MECHANICAL DEGRADATION l The polymer chain is ruptured




- Slides: 15

MECHANICAL DEGRADATION l The polymer chain is ruptured by mechanical means. The effect is to reduce the polymer molecular mass. l This is sometimes done deliberately. l Example: l MASTICATION of natural rubber but if it occurs in service the polymer properties will be adversely affected. l Usually the chain break is made permanent by oxygen attack and mechanical deg. Is more accurately described as MECHANOCHEMICAL DEGREDATION

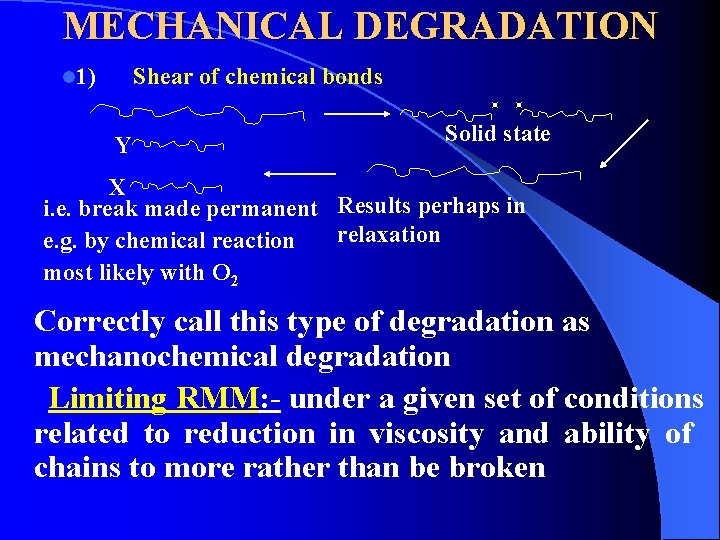
MECHANICAL DEGRADATION l 1) Shear of chemical bonds Y Solid state X i. e. break made permanent Results perhaps in relaxation e. g. by chemical reaction most likely with O 2 Correctly call this type of degradation as mechanochemical degradation Limiting RMM: - under a given set of conditions related to reduction in viscosity and ability of chains to more rather than be broken

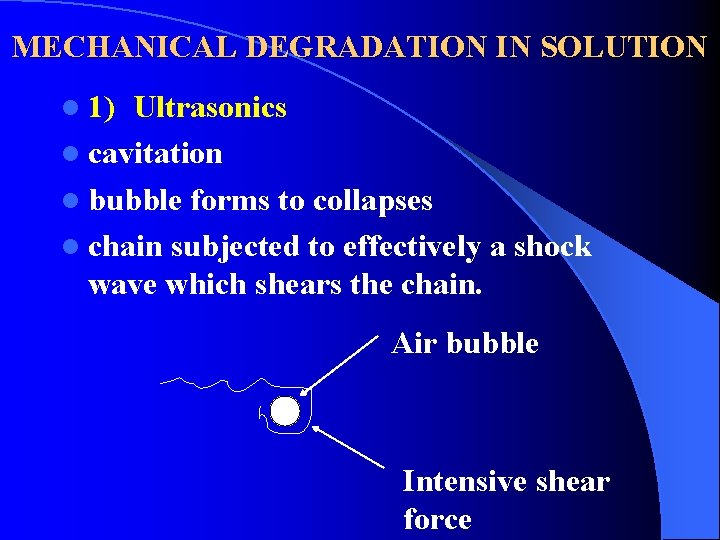
MECHANICAL DEGRADATION IN SOLUTION l 1) Ultrasonics l cavitation l bubble forms to collapses l chain subjected to effectively a shock wave which shears the chain. Air bubble Intensive shear force

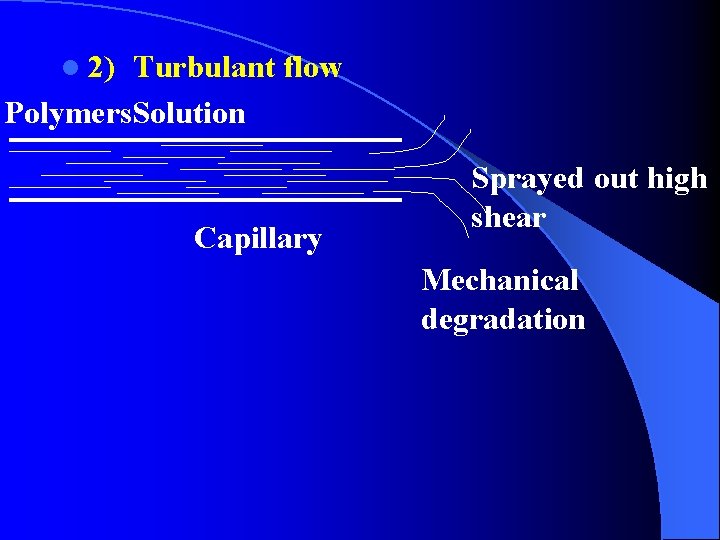
l 2) Turbulant flow Polymers. Solution Capillary Sprayed out high shear Mechanical degradation

l 3) Violent shaking or stirring l It will be the highest molecular masses which will be affected. , and hence whilst the average molecular mass will fall the molecular mass distribution will change. l a) average value falls l b) distribution narrows l In making up solutions for molecular mass characterization the addition of antioxidants may not be permitted. I. e. the value of Mn would be lowered.

Mechanical Degradation in the Solid State l Example l Mastication: l Deliberate break down of chains to reduce viscosity to aid dispersion of compounding ingredients. l Example l In use of N. R. i. e. RMM up to 5 X 106 l With synthetic polymer RMM controlled in polymerization reaction in order to avoid reed to masticate polymer.

EFFICIENCY OF MASTICATION l For a given set of shearing conditions efficiency of mastication depends on: - l 1) Shearing force. l 2) Molecular mass of original polymer. l 3) Temperature. l 4) Environment.

SHEARING FORCE l Greater the shearing force the greater the reduction in molecular mass.

MOLECULAR MASS l l l The greater the molecular mass the greater the reduction in molecular mass. Always falls to a limiting value. At low temperature Polymer molecules have high viscosity and therefore, chains are subjected to maximum shearing force (high efficiency). As the temperature increases viscosity falls and the mobility of chains increases and avoid the shearing force (efficiency decreases). At high temperature Degradation is caused by thermal oxidative breakdown Mastication continually exposing new surfaces for attack (efficiency increases).

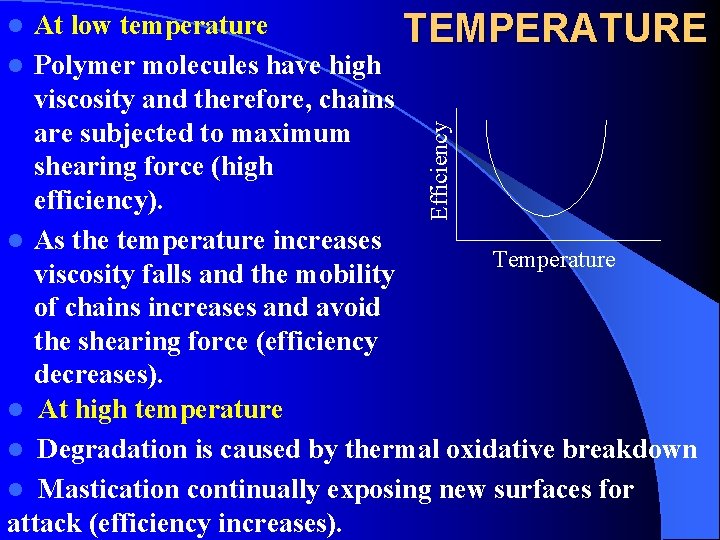
At low temperature TEMPERATURE l Polymer molecules have high viscosity and therefore, chains are subjected to maximum shearing force (high efficiency). l As the temperature increases Temperature viscosity falls and the mobility of chains increases and avoid the shearing force (efficiency decreases). l At high temperature l Degradation is caused by thermal oxidative breakdown l Mastication continually exposing new surfaces for attack (efficiency increases). Efficiency l

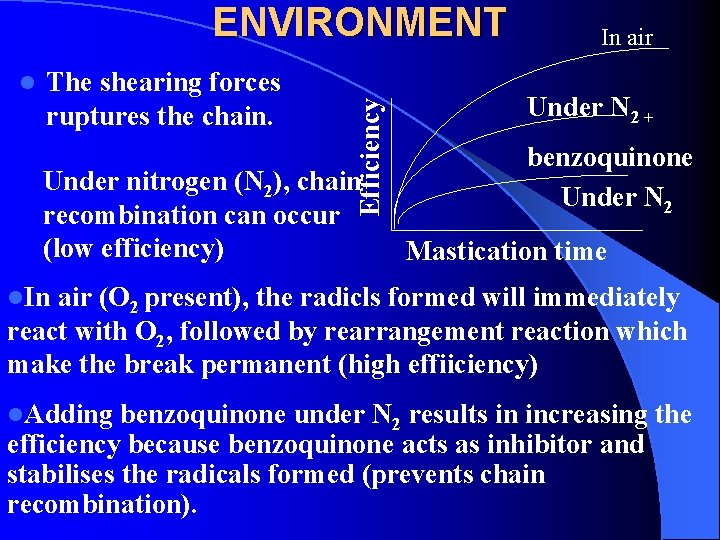
l The shearing forces ruptures the chain. Efficiency ENVIRONMENT Under nitrogen (N 2), chain recombination can occur (low efficiency) In air Under N 2 + benzoquinone Under N 2 Mastication time l. In air (O 2 present), the radicls formed will immediately react with O 2, followed by rearrangement reaction which make the break permanent (high effiiciency) l. Adding benzoquinone under N 2 results in increasing the efficiency because benzoquinone acts as inhibitor and stabilises the radicals formed (prevents chain recombination).

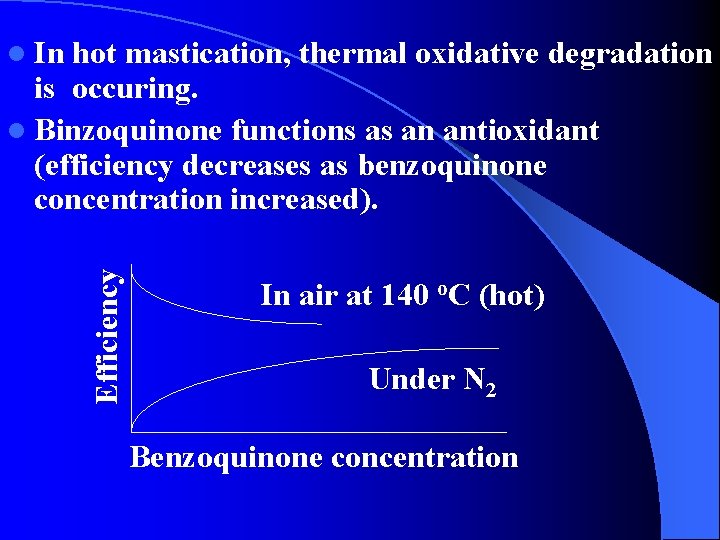
hot mastication, thermal oxidative degradation is occuring. l Binzoquinone functions as an antioxidant (efficiency decreases as benzoquinone concentration increased). Efficiency l In In air at 140 o. C (hot) Under N 2 Benzoquinone concentration

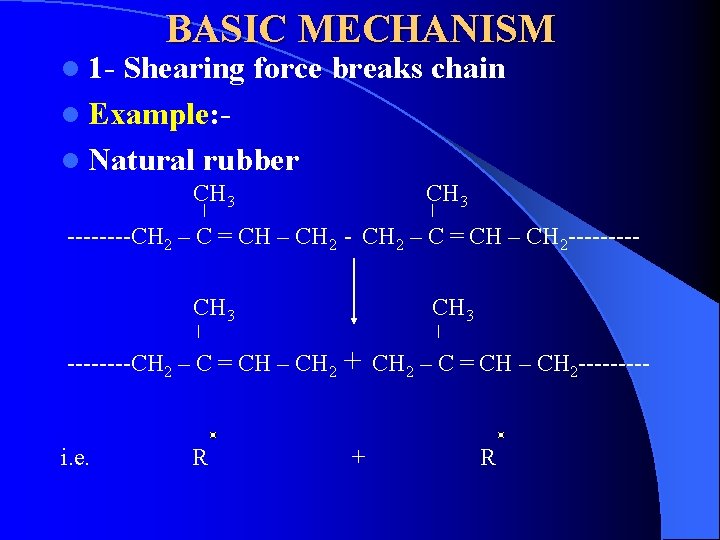
l 1 - BASIC MECHANISM Shearing force breaks chain l Example: l Natural rubber CH 3 ----CH 2 – C = CH – CH 2 - CH 2 – C = CH – CH 2 ----CH 3 ----CH 2 – C = CH – CH 2 i. e. R CH 3 + + CH 2 – C = CH – CH 2 ----- R

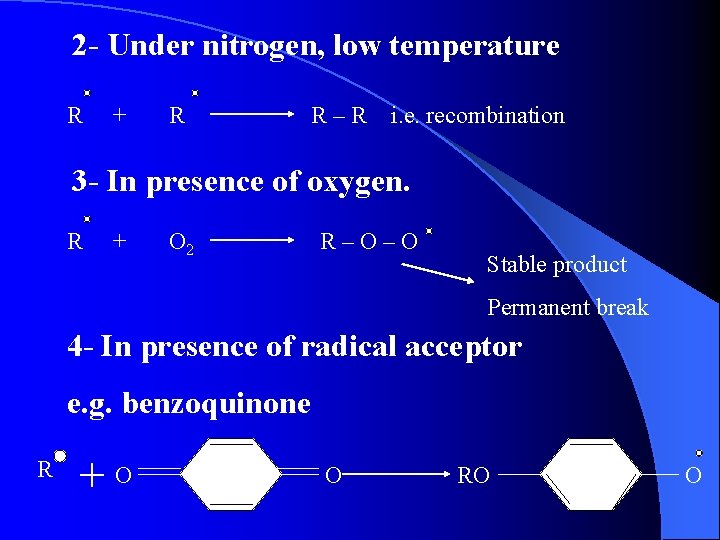
2 - Under nitrogen, low temperature R + R R–R i. e. recombination 3 - In presence of oxygen. R + O 2 R–O–O Stable product Permanent break 4 - In presence of radical acceptor e. g. benzoquinone R +O O RO O

Thank You See You Next Lecture