Causes of Change Energy and Change Changes and

























- Slides: 60

Causes of Change Energy and Change

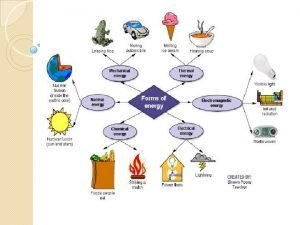
Changes and Energy Both chemical and physical changes are accompanied by either an increase or decrease in energy. Energy is the ability to do work or produce heat. There are two major types of energy: kinetic and potential. Kinetic energy is the energy of motion and depends on both the mass and the velocity of the object. Potential energy is stored energy. Matter possesses both types of energy. Heat and temperature are used to describe the energy content of matter.

What is temperature? Temperature is a measure of the average kinetic energy (energy of motion) of particles in matter. As the kinetic energy of the particles increases, the temperature increases. As the kinetic energy of the particles decreases, the temperature decreases. The lowest possible temperature (the temperature at which the particles stop moving) is called absolute zero. Temperature is an intensive property and does not depend on the amount of matter present.

How is temperature measured? Temperature is measured with a thermometer. Almost all substances expand with an increase in temperature (exception is water). Thermometers are designed so that the substances they contain (mercury, etc. ) expand contract more than the volume of the glass tube that contains them so that the column height of the substance changes.

Temperature Scales Several temperature scales have been devised: - Fahrenheit (o. F): weather is measured using this scale. - Celsius (o. C): the scale used in the metric system. -Kelvin (K): based on absolute zero; used in the International System (SI).

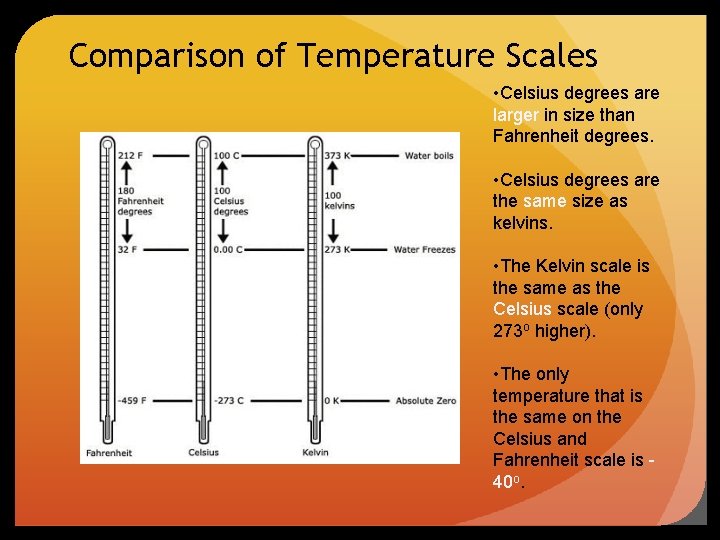
Comparison of Temperature Scales • Celsius degrees are larger in size than Fahrenheit degrees. • Celsius degrees are the same size as kelvins. • The Kelvin scale is the same as the Celsius scale (only 273 o higher). • The only temperature that is the same on the Celsius and Fahrenheit scale is 40 o.

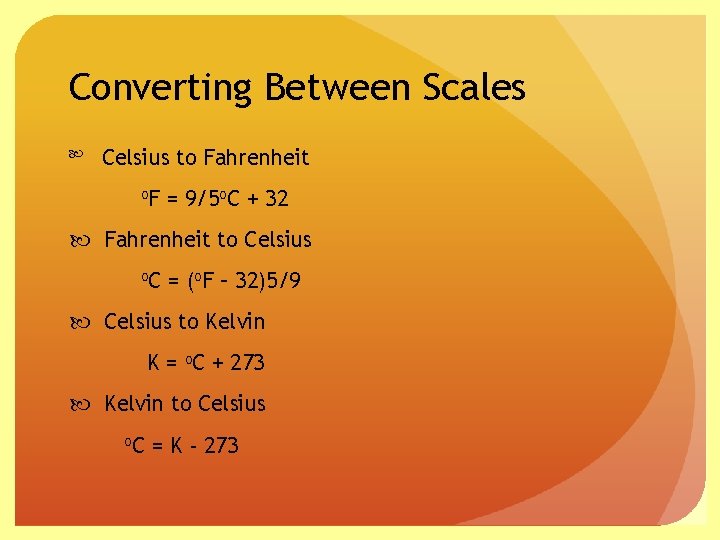
Converting Between Scales Celsius to Fahrenheit o. F = 9/5 o. C + 32 Fahrenheit to Celsius o. C = (o. F – 32)5/9 Celsius to Kelvin K = o. C + 273 Kelvin to Celsius o. C = K - 273

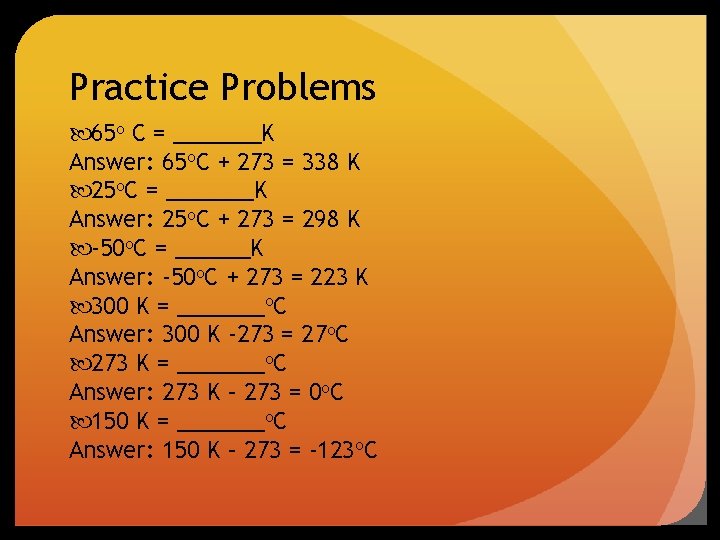
Practice Problems 65 o C = _______K Answer: 65 o. C + 273 = 338 K 25 o. C = _______K Answer: 25 o. C + 273 = 298 K -50 o. C = ______K Answer: -50 o. C + 273 = 223 K 300 K = _______o. C Answer: 300 K -273 = 27 o. C 273 K = _______o. C Answer: 273 K – 273 = 0 o. C 150 K = _______o. C Answer: 150 K – 273 = -123 o. C

How Are Heat and Temperature Related? Heat (q) is the total amount of energy that transfers from one object to another. Heat is an extensive property and changes based on the amount of matter present. Temperature measures the intensity of the energy and heat measures the quantity of energy. The temperature of an object determines the direction of heat transfer. When two objects of different temperatures are in contact, heat moves from the object at the higher temperature to the object at the lower temperature until they are both at the same temperature.

Methods of Heat Transfer Conduction Convection Radiation

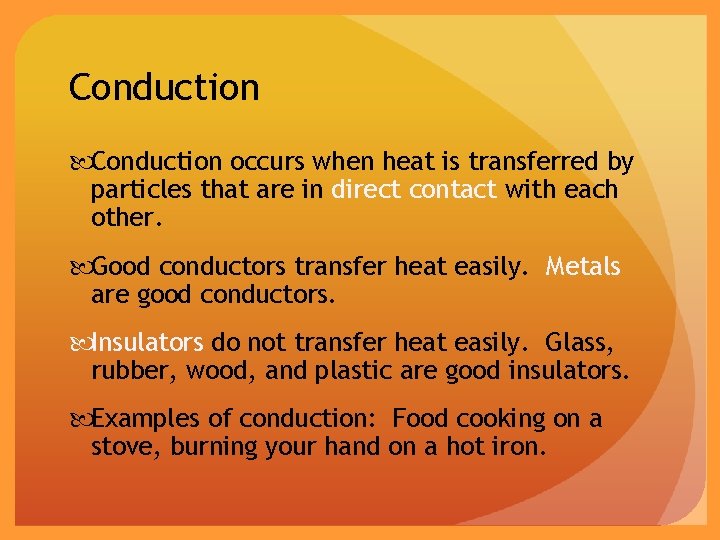
Conduction occurs when heat is transferred by particles that are in direct contact with each other. Good conductors transfer heat easily. Metals are good conductors. Insulators do not transfer heat easily. Glass, rubber, wood, and plastic are good insulators. Examples of conduction: Food cooking on a stove, burning your hand on a hot iron.

Walking on hot coals

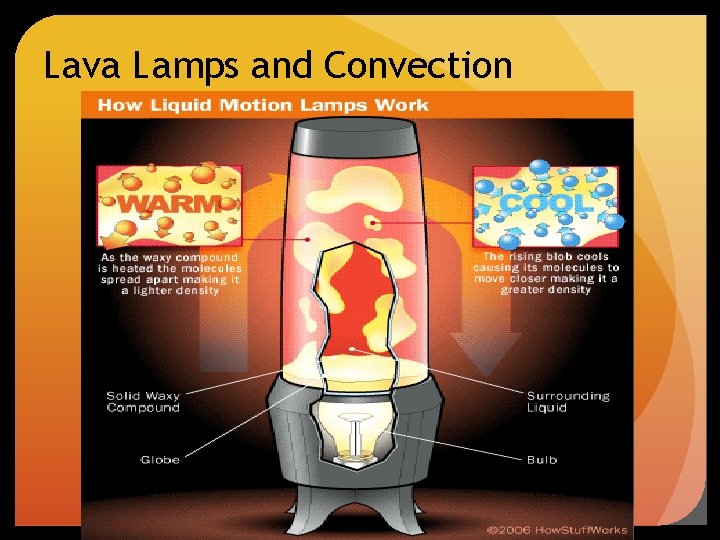
Convection occurs when heat is transferred through the movement of gas or liquid particles. Warm air/water is less dense and rises. Cool air/water is more dense and sinks. These differences in density create a circulating current. Examples: the heating of your home, deep ocean water is cooler than at the surface.

Lava Lamps and Convection

Radiation of heat occurs when heat is transferred in matter or space by means of electromagnetic waves. This type of heat travels outward from its source in all directions. Examples: a fireplace heating a room, the sun heating the earth.

A radiator exemplifies all three methods of heat transfer. 1. Heat radiates outward from the radiator to heat the room through radiation. 2. The man is warming his hands over the radiator through the process of convection. 3. If the man accidentally touches the surface of the radiator, he will burn his hands through the process of conduction.

Practice Questions Identify each of the following as conduction, convection, and radiation. 1. A hot air balloon. Answer: Convection 2. Warming yourself in front of a fireplace. Answer: Radiation 3. Cooking food in a microwave oven. Answer: Radiation 4. Frying bacon. Answer: Conduction 5. Burning your hand on a curling iron. Answer: Conduction 6. It is warmer in the attic than on the first floor of your house. Answer: Convection

Transfer of Energy in Chemical and Physical Changes All chemical and physical changes involve the release or absorption of heat. The system includes the substances involved in the change. The surroundings include everything else in the universe. The system and the surroundings make up the universe. Law of Conservation of Energy states that energy cannot be created or destroyed-instead it is transferred or changed from one form to another.

Endothermic vs. Exothermic Endothermic processes are those in which heat flows from the surroundings into a system (heat is absorbed). The system gains heat and the surroundings cool down. Exothermic processes are those in which heat flows from the system to the surroundings (heat is released). The system loses heat and the surroundings heat up.

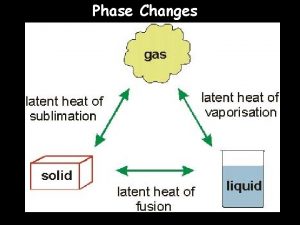
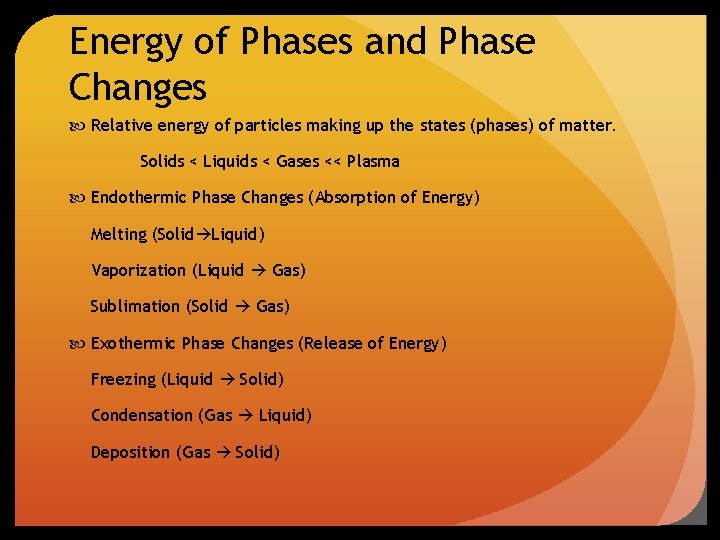
Energy of Phases and Phase Changes Relative energy of particles making up the states (phases) of matter. Solids < Liquids < Gases << Plasma Endothermic Phase Changes (Absorption of Energy) Melting (Solid Liquid) Vaporization (Liquid Gas) Sublimation (Solid Gas) Exothermic Phase Changes (Release of Energy) Freezing (Liquid Solid) Condensation (Gas Liquid) Deposition (Gas Solid)

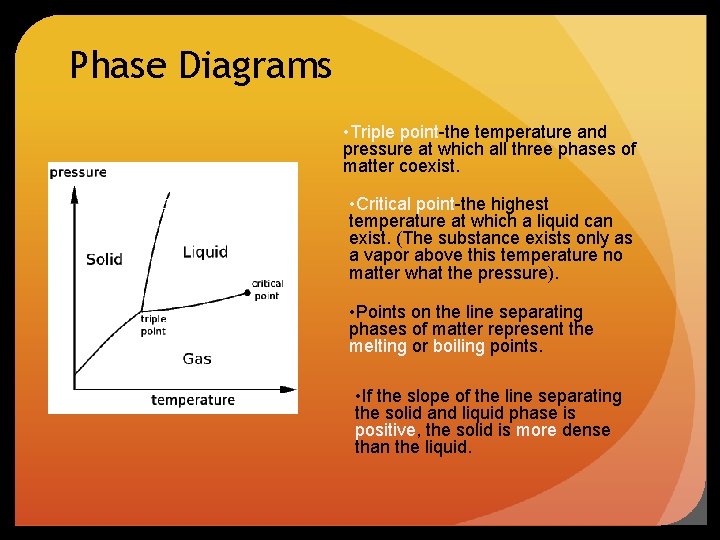
Phase Diagrams • Triple point-the temperature and pressure at which all three phases of matter coexist. • Critical point-the highest temperature at which a liquid can exist. (The substance exists only as a vapor above this temperature no matter what the pressure). • Points on the line separating phases of matter represent the melting or boiling points. • If the slope of the line separating the solid and liquid phase is positive, the solid is more dense than the liquid.

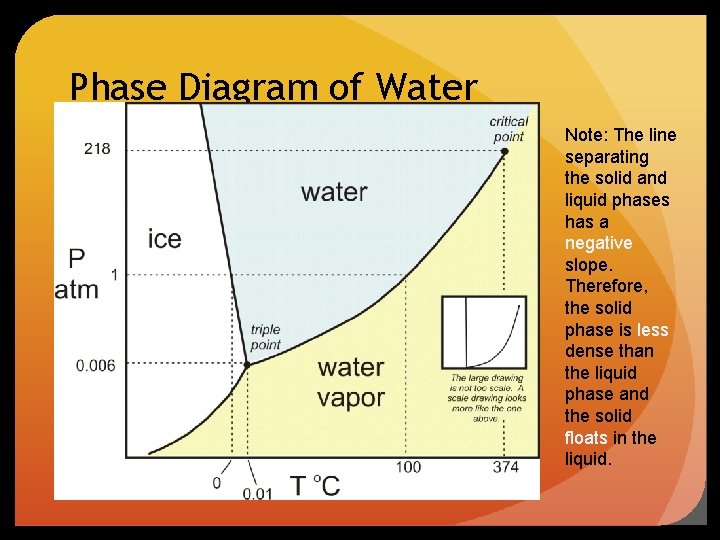
Phase Diagram of Water Note: The line separating the solid and liquid phases has a negative slope. Therefore, the solid phase is less dense than the liquid phase and the solid floats in the liquid.

Practice Interpreting Phase Diagrams For additional interactive practice, visit the following website. http: //www. sciencegeek. net/Chemistry/taters/phasediagram. htm

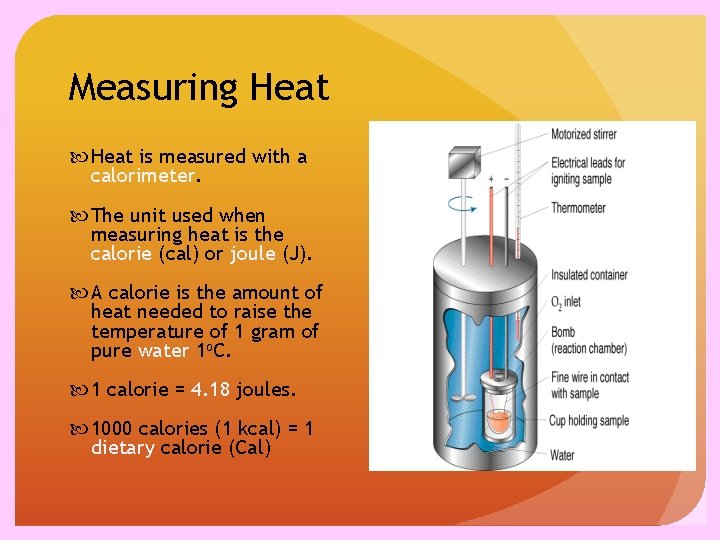
Measuring Heat is measured with a calorimeter. The unit used when measuring heat is the calorie (cal) or joule (J). A calorie is the amount of heat needed to raise the temperature of 1 gram of pure water 1 o. C. 1 calorie = 4. 18 joules. 1000 calories (1 kcal) = 1 dietary calorie (Cal)

Heat Capacity The amounto of heat needed to raise the temperature of an object 1 C is called the heat capacity of the object. The heat capacity depends on the mass and the chemical make-up of the substance. Heat capacity is an extensive property. Specific heat capacity is the amount of heat it takes to o raise the temperature of 1 g of a substance 1 C. Specific heat capacity is an intensive property. Water has a high specific heat capacity compared to other substances-therefore, it requires more heat to change the temperature of a sample of water.

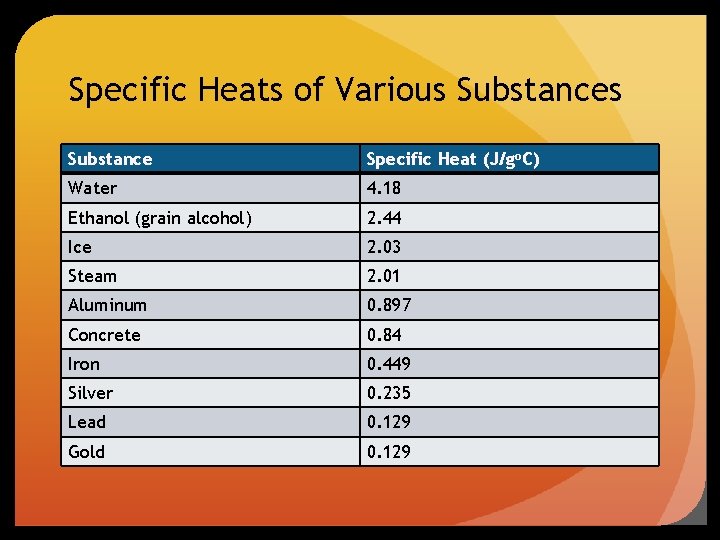
Specific Heats of Various Substance Specific Heat (J/go. C) Water 4. 18 Ethanol (grain alcohol) 2. 44 Ice 2. 03 Steam 2. 01 Aluminum 0. 897 Concrete 0. 84 Iron 0. 449 Silver 0. 235 Lead 0. 129 Gold 0. 129

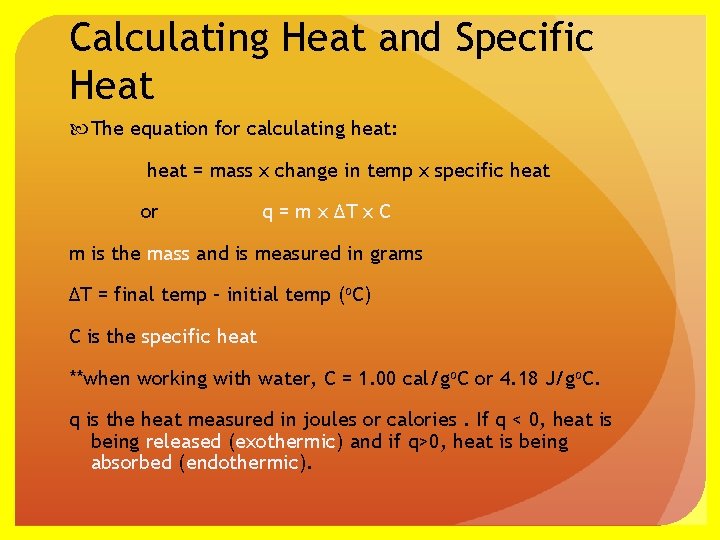
Calculating Heat and Specific Heat The equation for calculating heat: heat = mass x change in temp x specific heat or q = m x ∆T x C m is the mass and is measured in grams ∆T = final temp – initial temp (o. C) C is the specific heat **when working with water, C = 1. 00 cal/go. C or 4. 18 J/go. C. q is the heat measured in joules or calories. If q < 0, heat is being released (exothermic) and if q>0, heat is being absorbed (endothermic).

Practice Problem #1 If the temperature of 34. 4 g of ethanol increases from 25. 5 o. C to 78. 8 o. C, how much heat has been absorbed by the ethanol?

Answer to Practice Problem #1 q=? m= 34. 4 g ∆ T = 78. 8 -25. 5 o. C C=2. 44 J/g o. C q = mx∆Tx. C q = 34. 4 (78. 8 -25. 5)2. 44 q= 34. 4(53. 3)2. 44 q=4, 470 J or 4. 47 k. J

Practice Problem #2 A 155 g sample of an unknown substance was heated from 25. 0 o. C to 40. 0 o. C. In the process, the substance absorbed 5696 J of energy. What is the specific heat of the substance?

Answer to Practice Problem 2 q= 5696 J m= 155 g ∆T = 40. 0 – 25. 0 o. C C=? q = mx∆Tx. C 5696 = 155 (40. 0 -25. 0) x 5696 = 155 (15. 0) x 5696 = 2325 x x = 5696/2325 = 2. 45 J/g o. C

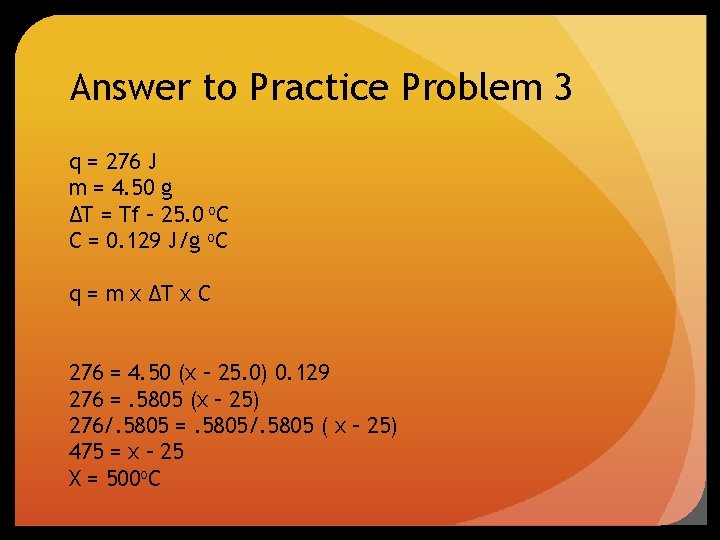
Practice Problem #3 A 4. 50 g nugget of pure gold absorbed 276 J of heat. The initial temperature was 25. 0 o. C. What was the final temperature?

Answer to Practice Problem 3 q = 276 J m = 4. 50 g ∆T = Tf – 25. 0 o. C C = 0. 129 J/g o. C q = m x ∆T x C 276 = 4. 50 (x – 25. 0) 0. 129 276 =. 5805 (x – 25) 276/. 5805 =. 5805/. 5805 ( x – 25) 475 = x – 25 X = 500 o. C

Practice Problem #4 When a 58. 8 g piece of hot alloy is placed in 125 g of cold water in a calorimeter, the temperature of the alloy decreases by 106. 1 o. C, while the temperature of the water increases by 10. 5 o. C. What is the specific heat of the alloy?

Answer to Practice Problem #4 In order to solve this problem, it is important to recognize that the alloy lost heat (temperature decreased) and the water gained heat (temperature increased). Due to the law of conservation of energy, heat lost = heat gained Heat gained by water: q = m x ∆T x C q = 125 x 10. 5 x 4. 18 = 5486 J Heat lost by alloy: q = m x ∆T x C 5486 = 58. 8 x (106. 1) x C C = 0. 879 J/go. C

Latent Heat and Changes in State Latent heat is the energy involved in matter undergoing changes in state. Heat of fusion is the energy required to change 1 g of a substance from a solid to a liquid. The heat of fusion is unique to the substance. q = Hfusion x mass

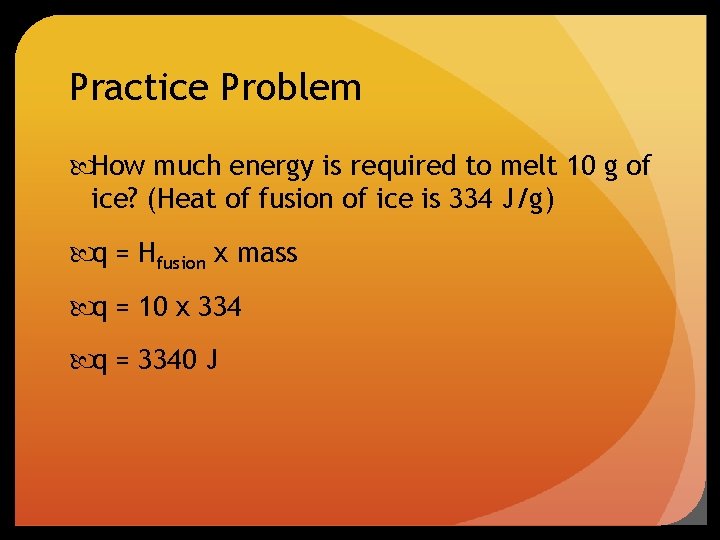
Practice Problem How much energy is required to melt 10 g of ice? (Heat of fusion of ice is 334 J/g) q = Hfusion x mass q = 10 x 334 q = 3340 J

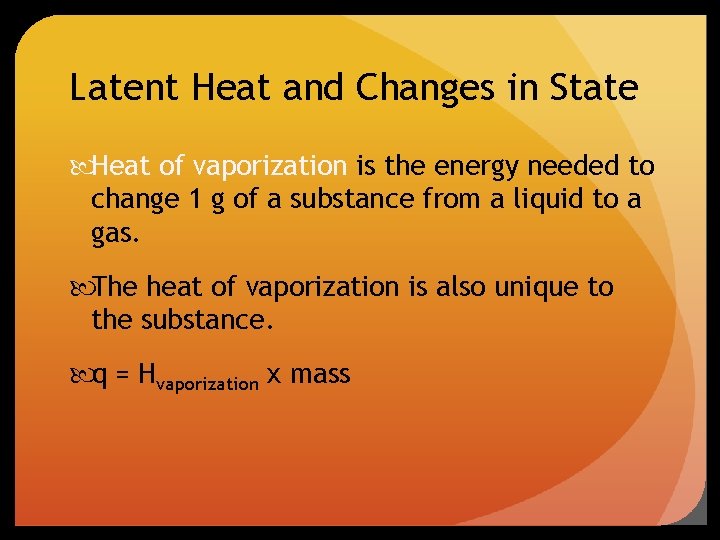
Latent Heat and Changes in State Heat of vaporization is the energy needed to change 1 g of a substance from a liquid to a gas. The heat of vaporization is also unique to the substance. q = Hvaporization x mass

Practice Problem How much energy is required to boil 10 g of water? (Hvap for water is 2260 J/g) q = Hvap x m q = 2260 x 10 q = 22600 J

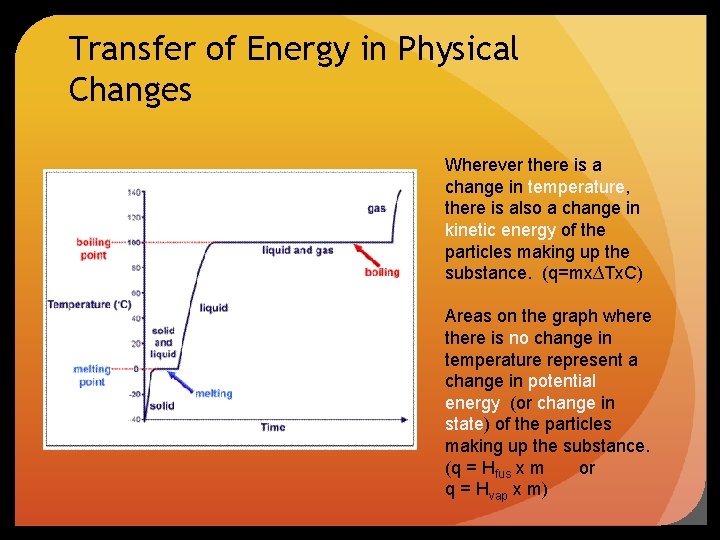
Transfer of Energy in Physical Changes Wherever there is a change in temperature, there is also a change in kinetic energy of the particles making up the substance. (q=mx∆Tx. C) Areas on the graph where there is no change in temperature represent a change in potential energy (or change in state) of the particles making up the substance. (q = Hfus x m or q = Hvap x m)

Practice Problem (Honors Chemistry only) Calculate the energy required to change 10 g of ice at -10 o. C to steam at 110 o. C. Hint: This problem must be solved in five separate steps.

Answer to practice problem Step 1: Convert 10 g ice at -10 o. C to ice at 0 o. C. Answer: q = mx∆Tx. C q = 10 x (0 - -10 o. C) x 2. 03 = 203 J Step 2: Melt the 10 g of ice. Answer: q = mx. Hfus q = 10 x 334 = 3340 J Step 3: Raise the temp of the water from 0 o. C to 100 o. C. Answer: q = mx∆Tx. C q = 10 x (100 -0) x 4. 18 = 4180 J Step 4: Boil the 10 g of water. Answer: q= mx. Hvap q = 10 x 2260 = 22600 J Step 5: Raise the temp of the steam from 100 o. C to 110 o. C. Answer: q = mx∆Tx. C q = 10 x (110 – 100) x 2. 01 = 201 J Step 6: Calculate the total. Answer: 203 + 3340 + 4180 + 22600 + 201 = 30524 = 31000 J

Practice Problem #2 Calculate the energy required to change 1 mole of ice at 0 o. C to steam at 100 o. C.

Answer to Practice Problem #2. Step 1: Melt the ice. Answer: q = mx. Hfus q = 18 g x 334 = 6012 J Step 2: Raise the temp of the water from 0 o. C to 100 o. C. Answer: q = m x ∆T x C q = 18 x (100 -0) x 4. 18 = 7524 J Step 3: Convert the liquid water to steam Answer: q = mx. Hvap q = 18 x 2260 = 40680 J Step 4: Calculate the total. Answer: 6012 + 7524 + 40680 = 54216 J = 54000 J

Practice Interpreting Energy Graphs Illustrating Phase Changes For additional interactive practice, visit the following website. http: //www. sciencegeek. net/Chemistry/taters/phasediagrams 2. htm

Thermochemical Equations An equation that includes the heat change involved in a chemical reaction is called a thermochemical equation. Heat of reaction (∆H) is the heat change for a particular equation. ∆H = Hproducts - Hreactants

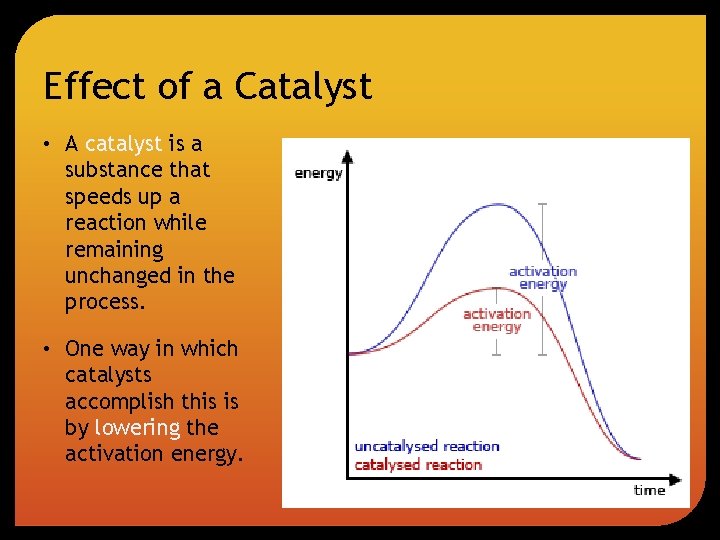
Potential Energy Diagrams All reactions begin with the breaking of the bonds of the reactants (endothermic step). In order for those bonds to break, collisions between the reacting molecules must occur and the reacting molecules must possess a minimum amount of energy (activation energy). During the collision, an unstable intermediate molecule forms (activated complex). The molecules then rearrange and form new bonds resulting in the formation of products (exothermic step).

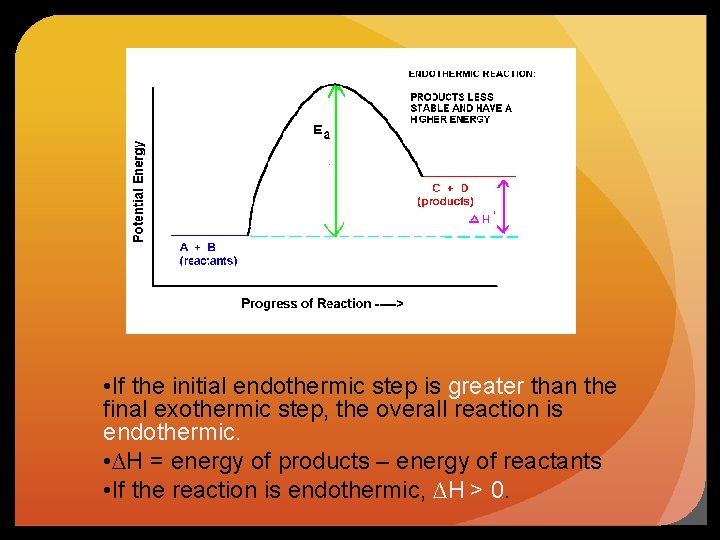
• If the initial endothermic step is greater than the final exothermic step, the overall reaction is endothermic. • ∆H = energy of products – energy of reactants • If the reaction is endothermic, ∆H > 0.

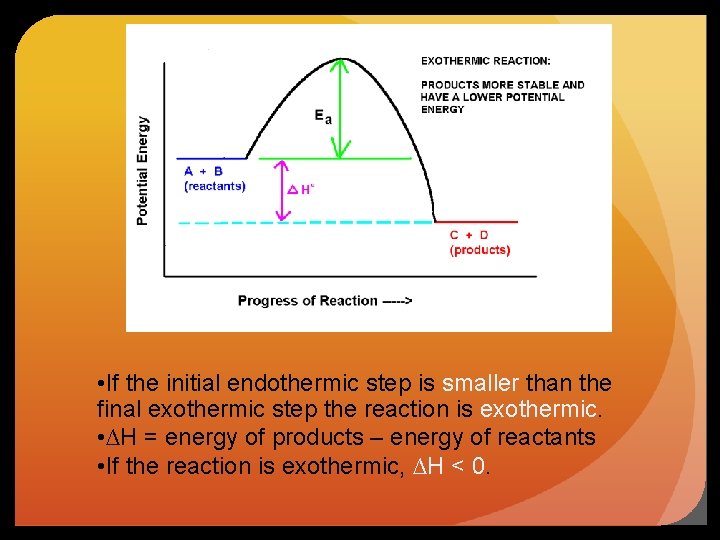
• If the initial endothermic step is smaller than the final exothermic step the reaction is exothermic. • ∆H = energy of products – energy of reactants • If the reaction is exothermic, ∆H < 0.

Effect of a Catalyst • A catalyst is a substance that speeds up a reaction while remaining unchanged in the process. • One way in which catalysts accomplish this is by lowering the activation energy.

Practice Interpreting Potential Energy Graphs For additional interactive practice, visit the following website. http: //www. sciencegeek. net/Chemistry/taters/energydiagram. htm

Writing Thermochemical Equations When 2 mol of solid magnesium combines with 1 mole of oxygen gas, 2 mol of solid magnesium oxide is formed and 1204 k. J of heat is released. Write thermochemical equation for this reaction. 2 Mg(s) + O 2(g) 2 Mg. O(s) + 1204 k. J Note: The energy is written on the reactant side of the equation for endothermic reactions.

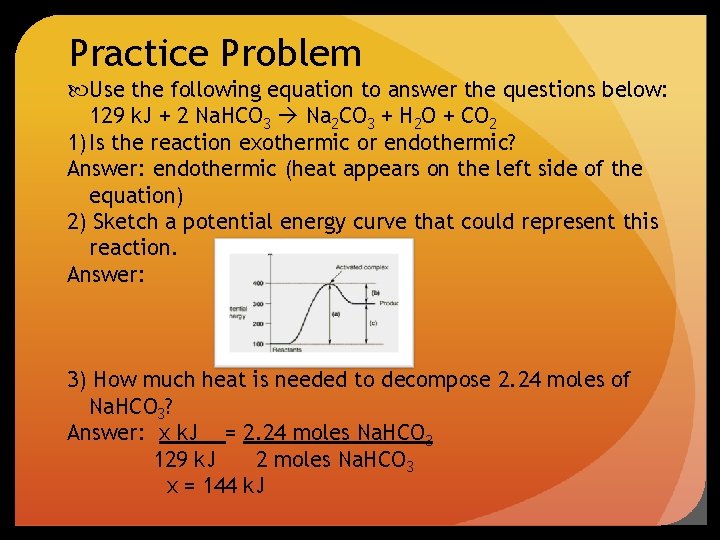
Practice Problem Use the following equation to answer the questions below: 129 k. J + 2 Na. HCO 3 Na 2 CO 3 + H 2 O + CO 2 1) Is the reaction exothermic or endothermic? Answer: endothermic (heat appears on the left side of the equation) 2) Sketch a potential energy curve that could represent this reaction. Answer: 3) How much heat is needed to decompose 2. 24 moles of Na. HCO 3? Answer: x k. J = 2. 24 moles Na. HCO 3 129 k. J 2 moles Na. HCO 3 x = 144 k. J

Review For additional interactive practice, visit the following website. http: //www. sciencegeek. net/Chemistry/taters/Unit 7 Thermochemistry. htm

Spontaneity Any physical or chemical change that, once begun, requires no outside intervention is a spontaneous process. Examples of spontaneous processes include: rusting, burning, etc. If you reverse the direction of a spontaneous reaction, it will result in a reaction that is not spontaneous. The change in enthalpy (heat) is not the only factor that determines spontaneity. Both exothermic and endothermic process can be spontaneous. For example: burning (exo-) and melting (endo-) are both spontaneous processes.

Entropy If both exothermic and endothermic changes can be spontaneous, there must be another factor that helps to determine spontaneity. The second driving force that determines spontaneity is entropy. Entropy (S) is a measure of the disorder of a system. The second law of thermodynamics states that spontaneous processes proceed in such a way as to increase the disorder (entropy) of the universe.

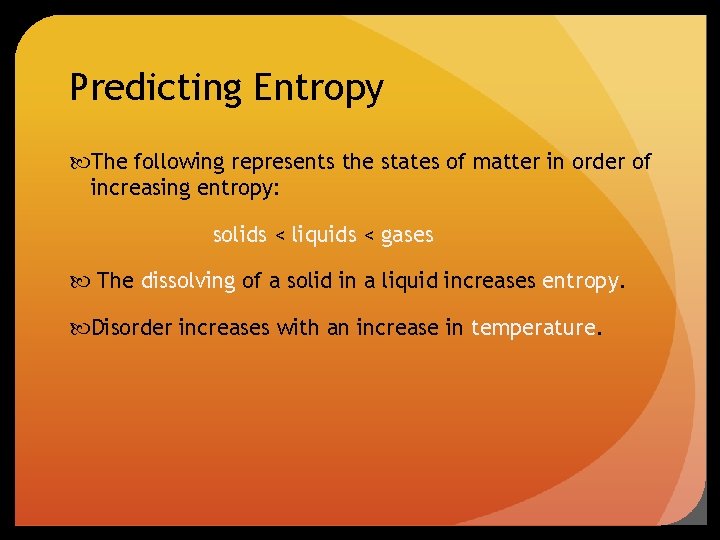
Predicting Entropy The following represents the states of matter in order of increasing entropy: solids < liquids < gases The dissolving of a solid in a liquid increases entropy. Disorder increases with an increase in temperature.

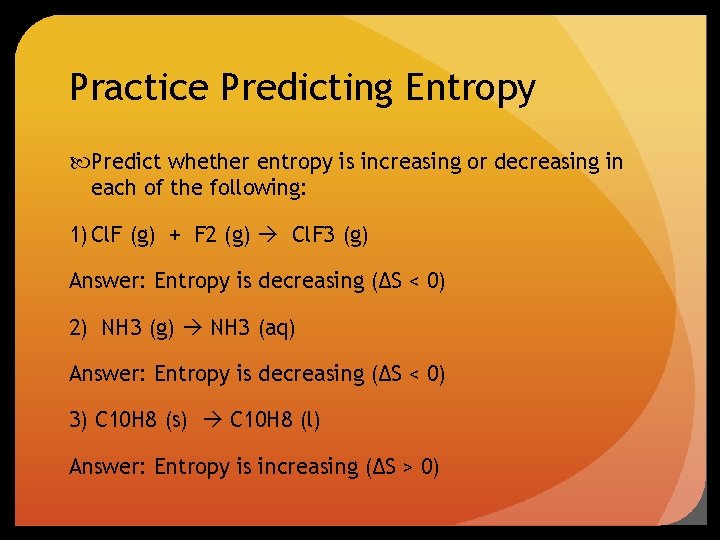
Practice Predicting Entropy Predict whether entropy is increasing or decreasing in each of the following: 1) Cl. F (g) + F 2 (g) Cl. F 3 (g) Answer: Entropy is decreasing (ΔS < 0) 2) NH 3 (g) NH 3 (aq) Answer: Entropy is decreasing (ΔS < 0) 3) C 10 H 8 (s) C 10 H 8 (l) Answer: Entropy is increasing (ΔS > 0)

Putting it Together Exothermic reactions in which the entropy is increasing are always spontaneous (ΔH < 0 and ΔS > 0) Endothermic reactions in which the entropy is decreasing are never spontaneous. (ΔH > 0 and ΔS < 0) Exothermic reactions in which the entropy is decreasing are only spontaneous at low temperatures. (ΔH < 0 and ΔS < 0) Endothermic reactions in which the entropy is increasing are only spontaneous at high temperatures. (ΔH > 0 and ΔS > 0)

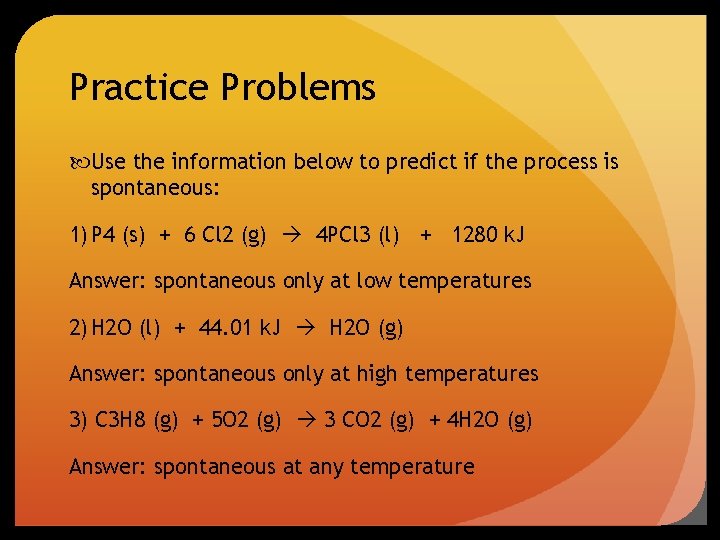
Practice Problems Use the information below to predict if the process is spontaneous: 1) P 4 (s) + 6 Cl 2 (g) 4 PCl 3 (l) + 1280 k. J Answer: spontaneous only at low temperatures 2) H 2 O (l) + 44. 01 k. J H 2 O (g) Answer: spontaneous only at high temperatures 3) C 3 H 8 (g) + 5 O 2 (g) 3 CO 2 (g) + 4 H 2 O (g) Answer: spontaneous at any temperature
 Elizabeth mulroney
Elizabeth mulroney Sample of chemical changes
Sample of chemical changes Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Proximate causation vs ultimate causation
Proximate causation vs ultimate causation Altruistic acts examples
Altruistic acts examples What is delta h
What is delta h Energy changes
Energy changes Energy changes
Energy changes What is thermochemistry
What is thermochemistry Changes aren't permanent but change is
Changes aren't permanent but change is The sudden release of energy stored in rocks causes a(n)
The sudden release of energy stored in rocks causes a(n) What causes the seasons to change? *
What causes the seasons to change? * Which of the following instruments measures air pressure?
Which of the following instruments measures air pressure? Human causes of climate change
Human causes of climate change Section 2 describing energy worksheet answers
Section 2 describing energy worksheet answers Primary energy and secondary energy
Primary energy and secondary energy Primary energy and secondary energy
Primary energy and secondary energy Helmholtz free energy
Helmholtz free energy Renewable energy and energy efficiency partnership
Renewable energy and energy efficiency partnership Kinetic energy examples
Kinetic energy examples Potential energy
Potential energy Mechanical advantage
Mechanical advantage Conservation of mechanical energy
Conservation of mechanical energy Energy forms and energy conversions
Energy forms and energy conversions Compare and contrast physical and chemical changes
Compare and contrast physical and chemical changes Family of orientation
Family of orientation Marriages and families changes choices and constraints
Marriages and families changes choices and constraints Phosphoanhydride
Phosphoanhydride Natural science grade 7 lesson plans term 3
Natural science grade 7 lesson plans term 3 Chapter 15 energy and chemical change
Chapter 15 energy and chemical change Energy and change grade 7
Energy and change grade 7 Absolute change and relative change formula
Absolute change and relative change formula Difference in physical and chemical changes
Difference in physical and chemical changes Supply and demand curve shifts
Supply and demand curve shifts Which is an example of a physical change
Which is an example of a physical change Rocks change due to temperature and pressure change
Rocks change due to temperature and pressure change Whats a chemical change
Whats a chemical change First-order change and second-order change examples
First-order change and second-order change examples Kinetic energy of a spring
Kinetic energy of a spring How to calculate gibbs free energy
How to calculate gibbs free energy Negative free energy change
Negative free energy change Efficiency equation
Efficiency equation How are thermal energy and temperature different
How are thermal energy and temperature different A hairdryer converts ____ energy into ____ energy.
A hairdryer converts ____ energy into ____ energy. Electric energy formula
Electric energy formula How to convert mechanical energy to electrical energy
How to convert mechanical energy to electrical energy Chapter 5 thermal energy answer key
Chapter 5 thermal energy answer key Delta g
Delta g As nutritional energy passes through the food chain it is
As nutritional energy passes through the food chain it is As a roller coaster goes downhill
As a roller coaster goes downhill Chapter 7 energy conservation of energy
Chapter 7 energy conservation of energy Indirect forms of solar energy
Indirect forms of solar energy ________ converts light energy into chemical energy. *
________ converts light energy into chemical energy. * Gravitational potential energy vs kinetic energy
Gravitational potential energy vs kinetic energy Potential energy to chemical energy examples
Potential energy to chemical energy examples Electric potential and electric field equation
Electric potential and electric field equation Physics energy definition
Physics energy definition Light energy examples
Light energy examples Gravitational potential energy vs kinetic energy
Gravitational potential energy vs kinetic energy What is useful energy
What is useful energy