CHAPTER 13 GAS MIXTURES 1 Composition of a





- Slides: 28

CHAPTER 13 GAS MIXTURES 1. Composition of a Gas Mixture 2. - Mass and Mole Fractions 2. 3. 3. 4. P-v-T Behavior of Gas Mixtures - Ideal and Real Gases Properties of Gas Mixtures (um, hm, sm, etc) - Ideal and Real Gases 에너지변환시스템연구실(ECOS) Energy Conversion System Lab. Chung H. Jeon

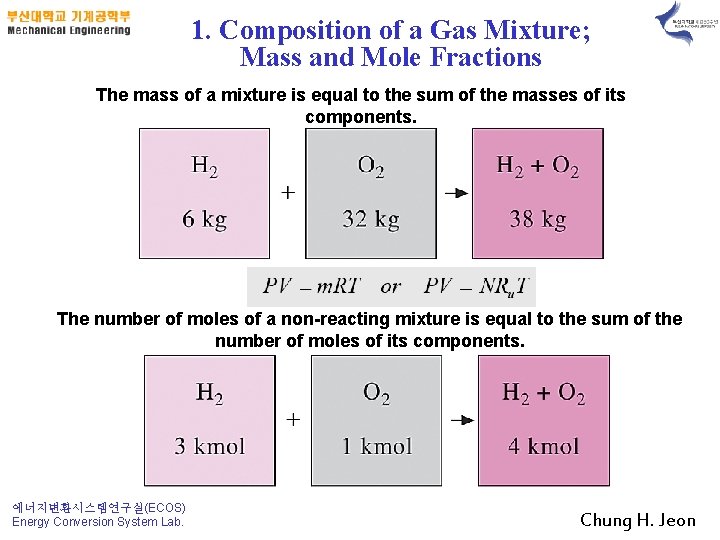
1. Composition of a Gas Mixture; Mass and Mole Fractions The mass of a mixture is equal to the sum of the masses of its components. The number of moles of a non-reacting mixture is equal to the sum of the number of moles of its components. 에너지변환시스템연구실(ECOS) Energy Conversion System Lab. Chung H. Jeon

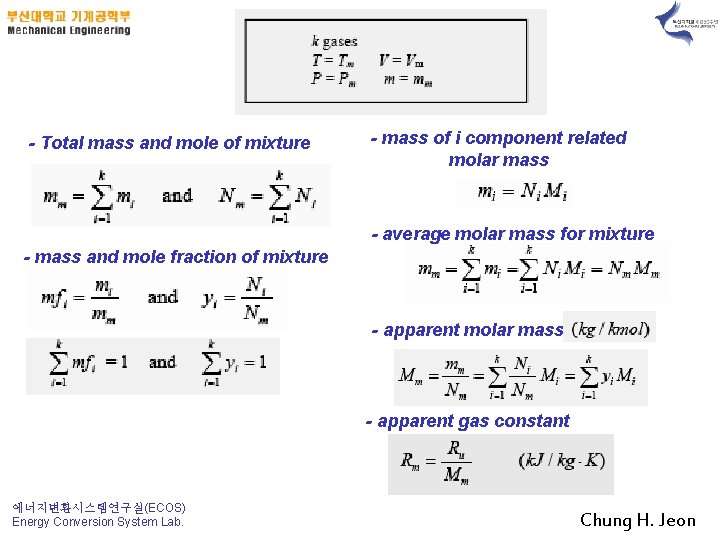
- Total mass and mole of mixture - mass of i component related molar mass - average molar mass for mixture - mass and mole fraction of mixture - apparent molar mass - apparent gas constant 에너지변환시스템연구실(ECOS) Energy Conversion System Lab. Chung H. Jeon

에너지변환시스템연구실(ECOS) Energy Conversion System Lab. Chung H. Jeon

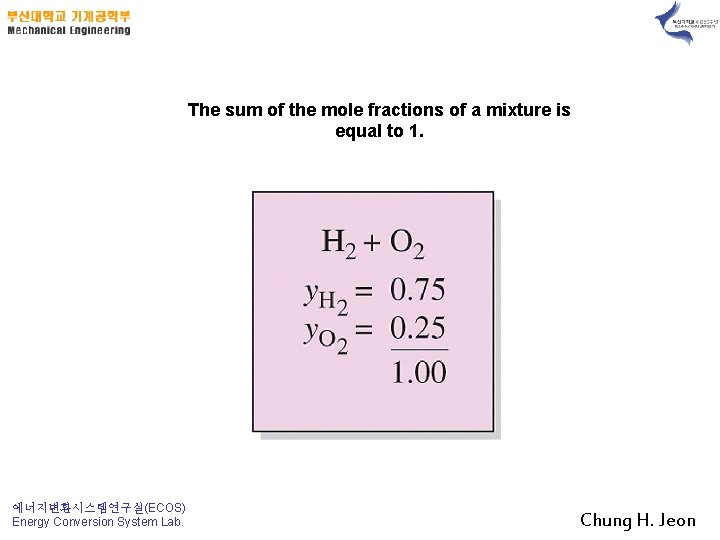
The sum of the mole fractions of a mixture is equal to 1. 에너지변환시스템연구실(ECOS) Energy Conversion System Lab. Chung H. Jeon

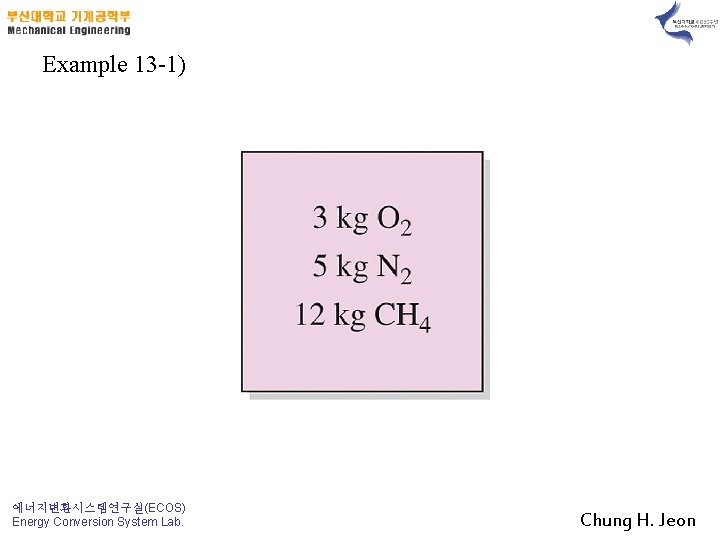
Example 13 -1) 에너지변환시스템연구실(ECOS) Energy Conversion System Lab. Chung H. Jeon

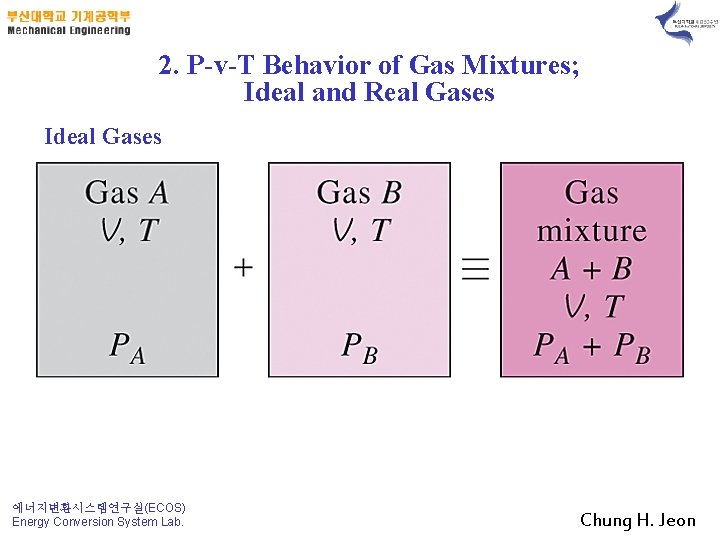
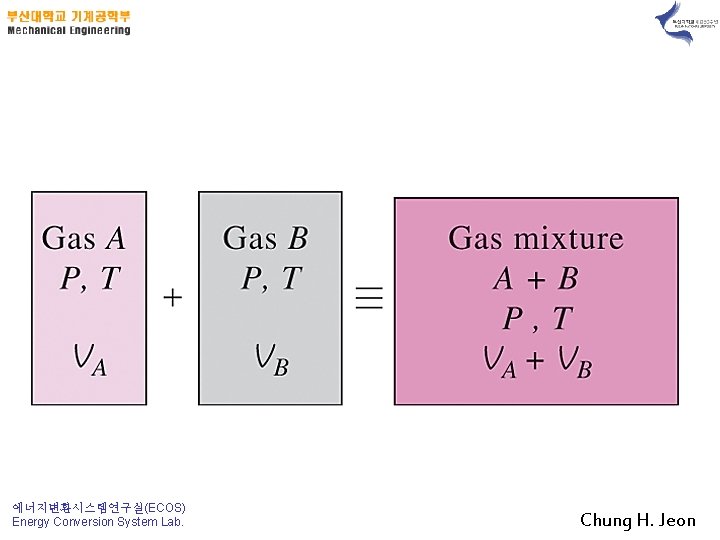
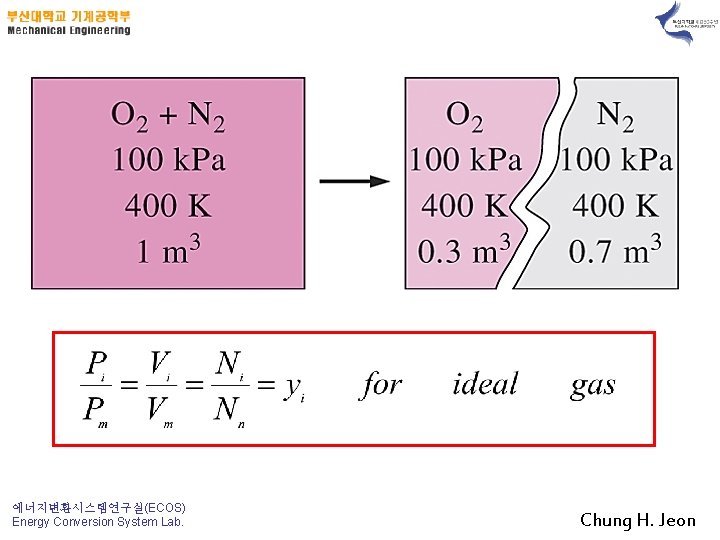
2. P-v-T Behavior of Gas Mixtures; Ideal and Real Gases Ideal Gases 에너지변환시스템연구실(ECOS) Energy Conversion System Lab. Chung H. Jeon

에너지변환시스템연구실(ECOS) Energy Conversion System Lab. Chung H. Jeon

에너지변환시스템연구실(ECOS) Energy Conversion System Lab. Chung H. Jeon

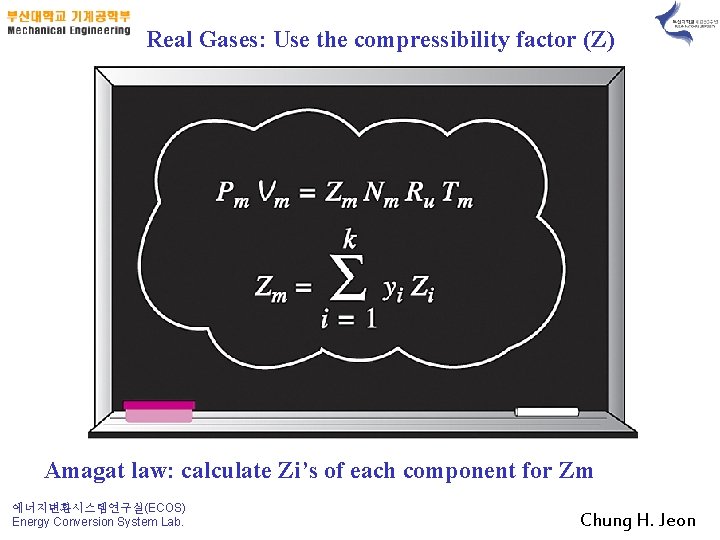
Real Gases: Use the compressibility factor (Z) Amagat law: calculate Zi’s of each component for Zm 에너지변환시스템연구실(ECOS) Energy Conversion System Lab. Chung H. Jeon

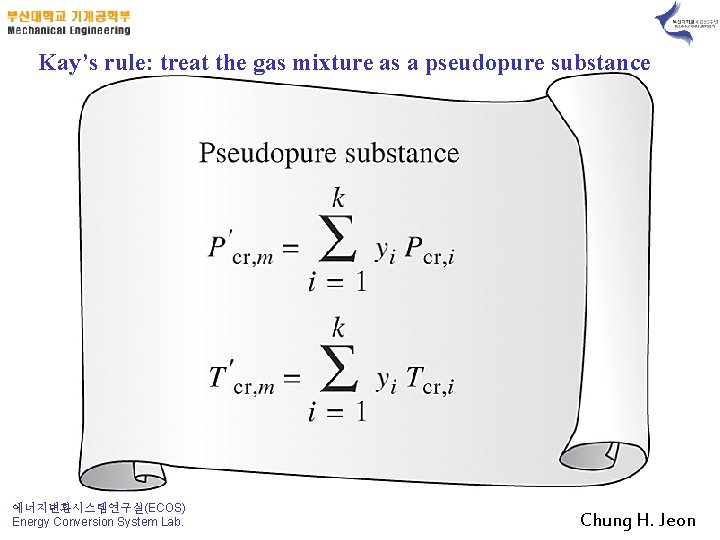
Kay’s rule: treat the gas mixture as a pseudopure substance 에너지변환시스템연구실(ECOS) Energy Conversion System Lab. Chung H. Jeon

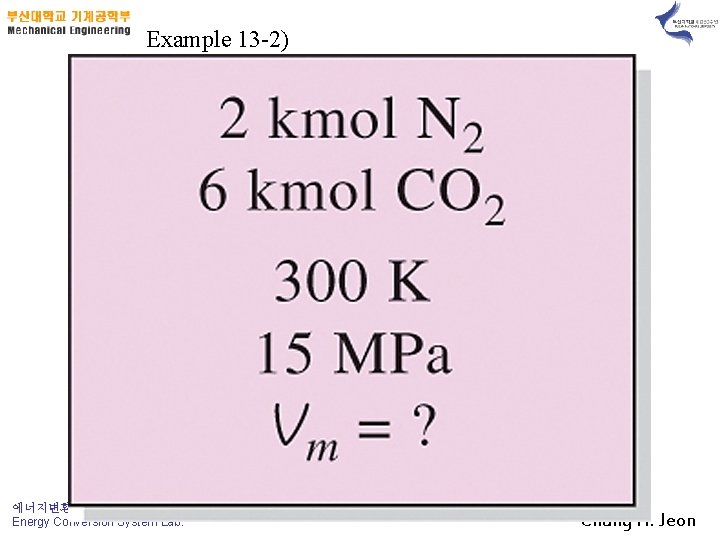
Example 13 -2) 에너지변환시스템연구실(ECOS) Energy Conversion System Lab. Chung H. Jeon

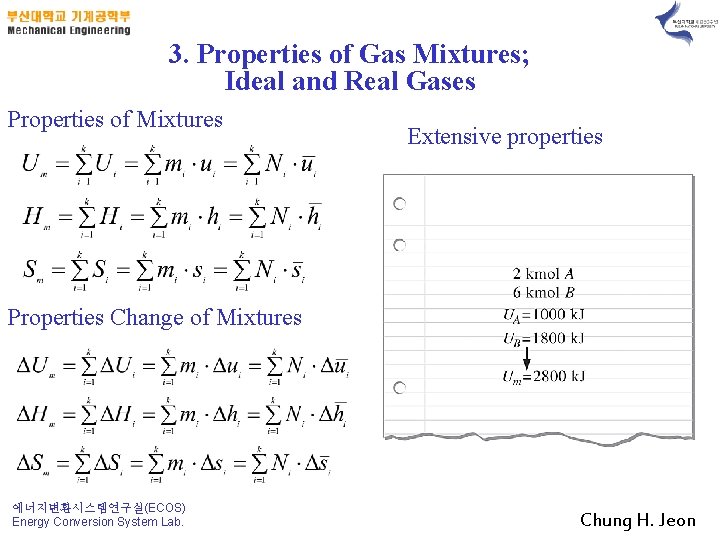
3. Properties of Gas Mixtures; Ideal and Real Gases Properties of Mixtures Extensive properties Properties Change of Mixtures 에너지변환시스템연구실(ECOS) Energy Conversion System Lab. Chung H. Jeon

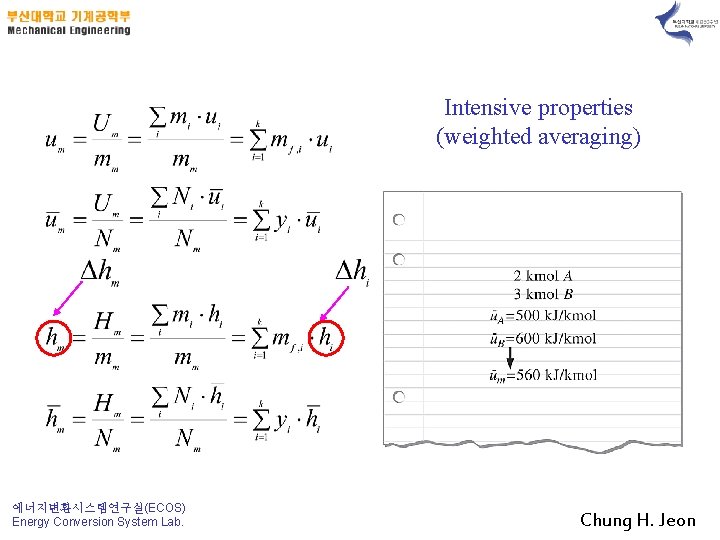
Intensive properties (weighted averaging) 에너지변환시스템연구실(ECOS) Energy Conversion System Lab. Chung H. Jeon

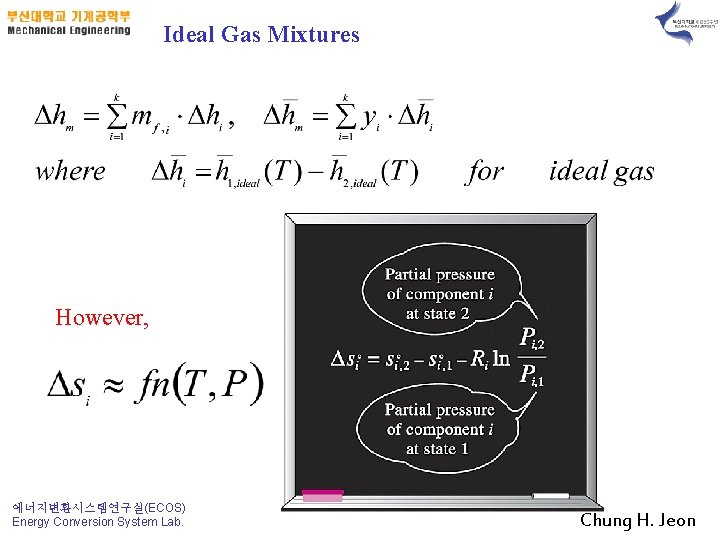
Ideal Gas Mixtures However, 에너지변환시스템연구실(ECOS) Energy Conversion System Lab. Chung H. Jeon

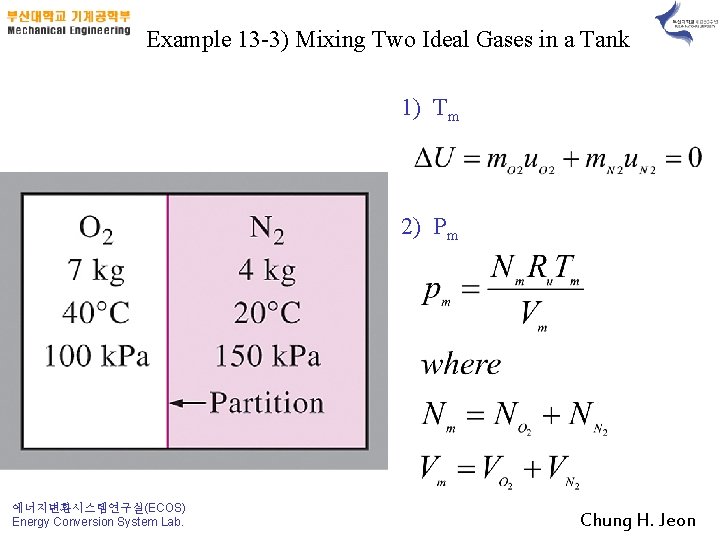
Example 13 -3) Mixing Two Ideal Gases in a Tank 1) Tm 2) Pm 에너지변환시스템연구실(ECOS) Energy Conversion System Lab. Chung H. Jeon

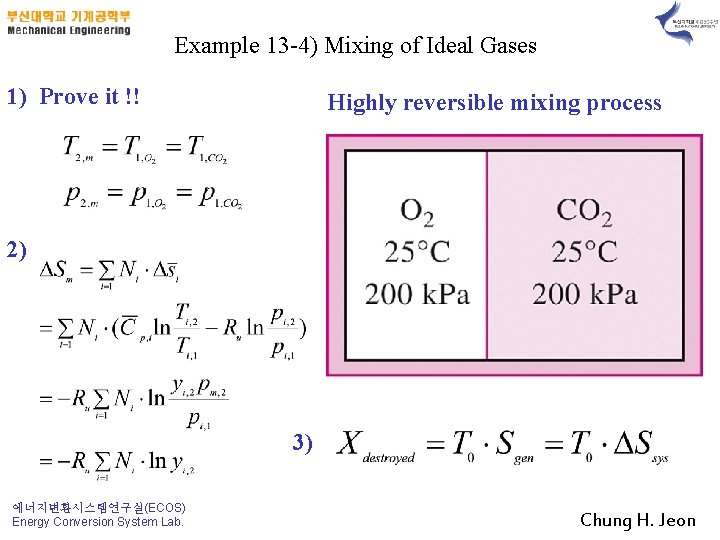
Example 13 -4) Mixing of Ideal Gases 1) Prove it !! Highly reversible mixing process 2) 3) 에너지변환시스템연구실(ECOS) Energy Conversion System Lab. Chung H. Jeon

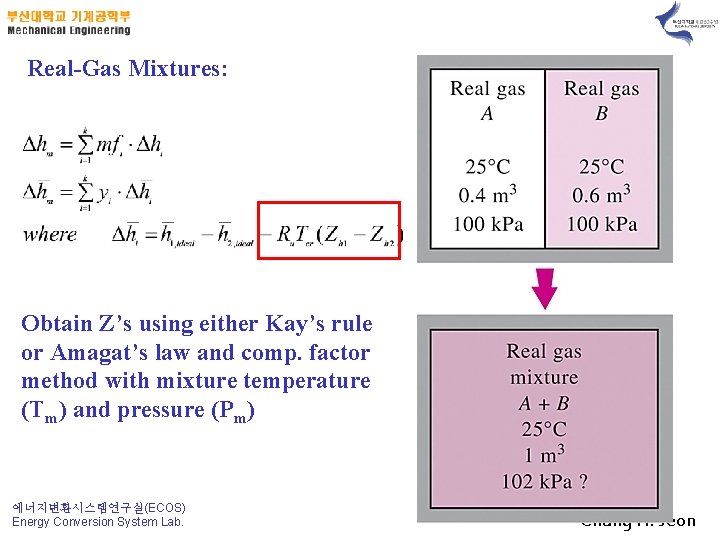
Real-Gas Mixtures: Obtain Z’s using either Kay’s rule or Amagat’s law and comp. factor method with mixture temperature (Tm) and pressure (Pm) 에너지변환시스템연구실(ECOS) Energy Conversion System Lab. Chung H. Jeon

Example 13 -5) Cooling of a non-ideal Gas Mixture 에너지변환시스템연구실(ECOS) Energy Conversion System Lab. Chung H. Jeon

Extra Slides 에너지변환시스템연구실(ECOS) Energy Conversion System Lab. Chung H. Jeon

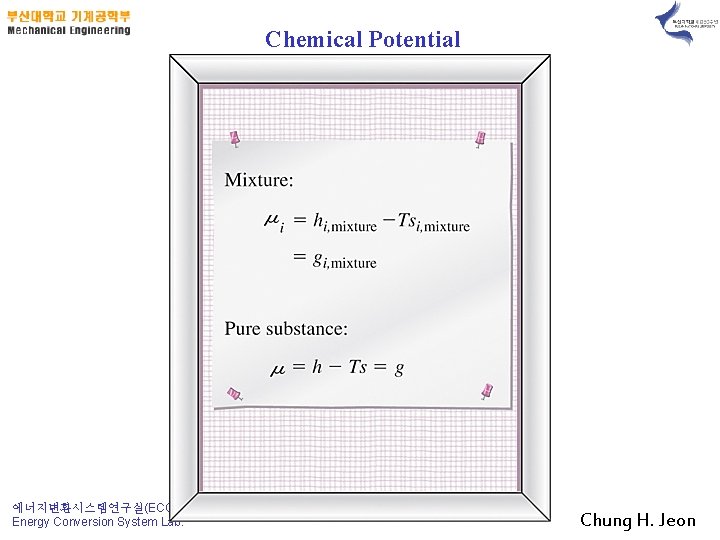
Chemical Potential 에너지변환시스템연구실(ECOS) Energy Conversion System Lab. Chung H. Jeon

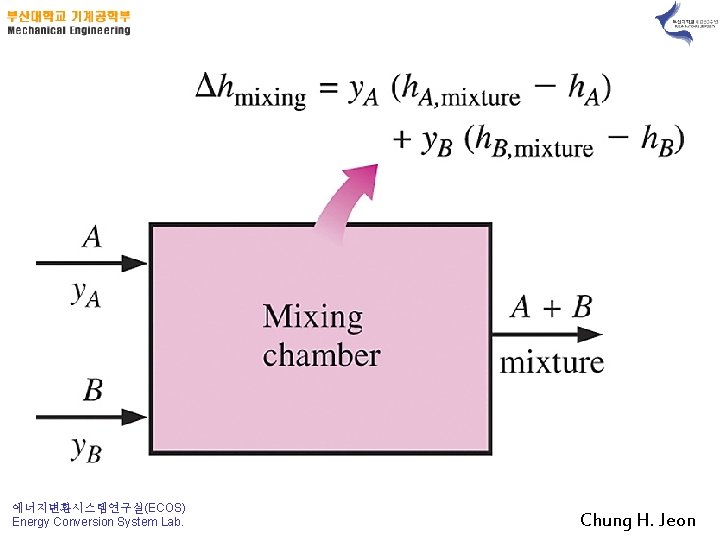
에너지변환시스템연구실(ECOS) Energy Conversion System Lab. Chung H. Jeon

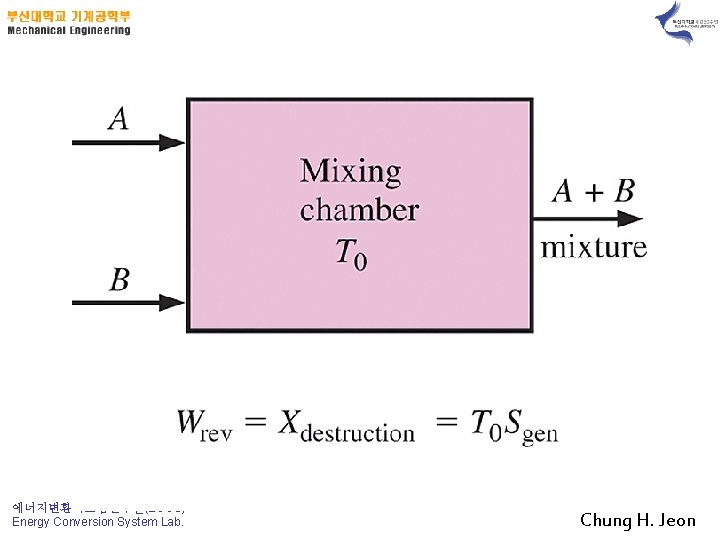
에너지변환시스템연구실(ECOS) Energy Conversion System Lab. Chung H. Jeon

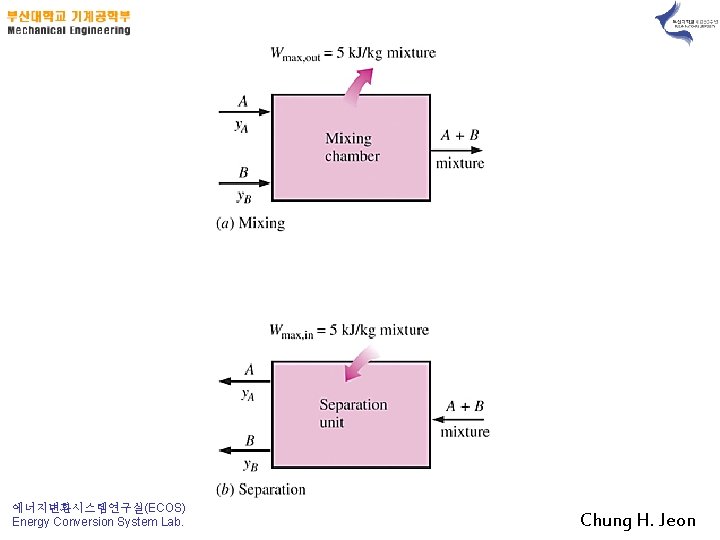
에너지변환시스템연구실(ECOS) Energy Conversion System Lab. Chung H. Jeon

에너지변환시스템연구실(ECOS) Energy Conversion System Lab. Chung H. Jeon

에너지변환시스템연구실(ECOS) Energy Conversion System Lab. Chung H. Jeon

에너지변환시스템연구실(ECOS) Energy Conversion System Lab. Chung H. Jeon

에너지변환시스템연구실(ECOS) Energy Conversion System Lab. Chung H. Jeon