Categories Radiographic contrast agents Local anesthetics Corticosteroids Topical






















- Slides: 30



Categories • Radiographic contrast agents • Local anesthetics • Corticosteroids • Topical antiseptics

RADIOGRAPHIC CONTRAST AGENTS • Iodinated contrast media (ICM) • Clinically used agents have between 180 to 400 mg/ml of iodine

Side Effects Osmotoxic reactions including: • Pain on injection • Hemolysis • Endothelial damage (capillary leak and edema) • Vasodilation (flushing, warmth, hypotension cardiovascular collapse) • Hypervolemia • Direct cardiodepressive effects

PHARMACOLOGY • Ionic ICM: are strictly contraindicated for all applications involving the central nervous system (CNS) and may cause severe or fatal neurotoxic reactions following intrathecal administration.

PHARMACOLOGY • Non-ionic ICM: are the agents of choice for interventional pain procedures due to their lower osmolality and toxicity. • Elimination is by glomerular filtration without reabsorption and there is virtually no metabolism. (normal 2 hr)

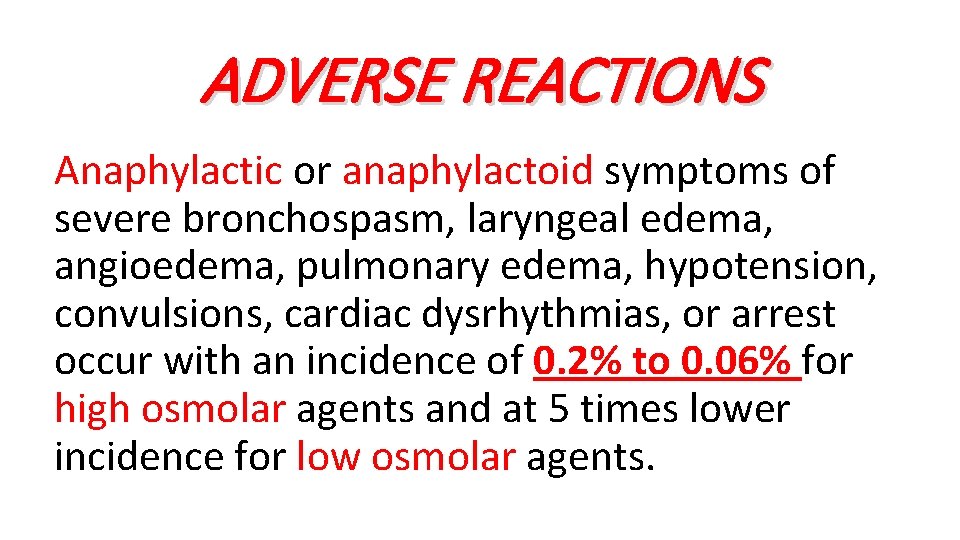
ADVERSE REACTIONS Anaphylactic or anaphylactoid symptoms of severe bronchospasm, laryngeal edema, angioedema, pulmonary edema, hypotension, convulsions, cardiac dysrhythmias, or arrest occur with an incidence of 0. 2% to 0. 06% for high osmolar agents and at 5 times lower incidence for low osmolar agents.

ADVERSE REACTIONS Risks: prior reaction to ICM, and with underlying disease including asthmatics, history of atopy, and advanced heart disease) If suspected these reactions need to be treated with oxygen, intravenous fluids, antihistamines -H 1 and H 2 blockers-, adrenergic drugs –epinephrine-, and corticosteroids, and full cardiopulmonary resuscitation as needed.


PREVENTION STRATEGIES FOR ADVERSE REACTIONS Ionic ICM have 4 times the incidence of these reactions compared to nonionic agents.

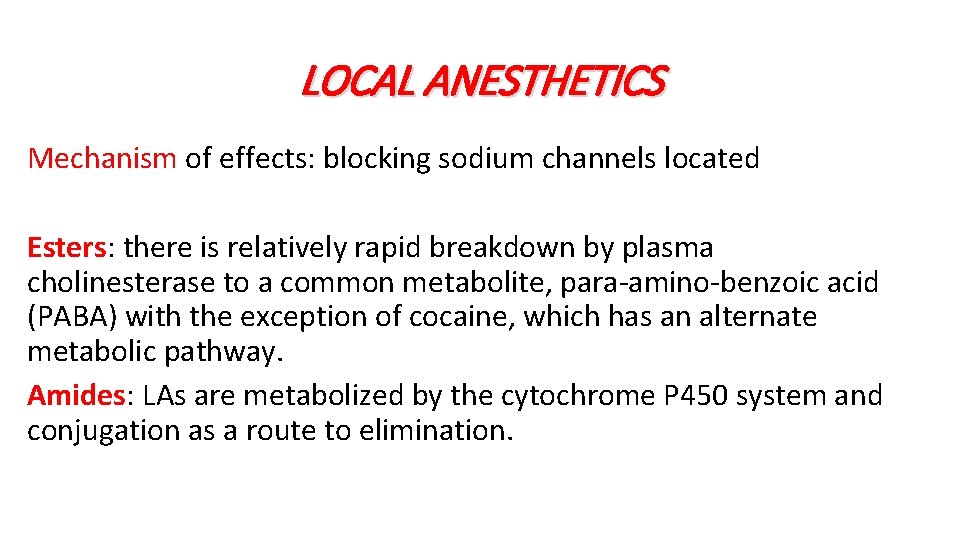
LOCAL ANESTHETICS Mechanism of effects: blocking sodium channels located Esters: there is relatively rapid breakdown by plasma cholinesterase to a common metabolite, para-amino-benzoic acid (PABA) with the exception of cocaine, which has an alternate metabolic pathway. Amides: LAs are metabolized by the cytochrome P 450 system and conjugation as a route to elimination.

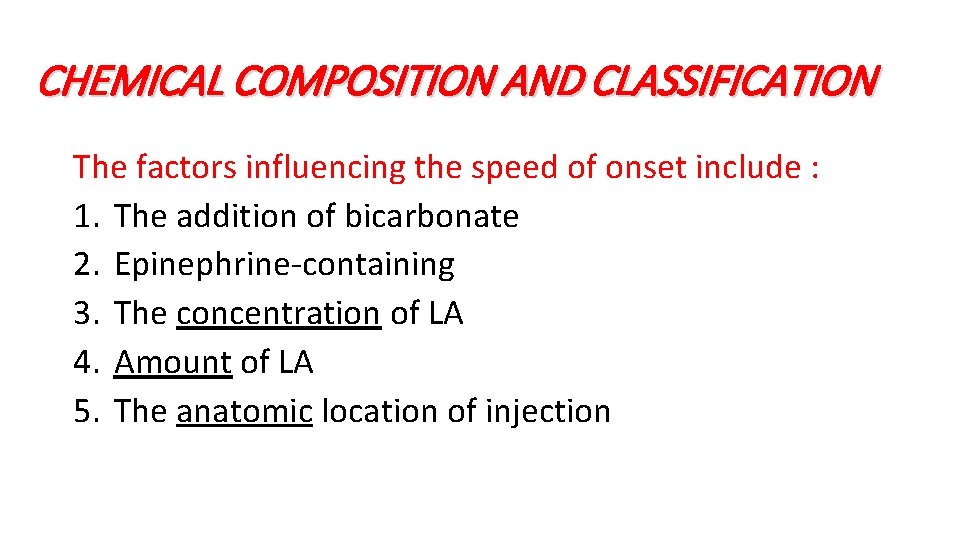
CHEMICAL COMPOSITION AND CLASSIFICATION The factors influencing the speed of onset include : 1. The addition of bicarbonate 2. Epinephrine-containing 3. The concentration of LA 4. Amount of LA 5. The anatomic location of injection

CHEMICAL COMPOSITION AND CLASSIFICATION Ropivacaine, is stated to be 1. More motor sparing than bupivacaine and 2. Less cardiotoxicity at equipotent doses.

CHEMICAL COMPOSITION AND CLASSIFICATION Mixtures of LAs to produce quick onset and/or a prolonged duration have been intermittently advocated: bupivacaine/lidocaine or ropivacaine/lidocaine vs bupivacaine or ropivacaine alone provides a quicker onset but shorter duration of action.

CHEMICAL COMPOSITION AND CLASSIFICATION Studies on epidural use suggest no significant difference when used in combination in terms of speed of onset or change in duration of action.


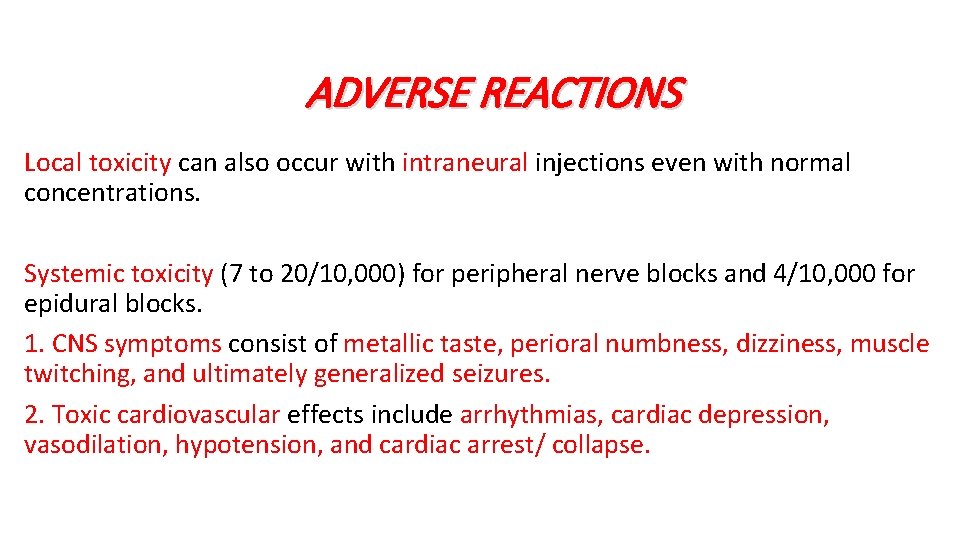
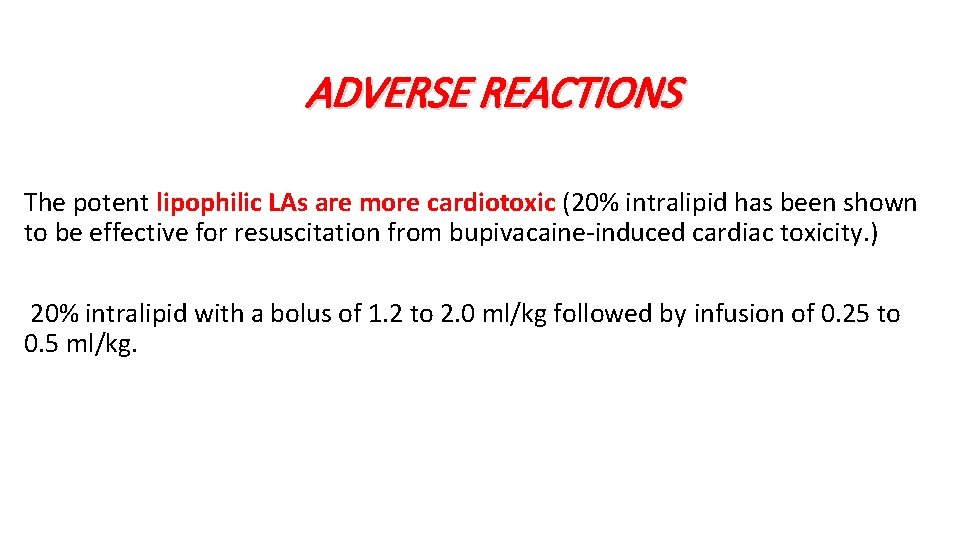
ADVERSE REACTIONS Local toxicity can also occur with intraneural injections even with normal concentrations. Systemic toxicity (7 to 20/10, 000) for peripheral nerve blocks and 4/10, 000 for epidural blocks. 1. CNS symptoms consist of metallic taste, perioral numbness, dizziness, muscle twitching, and ultimately generalized seizures. 2. Toxic cardiovascular effects include arrhythmias, cardiac depression, vasodilation, hypotension, and cardiac arrest/ collapse.

ADVERSE REACTIONS The potent lipophilic LAs are more cardiotoxic (20% intralipid has been shown to be effective for resuscitation from bupivacaine-induced cardiac toxicity. ) 20% intralipid with a bolus of 1. 2 to 2. 0 ml/kg followed by infusion of 0. 25 to 0. 5 ml/kg.

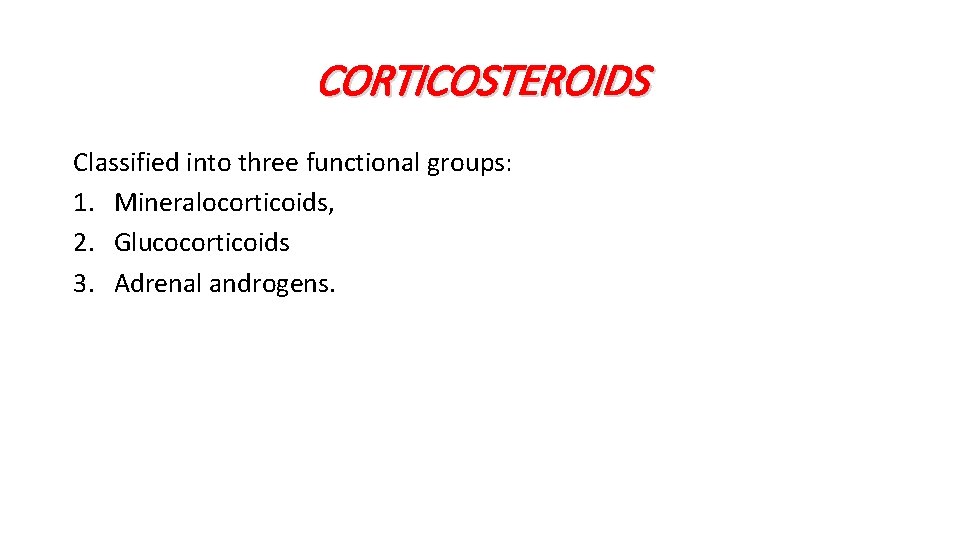
CORTICOSTEROIDS Classified into three functional groups: 1. Mineralocorticoids, 2. Glucocorticoids 3. Adrenal androgens.

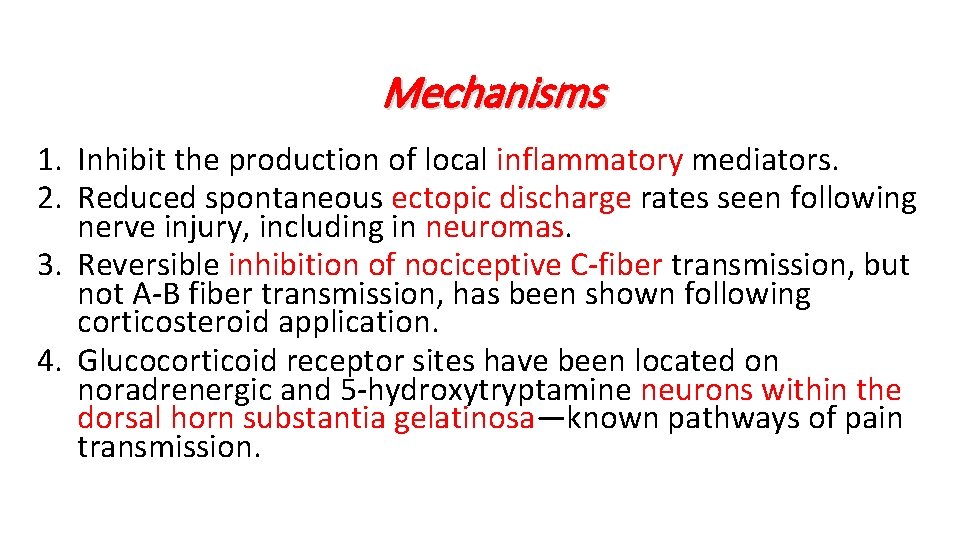
Mechanisms 1. Inhibit the production of local inflammatory mediators. 2. Reduced spontaneous ectopic discharge rates seen following nerve injury, including in neuromas. 3. Reversible inhibition of nociceptive C-fiber transmission, but not A-B fiber transmission, has been shown following corticosteroid application. 4. Glucocorticoid receptor sites have been located on noradrenergic and 5 -hydroxytryptamine neurons within the dorsal horn substantia gelatinosa—known pathways of pain transmission.

CORTICOSTEROIDS Serious adverse events: 1. Transforaminal epidural injections with particulate corticosteroid solutions. An inadvertent injection of a steroid particulate into the artery of Adamkiewicz during thoracic or lumbar transforaminal epidural steroid injection could result in spinal cord ischemia leading to profound lower extremity motor deficits, even paraplegia.

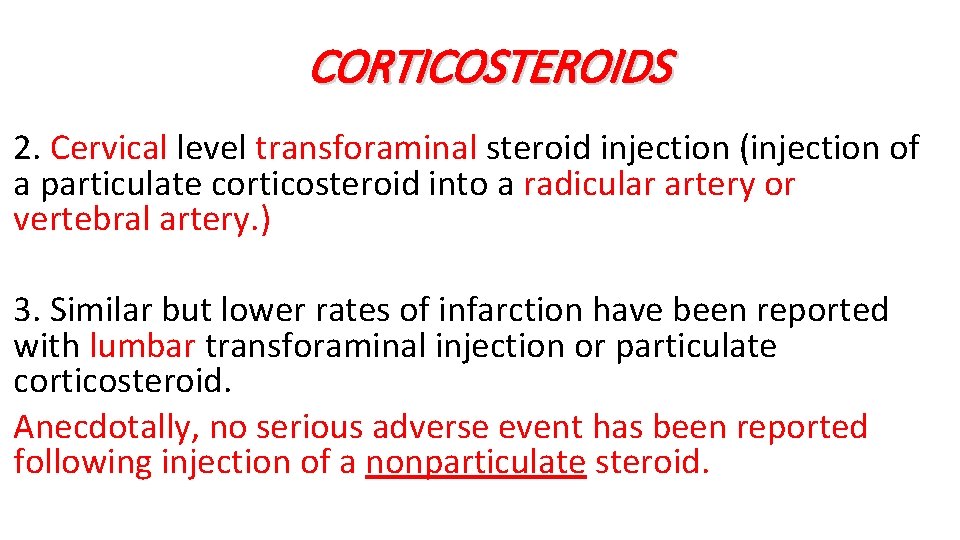
CORTICOSTEROIDS 2. Cervical level transforaminal steroid injection (injection of a particulate corticosteroid into a radicular artery or vertebral artery. ) 3. Similar but lower rates of infarction have been reported with lumbar transforaminal injection or particulate corticosteroid. Anecdotally, no serious adverse event has been reported following injection of a nonparticulate steroid.

ADVERSE REACTIONS 1. Sterile meningitis and arachnoiditis have been reported following intrathecal injection of methylprednisolone, although these may possibly be related to the polyethylene additive of the preparation. 2. Brief euphoric or manic reactions have been reported following high-dose conticosteroid therapy. 3. Although rare, analphylactoid reactions have been reported following intravenous, intramuscular, and soft-tissue conrticosteroid injections. (supportive therapies (i. e. , airway, breathing, circulation, supplemental oxygen), including advanced cardiac life support guidelines when indicated)



SKIN ANTISEPTIC AGENTS Infections relatively rare: epidural abscess, discitis, osteomyelitis, and meningitis. Infection control: selection of the skin antiseptic agent. meticulous hand washing, skin preparation, instrument sterilization, strict aseptic technique, and timely antibiotic prophylaxis when indicated.

SKIN ANTISEPTIC AGENTS Products containing iodophors and chlorhexidine gluconate. Additionally, agents are further classified as either aqueous-based or alcohol-based solutions. 1. Aqueous-based iodophors, such as povidone-iodine, can be safely used on mucous membrane surfaces. 2. Alcohol-based solutions offer a quicker onset and often more sustained antimicrobial activity.


 Nephrotic syndrome in child
Nephrotic syndrome in child Anesthesia
Anesthesia Local anesthesia classification
Local anesthesia classification Contraindications of spinal anesthesia
Contraindications of spinal anesthesia Site:slidetodoc.com
Site:slidetodoc.com Baricity of local anesthetics
Baricity of local anesthetics Topical agent definition
Topical agent definition Topical agents are
Topical agents are Define topical agents with example
Define topical agents with example Nitrous oxide properties
Nitrous oxide properties Barbiturates moa
Barbiturates moa Oil gas partition coefficient inhaled anesthetics
Oil gas partition coefficient inhaled anesthetics Mac anesthesia minimum alveolar concentration
Mac anesthesia minimum alveolar concentration Labial mounting method
Labial mounting method Left lateral position
Left lateral position Radiographic film
Radiographic film Geometric unsharpness of margins in radiographic image:
Geometric unsharpness of margins in radiographic image: Outer canthus
Outer canthus Guide shoe marks artifacts
Guide shoe marks artifacts 8:1 grid
8:1 grid Transhepatic cholangiography
Transhepatic cholangiography Sid vs oid
Sid vs oid Reverse towne projection
Reverse towne projection Orbitomeatal line
Orbitomeatal line Darkroom entrance
Darkroom entrance Notching of the crestal lamina dura
Notching of the crestal lamina dura Radiographic films
Radiographic films Double contrast vs single contrast
Double contrast vs single contrast Strategies for competing in international markets
Strategies for competing in international markets George‚äôs gyros
George‚äôs gyros A think local act local multicountry type of strategy
A think local act local multicountry type of strategy