CEE NETWORK ISPOR CEE Network MCDA initiatives Multicriteria














- Slides: 30

CEE NETWORK ISPOR CEE Network MCDA initiatives: Multi-criteria decision payer preferences for rare diseases in Central East Europe countries Vladimir Zah, Ph. D(c) CEESTAHC, Krakow, Poland, Dec 13, 2016

Conflict of Interest All views expressed are personal and may not represent ISPOR views, opinions or policies. Author has no financial or other conflict of interest. CEESTAHC, Krakow, Poland, Dec 13, 2016

Attention CEESTAHC, Krakow, Poland, Dec 13, 2016

Ø How did we get here? Ø About MCDA Ø Structuring evidence with MCDA (Multi Criteria Decision Making) Ø MCDA CEE Initiative in rare diseases Ø Next Steps CEESTAHC, Krakow, Poland, Dec 13, 2016

How did we get here? • 1970 s/80 s – debate in economics and ethics literature about relevant criteria for making resource allocation decisions in health care – focus on clinical and cost-effectiveness • 1990 s/2000 s – emergence of health technology assessment bodies; growing recognition that other criteria are important, relating to equity, acceptability, burden, sustainability etc. • 2010 s – growing interest in decision analytic methods for considering multiple criteria – driven primarily by the NICE in the UK and moves to Value Based Pricing • Multi Criteria Decision Analysis (MCDA) is a methodology designed to help decision-makers when making complex choices – first developed in the 1960 s/70 s CEESTAHC, Krakow, Poland, Dec 13, 2016

How did we get here? Life MCDA Decisions (if multiple boyfriend alternatives) CEESTAHC, Krakow, Poland, Dec 13, 2016

How did we get here? CEESTAHC, Krakow, Poland, Dec 13, 2016

How did we get here? Healthcare MCDA Decisions (Patient perspective) CEESTAHC, Krakow, Poland, Dec 13, 2016 1) Immediate Cure 2) Most Effective 3) Most Expensive 4) No Side Effects 5) Unmet Need fulfilled (rare diseases) 6) Always Available 7) No Risk of Death 8) No Disease Recurrence 9) No Waiting 10)No Responsibility (Adherence/Compliance) 11)No Life Style Change

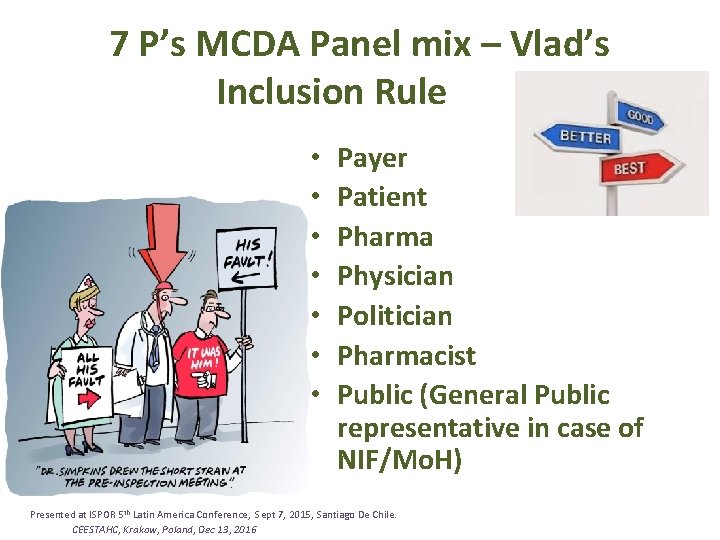
7 P’s MCDA Panel mix – Vlad’s Inclusion Rule • • Payer Patient Pharma Physician Politician Pharmacist Public (General Public representative in case of NIF/Mo. H) Presented at ISPOR 5 th Latin America Conference, Sept 7, 2015, Santiago De Chile. CEESTAHC, Krakow, Poland, Dec 13, 2016

Structuring evidence with MCDA (Multi Criteria Decision Making) Two main stages in MCDA 1. Problem structuring: 2. a) generating a set of criteria against which the alternatives are to be evaluated and compared b) generating a set of alternatives 2. Model building: constructing some form of model which represents decision-makers’ objectives and their value judgements Key methodological considerations: – methods used to describe decision-makers preferences and elicit importance weights for decision-making criteria – type of aggregation model used to combine criteria scores CEESTAHC, Krakow, Poland, Dec 13, 2016

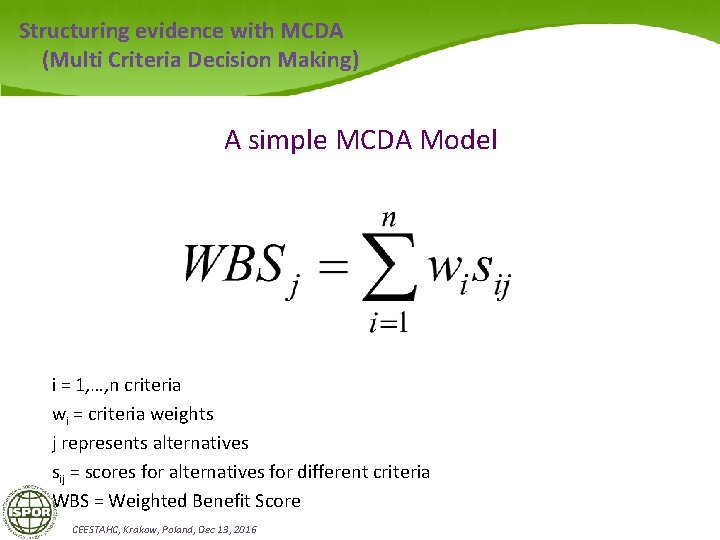
Structuring evidence with MCDA (Multi Criteria Decision Making) A simple MCDA Model i = 1, …, n criteria wi = criteria weights j represents alternatives sij = scores for alternatives for different criteria WBS = Weighted Benefit Score CEESTAHC, Krakow, Poland, Dec 13, 2016

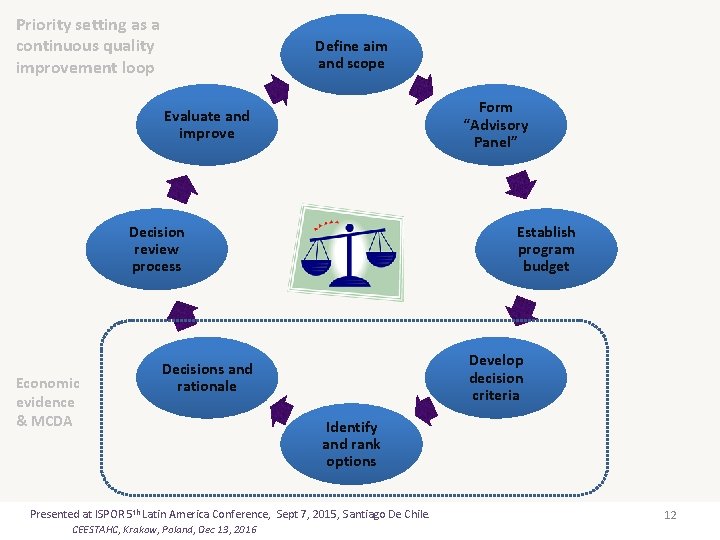
Priority setting as a continuous quality improvement loop Define aim and scope Form “Advisory Panel” Evaluate and improve Decision review process Economic evidence & MCDA Establish program budget Develop decision criteria Decisions and rationale Identify and rank options Presented at ISPOR 5 th Latin America Conference, Sept 7, 2015, Santiago De Chile. CEESTAHC, Krakow, Poland, Dec 13, 2016 12

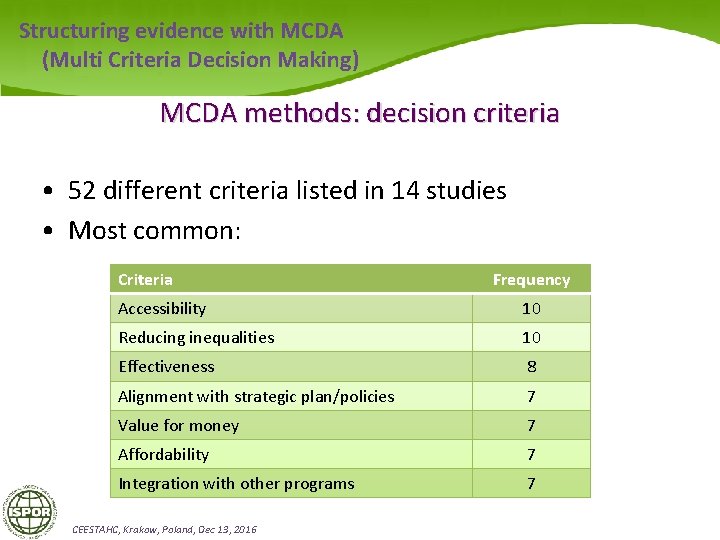
Structuring evidence with MCDA (Multi Criteria Decision Making) MCDA methods: decision criteria • 52 different criteria listed in 14 studies • Most common: Criteria Frequency Accessibility 10 Reducing inequalities 10 Effectiveness 8 Alignment with strategic plan/policies 7 Value for money 7 Affordability 7 Integration with other programs 7 CEESTAHC, Krakow, Poland, Dec 13, 2016

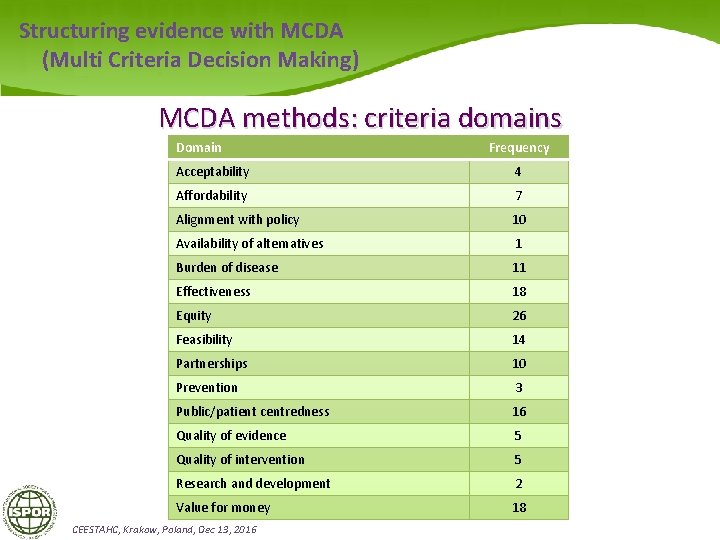
Structuring evidence with MCDA (Multi Criteria Decision Making) MCDA methods: criteria domains Domain Frequency Acceptability 4 Affordability 7 Alignment with policy 10 Availability of alternatives 1 Burden of disease 11 Effectiveness 18 Equity 26 Feasibility 14 Partnerships 10 Prevention 3 Public/patient centredness 16 Quality of evidence 5 Quality of intervention 5 Research and development 2 Value for money 18 CEESTAHC, Krakow, Poland, Dec 13, 2016

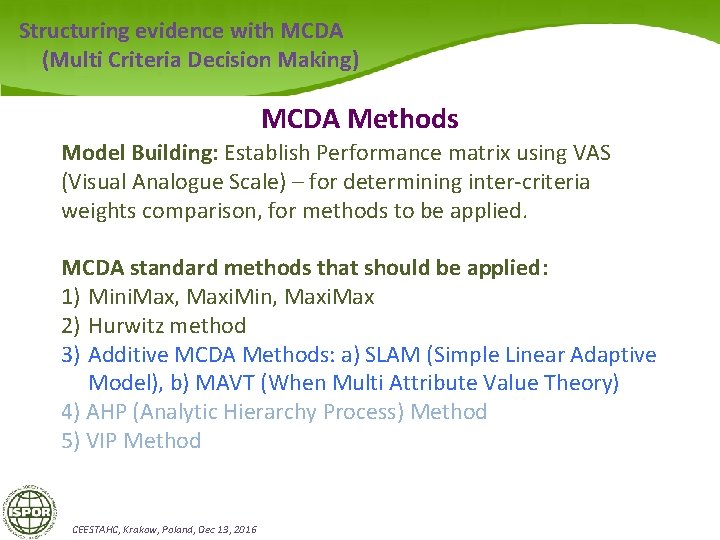
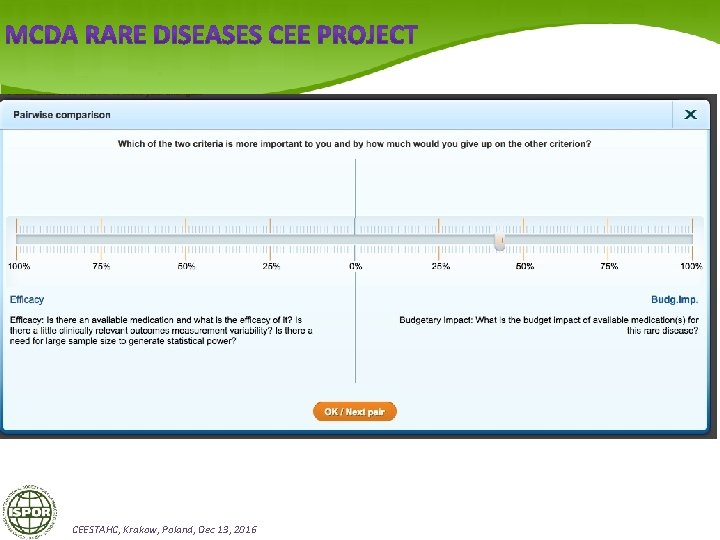
Structuring evidence with MCDA (Multi Criteria Decision Making) MCDA Methods Model Building: Establish Performance matrix using VAS (Visual Analogue Scale) – for determining inter-criteria weights comparison, for methods to be applied. MCDA standard methods that should be applied: 1) Mini. Max, Maxi. Min, Maxi. Max 2) Hurwitz method 3) Additive MCDA Methods: a) SLAM (Simple Linear Adaptive Model), b) MAVT (When Multi Attribute Value Theory) 4) AHP (Analytic Hierarchy Process) Method 5) VIP Method CEESTAHC, Krakow, Poland, Dec 13, 2016

Structuring evidence with MCDA (Multi Criteria Decision Making) What are some of the challenges? • Some methodological challenges - who’s criteria count – society, patients, decisionmakers etc. - methods used to elicit and describe decision-makers preferences, including the relationship between objectives and criteria - methods used to elicit importance weights for decisionmaking criteria - type of aggregation model used to combine criteria scores CEESTAHC, Krakow, Poland, Dec 13, 2016

MCDA CEE Initiative Title: “Payer preferences for Multi-Criteria Decision Making” Introduction: In the areas of medical devices, rare disease and oncology there are many instances of a negative or no cost-effectiveness value proposition. However, in the past, decision makers had to subconsciously pick criteria due to which such products would find a way to be reimbursed. In order to make this process transparent, MCDA was introduced in the pharmaceutical and medical device industry. Objective: To establish and publish decision makers perception of criteria and their importance when evaluating rare diseases, oncological products and medical devices. CEESTAHC, Krakow, Poland, Dec 13, 2016

MCDA CEE Initiative Description: MCDA “core" criteria will be established for CEE region, by analyzing responses from all CEE country decision makers. Further, default inter-criteria importance will be derived for each of the three product groups: 1) Rare Disease medications 2) Oncology medications 3) Medical Devices Results: Preliminary results presented at ISPOR EU meeting Vienna 2016. Lot’s of work ahead… CEESTAHC, Krakow, Poland, Dec 13, 2016

MCDA Rationale Background: In order to mitigate the risks that some P&R criteria shadows others in the decision making process, more and more jurisdictions implement a specific P&R pathway for orphan medical products (OMPs). 36 million individuals suffering from as many as 5000– 8000 different types of rare diseases in EU. On average up to 30% of them still are not treated with registered OMPs. Initiatives: A process designed exclusively for the Highly Specialised Technologies which is distinct from the appraisal conducted for other medicines used to treat more common diseases in England Wales. The established framework goes beyond the evaluation of clinical evidence and refers to the number of additional criteria such as the impact of the technology beyond direct health benefits, disease severity and patient’s disability. Instead of the cost-effectiveness analysis, it introduces the assessment of the budget impact and patient access schemes. Revealed preferences towards the appraisal of orphan drugs in Poland- MCDA analysis (in submission) CEESTAHC, Krakow, Poland, Dec 13, 2016

MCDA Rationale CEE countries lags behind the rest of EU regarding accessibility to treatment for rare disease patients. For instance, it was revealed that just 22 out of registered 61 and 28 out of 72 registered OMP were available in Bulgaria and Latvia respectively. Revealed preferences towards the appraisal of orphan drugs in Poland- MCDA analysis (in submission) OMP – Orphan Medical Product CEESTAHC, Krakow, Poland, Dec 13, 2016

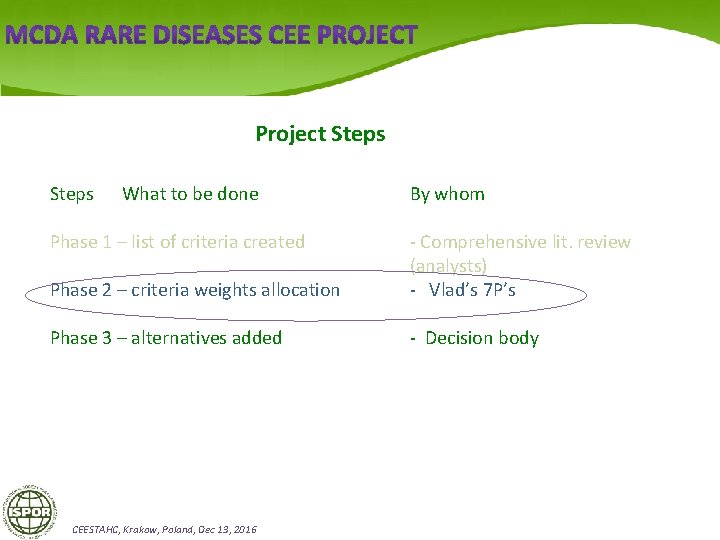
Project Steps What to be done Phase 1 – list of criteria created By whom Phase 2 – criteria weights allocation - Comprehensive lit. review (analysts) - Vlad’s 7 P’s Phase 3 – alternatives added - Decision body CEESTAHC, Krakow, Poland, Dec 13, 2016

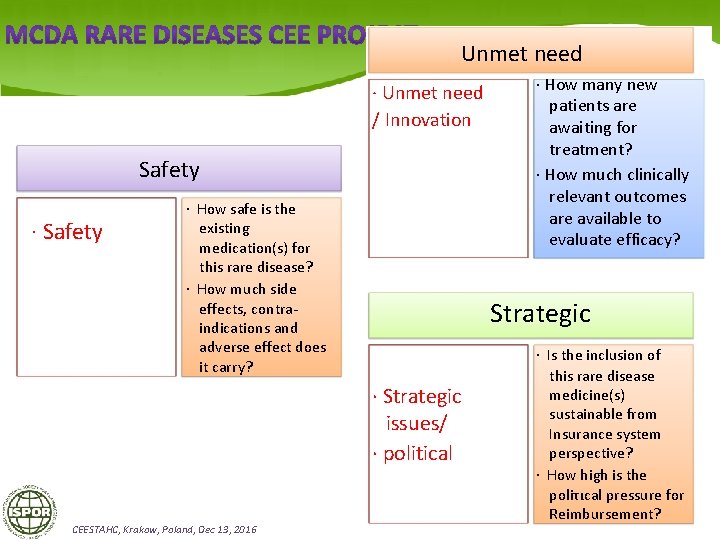
Efficacy · · · Efficacy Very low. Low. ModerateΗigh. Very high · Is there an available medication and what is the efficacy of it? · Is there a little clinically relevant outcomes measurement variability? · Is there a need for large sample size to generate statistical power? CEESTAHC, Krakow, Poland, Dec 13, 2016 Budgetary Impact · What is the budget impact of available medication(s) for this rare disease?

Unmet need · Unmet need / Innovation Safety · How safe is the existing medication(s) for this rare disease? · How much side effects, contraindications and adverse effect does it carry? Strategic · Strategic issues/ · political CEESTAHC, Krakow, Poland, Dec 13, 2016 · How many new patients are awaiting for treatment? · How much clinically relevant outcomes are available to evaluate efficacy? · Is the inclusion of this rare disease medicine(s) sustainable from Insurance system perspective? · How high is the poliτιcal pressure for Reimbursement?

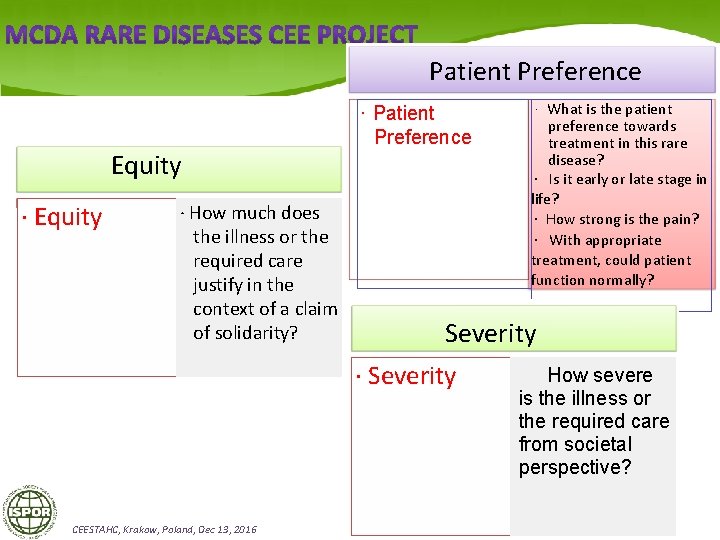
Patient Preference Equity · How much does the illness or the required care justify in the context of a claim of solidarity? · Patient Preference preference towards treatment in this rare disease? · Is it early or late stage in life? · How strong is the pain? · With appropriate treatment, could patient function normally? Severity · Severity CEESTAHC, Krakow, Poland, Dec 13, 2016 · What is the patient How severe is the illness or the required care from societal perspective?

1 0. 5 0. 4 0. 3 0. 2 0. 5 16. 07% 0. 5 1 0. 4 0. 3 0. 2 0. 4 16. 43% Poland CEESTAHC, Krakow, Poland, Dec 13, 2016 0. 6 1 0. 5 0. 3 0. 6 12. 86% 0. 6 0. 5 1 0. 4 0. 3 0. 5 13. 57% 0. 7 0. 5 0. 6 1 0. 3 0. 6 11. 79% 0. 8 0. 7 1 0. 5 0. 7 7. 50% 0. 8 0. 7 0. 5 1 0. 7 7. 50% 0. 5 0. 6 0. 4 0. 5 0. 4 0. 3 1 14. 29%

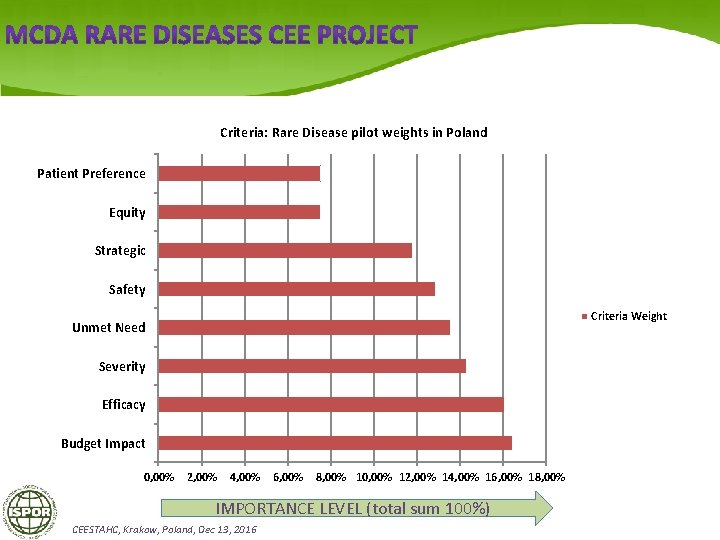
Criteria: Rare Disease pilot weights in Poland Patient Preference Equity Strategic Safety Criteria Weight Unmet Need Severity Efficacy Budget Impact 0, 00% 2, 00% 4, 00% 6, 00% 8, 00% 10, 00% 12, 00% 14, 00% 16, 00% 18, 00% IMPORTANCE LEVEL (total sum 100%) CEESTAHC, Krakow, Poland, Dec 13, 2016

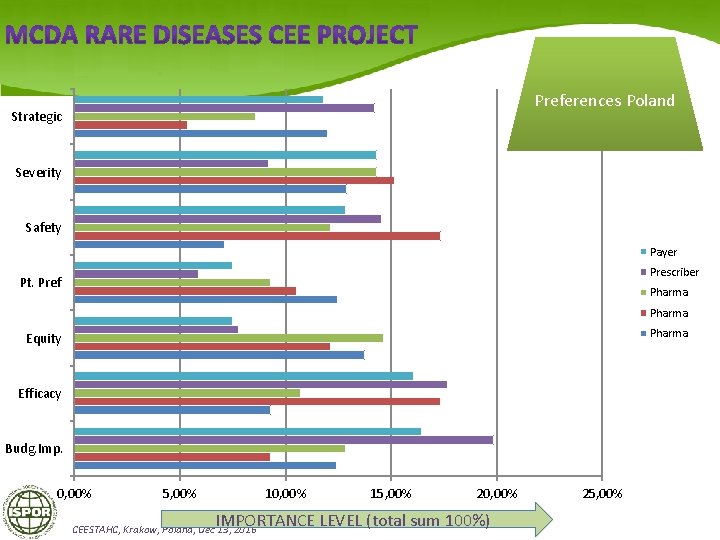
Preferences Poland Strategic Severity Safety Payer Prescriber Pt. Pref Pharma Equity Efficacy Budg. Imp. 0, 00% 5, 00% 10, 00% 15, 00% 20, 00% IMPORTANCE LEVEL (total sum 100%) CEESTAHC, Krakow, Poland, Dec 13, 2016 25, 00%

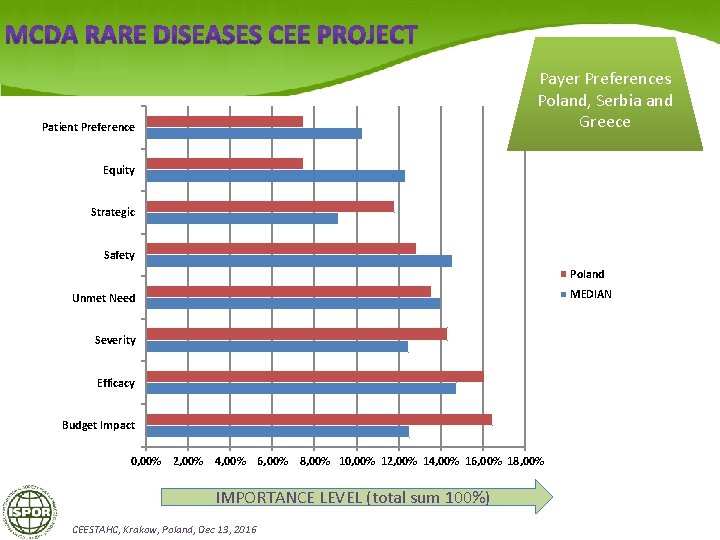
Payer Preferences Poland, Serbia and Greece Patient Preference Equity Strategic Safety Poland MEDIAN Unmet Need Severity Efficacy Budget Impact 0, 00% 2, 00% 4, 00% 6, 00% 8, 00% 10, 00% 12, 00% 14, 00% 16, 00% 18, 00% IMPORTANCE LEVEL (total sum 100%) CEESTAHC, Krakow, Poland, Dec 13, 2016

Conclusion CEESTAHC, Krakow, Poland, Dec 13, 2016

Conclusion Multi-Criteria Decision Analysis (MCDA) is considered as the most 7 promising P’s MCDA Panel mix – Vlad’s methodological approach in the Inclusion search for the. Rule trade off between the economic and non-economic criteria. Steps What to be done By whommust be clear. Lessons learned: Predefined criteria descriptions Phase 1 – list of criteria created - Comprehensive 7 P’s MCDA Panel mix – lit. review (analysts) Vlad’s Inclusion Rule Phase 2 – criteria weights allocation - Vlad’s 7 P’s • Payer • Patient Phase 3 – alternatives added - Decision body • Pharma • Physician • Politician • Pharmacist • Public (General Public representative in case of NIF/Mo. H) THANK YOU! | Questions CEESTAHC, Krakow, Poland, Dec 13, 2016