Phase Changes What Causes Phase Changes We have







- Slides: 10

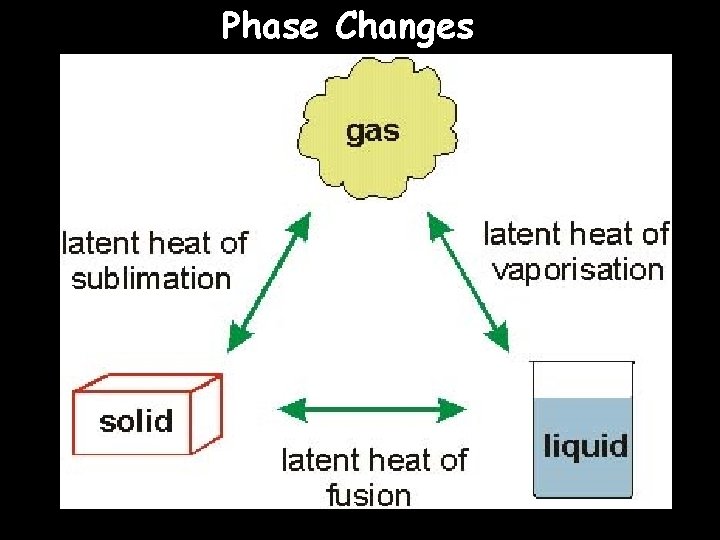
Phase Changes

What Causes Phase Changes? We have seen that the different states of matter are caused by the behavior of the particles in that state of matter. We are really looking at two factors: 1) How closely-packed together the molecules are 2) How quickly (and freely) the molecules move around If we can change the way that molecules move, we can also change how closely-packed together the molecules are We can change the way molecules move by adding energy, -- usually we add thermal (heat) energy

How Thermal Energy Changes Phases Thermal energy is always transferred from one object to another. -- travels from the warmer substance to the cooler substance When enough thermal energy has been transferred, the new substance gains that energy and changes state -- the substance and can also lose thermal energy and change state In terms of thermal energy, gases have the most thermal energy, followed by liquids, and then solids have the least thermal energy *** technically plasma has the most energy***

Solid/Liquid Phase Change A phase change from a solid to a liquid is called melting -- we have all seen melting with snow turning into water -- melting occurs at a specific temperature called the melting point A phase change from a liquid to a solid is called freezing -- we have all seen freezing when we make ice cubes -- freezing occurs at a specific temperature called the freezing point The freezing point and melting point of the same substance ARE EQUAL

Liquid/Gas Phase Change The change from a liquid to a gas is called vaporization -- this is how clouds form -- the temperature at which vaporization occurs is called the boiling point The change from a gas to a liquid is called condensation -- we have seen this when our bathroom mirrors fog up after a hot shower -- the temperature at which condensation occurs is called the condensation point The condensation point and boiling point of the same substance ARE EQUAL

Types of Vaporization There are two types of vaporization based on the method in which a substance turns from a liquid into a gas. In evaporation, only the molecules on the surface of the liquid vaporize -- this is how puddles turn into gas In boiling the molecules inside the liquid as well as on the surface of the liquid vaporize -- when you make pasta, this is how the steam occurs Usually, evaporation is a much slower process than boiling, because more molecules are involved in boiling

Gas/Solid Phase Change When a substance goes from being a solid to being a gas, the process of sublimation occurs -- you cannot actually see sublimation Sublimation happens because the solid substance gains energy so quickly that it does not have time to turn into a liquid -- happens in dry ice (solid carbon dioxide) -- also occurs in moth balls

Why Phase Changes Are Important Phase changes are important because they help identify substances. . . -- every substance has a unique melting/freezing point and a unique boiling/condensation point -- if you watch a substance carefully for when it melts or boils, you can identify that substance based on the temperature at which the phase change occurs -- For example, water melts and freezes at 0°C (32°F) and boils at 100°C (212°F)

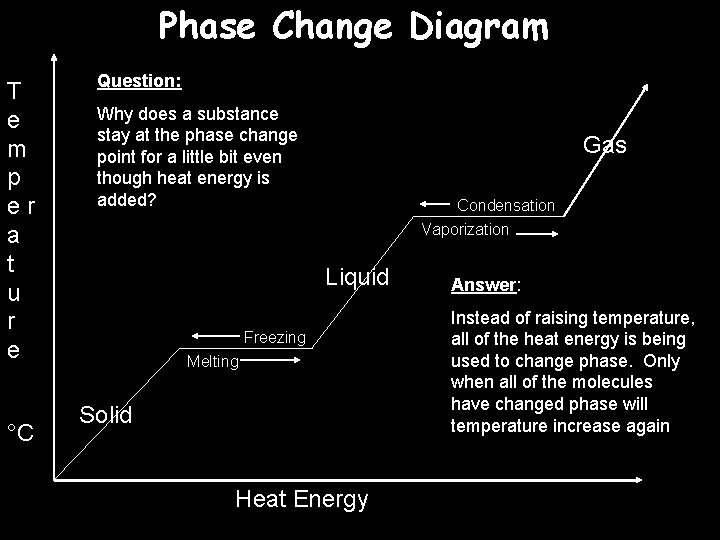
Phase Change Diagram T e m p er a t u r e °C Question: Why does a substance stay at the phase change point for a little bit even though heat energy is added? Gas Condensation Vaporization Liquid Freezing Melting Solid Heat Energy Answer: Instead of raising temperature, all of the heat energy is being used to change phase. Only when all of the molecules have changed phase will temperature increase again

You will have a quiz next class Be prepared to replicate the Phase Change Diagram on the previous slide.