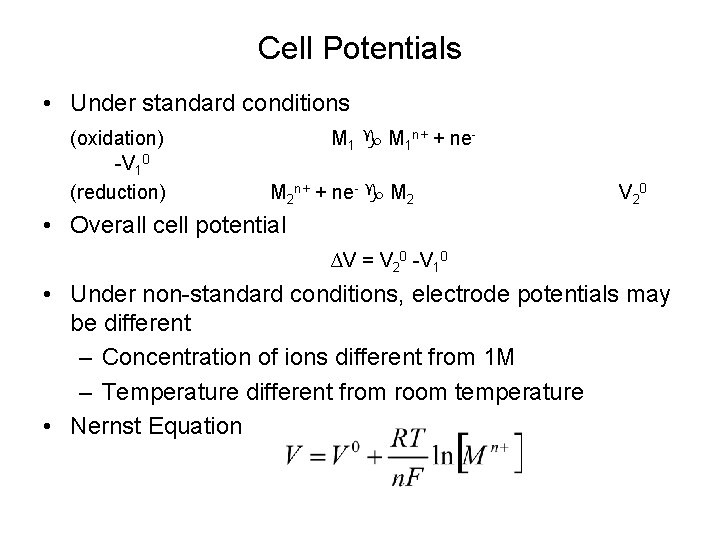
Cell Potentials • Under standard conditions (oxidation) -V 10 (reduction) M 1 n+ + ne. M 2 n+ + ne- M 2 V 2 0 • Overall cell potential ∆V = V 20 -V 10 • Under non-standard conditions, electrode potentials may be different – Concentration of ions different from 1 M – Temperature different from room temperature • Nernst Equation
![Nernst Equation M 1 n and M 2 n are the Nernst Equation • • • [M 1 n+] and [M 2 n+] are the](https://slidetodoc.com/presentation_image_h2/033a9820a32b510f2f3434d1701cb6a9/image-14.jpg)
Nernst Equation • • • [M 1 n+] and [M 2 n+] are the concentrations of the two ions n is the number of electrons involved in the reaction Faraday’s constant F is the charge on 1 mole of electrons F = (6. 023 x 1023 electrons/mol) x (1. 602 x 10 -19 C/electron) = 96, 500 C/mol R is the universal gas constant T is temperature in (K)

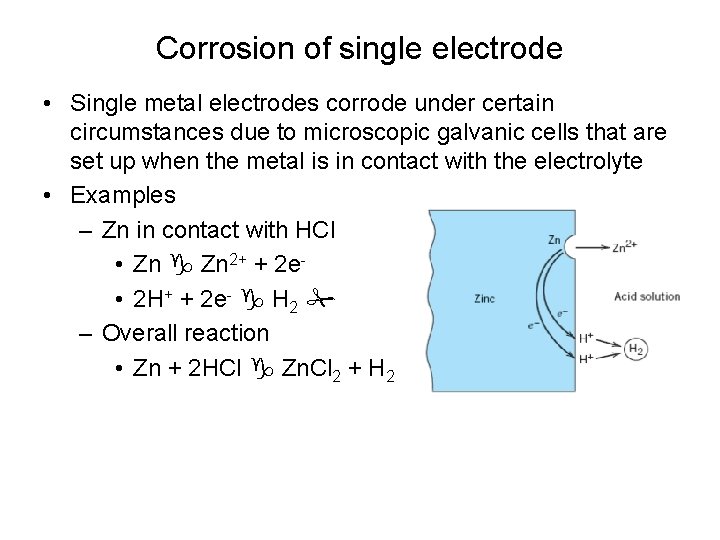
Corrosion of single electrode • Single metal electrodes corrode under certain circumstances due to microscopic galvanic cells that are set up when the metal is in contact with the electrolyte • Examples – Zn in contact with HCl • Zn 2+ + 2 e • 2 H+ + 2 e- H 2 – Overall reaction • Zn + 2 HCl Zn. Cl 2 + H 2

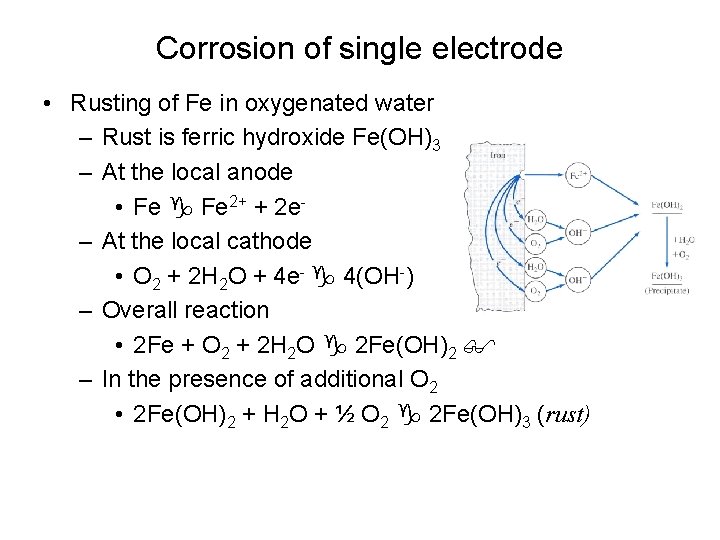
Corrosion of single electrode • Rusting of Fe in oxygenated water – Rust is ferric hydroxide Fe(OH)3 – At the local anode • Fe 2+ + 2 e– At the local cathode • O 2 + 2 H 2 O + 4 e- 4(OH-) – Overall reaction • 2 Fe + O 2 + 2 H 2 O 2 Fe(OH)2 – In the presence of additional O 2 • 2 Fe(OH)2 + H 2 O + ½ O 2 2 Fe(OH)3 (rust)

Examples of Galvanic cells • Identify anodic and cathodic reactions and determine if corrosion will occur – Cu and Zn immersed in dilute solution of Cu. SO 4 • Anode: • Cathode: • Cell potential – Cu in oxygenated water • Anode: • Cathode: • Cell potential:

Examples of Galvanic cells – Mg and Fe connected by an external wire and immersed in 1% Na. Cl solution • Anode: • Cathode: • Cell potential:

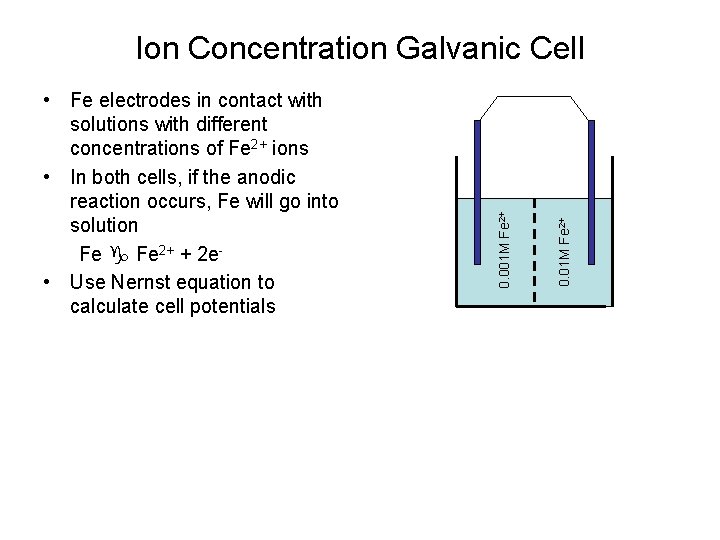
0. 01 M Fe 2+ • Fe electrodes in contact with solutions with different concentrations of Fe 2+ ions • In both cells, if the anodic reaction occurs, Fe will go into solution Fe 2+ + 2 e • Use Nernst equation to calculate cell potentials 0. 001 M Fe 2+ Ion Concentration Galvanic Cell

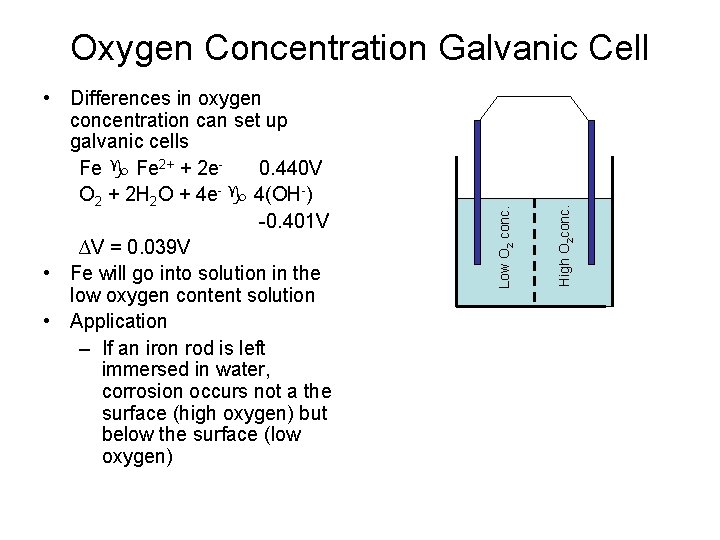
High O 2 conc. • Differences in oxygen concentration can set up galvanic cells Fe 2+ + 2 e 0. 440 V O 2 + 2 H 2 O + 4 e- 4(OH-) -0. 401 V ∆V = 0. 039 V • Fe will go into solution in the low oxygen content solution • Application – If an iron rod is left immersed in water, corrosion occurs not a the surface (high oxygen) but below the surface (low oxygen) Low O 2 conc. Oxygen Concentration Galvanic Cell
 Dry corrosion and wet corrosion
Dry corrosion and wet corrosion Differentiate between dry corrosion and wet corrosion.
Differentiate between dry corrosion and wet corrosion. Estimation of degradation function
Estimation of degradation function Edman degradation steps
Edman degradation steps Prdp biochemistry
Prdp biochemistry Mechanical degradation
Mechanical degradation Metabolismn
Metabolismn How environmental degradation occurs
How environmental degradation occurs Optimum notch filter in image processing
Optimum notch filter in image processing Light induced degradation
Light induced degradation Edman degradation
Edman degradation Salting out proteins
Salting out proteins Conclusion of environmental degradation
Conclusion of environmental degradation How environmental degradation occurs
How environmental degradation occurs Land degradation definition
Land degradation definition Starch degradation
Starch degradation Jurawatt
Jurawatt Abnormal degradation of disaccharides
Abnormal degradation of disaccharides Edman degradation
Edman degradation Tag degradation
Tag degradation Glycogen degradation
Glycogen degradation