Case Presentation Ted D Williams Pharm D RPH
























- Slides: 35

Case Presentation Ted D. Williams, Pharm. D, RPH Syracuse VAMC

Demographics • • • SM 57 years old male Date of Birth: OCT 6, 1951 Sex: MALE Wt. unavailable Ht. 74

Chief Complaint • An NF for rosiglitazone was submitted to pharmacy 8/27/09 • Patient had a recent ER visits with a diagnosis of renal impairment , BUN of 28 and a creatinine of 1. 6. • Patient was discharged from St. Joseph’s with a new Avandia (rosiglitazone) prescription. • “Patient cannot take glyburide as it causes hypoglycemia episodes”

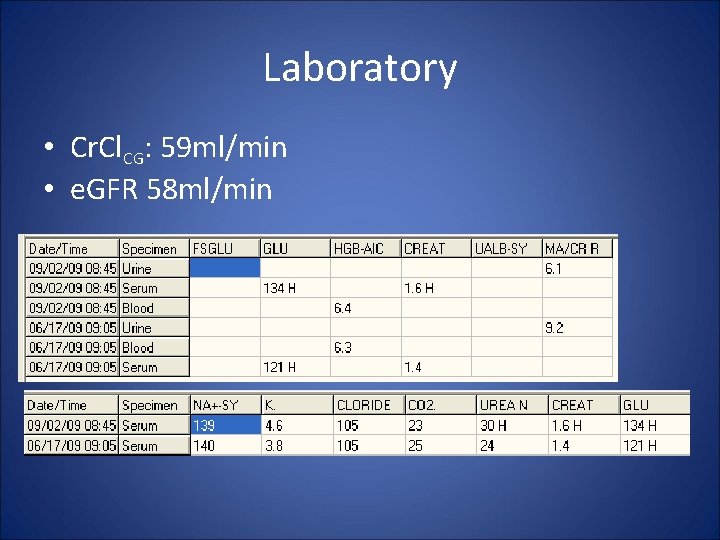
Laboratory • Cr. Cl. CG: 59 ml/min • e. GFR 58 ml/min

Past Medical History • • • Diabetes Mellitus Hypertension, Essential Hyperlipidemia Coronary Artery Disease Allergic rhinitis Osteoarthritis • Diagnosis dates are not available locally or through remote VISTA data

Past Rx History • Active – Albuterol MDI PRN (no dx) – Aspirin 81 mg EC PO daily – Cetirizine 10 mg PO daily – HCTZ/Lisinopril 25 mg/20 mg daily – Ibuprofen 800 mg PO TID PRN – Simvastatin 20 mg PO QHS • Inactive – Metformin 1000 mg PO BID (D/C 8/27/09) – Glipizide 5 mg PO daily (D/C 8/27/09)

Additional Information • Very little information is available on this patient – Eight progress notes locally – No scanned documents from hospitalization • A progress note on 5/14/2009 indicated that the patient has been taking metformin and glipizide since 2005 • ADR – Codeine N/V, Syncope

Treatment Options

Rosiglitazone • MOA – PPAR- Agonist • Increase peripheral tissue insulin uptake • Reduce plaque formation(? ) • Side effects – Edema (15%) • Contraindicated in heart failure – – Weight Gain (ADOPT Trial 3. 5 kg) Bone Fractures in women Increased cardiovascular risk Case reports of macular edema • Non-Formulary

Meformin • Why Metformin – Morbidity & Mortality – Weight Loss – Cost – PO administration – No hypoglycemia • Why Not Metformin – GI Upset – Lactic Acidosis (LA)…

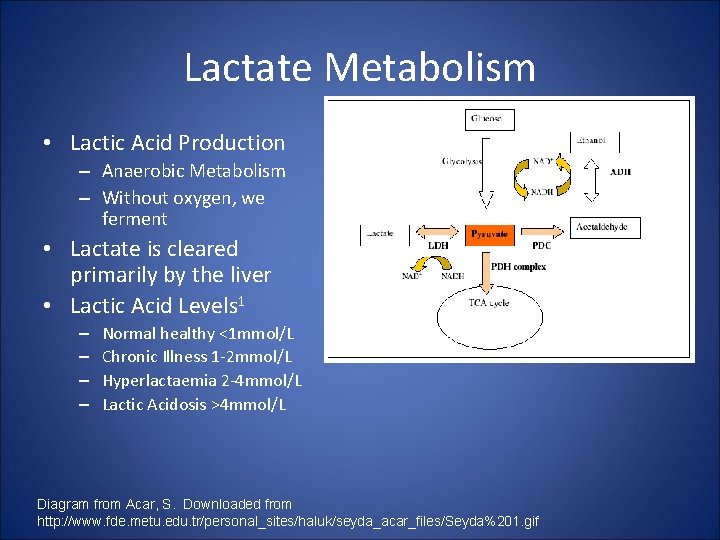
Lactate Metabolism • Lactic Acid Production – Anaerobic Metabolism – Without oxygen, we ferment • Lactate is cleared primarily by the liver • Lactic Acid Levels 1 – – Normal healthy <1 mmol/L Chronic Illness 1 -2 mmol/L Hyperlactaemia 2 -4 mmol/L Lactic Acidosis >4 mmol/L Diagram from Acar, S. Downloaded from http: //www. fde. metu. edu. tr/personal_sites/haluk/seyda_acar_files/Seyda%201. gif

Lactic Acidosis Risk Factors – Hypoxic • Promoting Lactic Acid Production – Resulting in Type A Lactic Acidosis – Ischemia & reduced tissue perfusion • Shock • ACS • Reduced Cardiac Output (HF) – Respiratory Failure • COPD • Asthma Nicks, BA, Mc. Ginnis, HD, Borron, SW, Megarbane, B. Lactic Acidosis. e. Medicine Updated 05/08/2009. Downloaded from http: //emedicine. medscape. com/article/768159 -overview

Lactic Acidosis Risk Factors – Non-Hypoxic • Impaired Clearance – Resulting in Type B Lactic Acidosis • Renal Dysfunction • Acid Base Disturbance • Liver Dysfunction – Inadequate lactate clearance • Malignancies • Drug Induced Nicks, BA, Mc. Ginnis, HD, Borron, SW, Megarbane, B. Lactic Acidosis. e. Medicine Updated 05/08/2009. Downloaded from http: //emedicine. medscape. com/article/768159 -overview

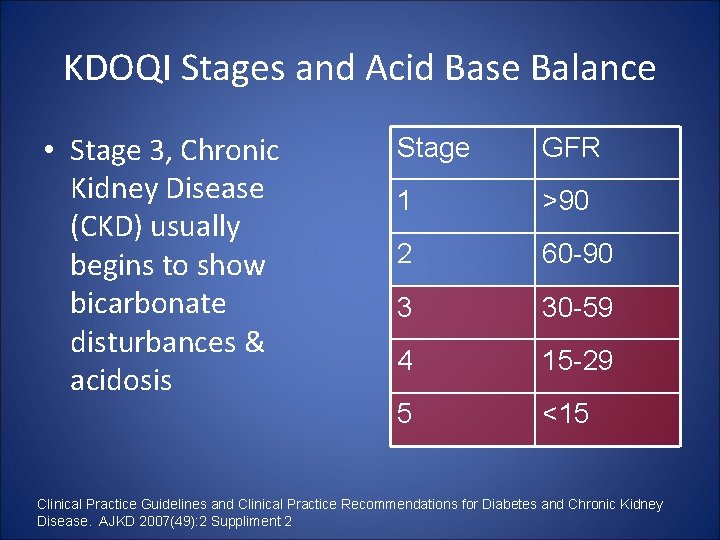
KDOQI Stages and Acid Base Balance • Stage 3, Chronic Kidney Disease (CKD) usually begins to show bicarbonate disturbances & acidosis Stage GFR 1 >90 2 60 -90 3 30 -59 4 15 -29 5 <15 Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease. AJKD 2007(49): 2 Suppliment 2

Prevalence of LA • Estimates vary between 1 -9 cases per 100, 000 patient years in treated diabetics (metformin and non-metformin)1 1. Salpeter, SR, Greyber, E, Pasternak, GA, Salpeter EE. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus (Review). Cochrane Collaboration 2006 (updated September 2007, re-published 2009)

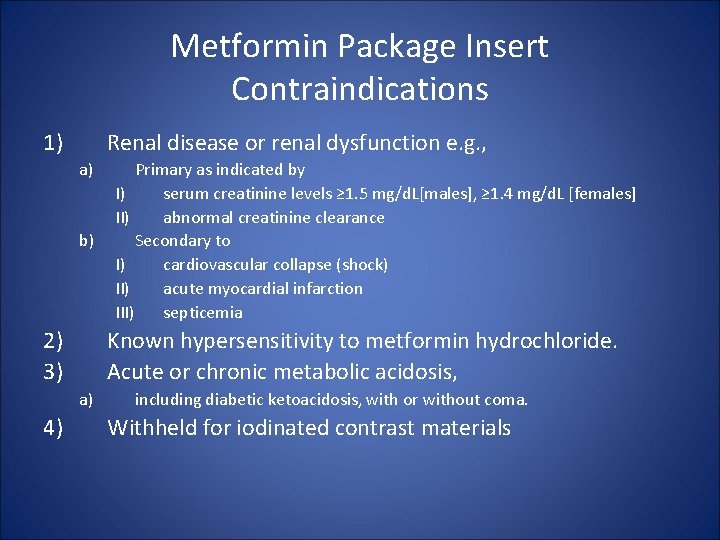
Metformin Package Insert Contraindications 1) Renal disease or renal dysfunction e. g. , a) b) 2) 3) Known hypersensitivity to metformin hydrochloride. Acute or chronic metabolic acidosis, a) 4) Primary as indicated by I) serum creatinine levels ≥ 1. 5 mg/d. L[males], ≥ 1. 4 mg/d. L [females] II) abnormal creatinine clearance Secondary to I) cardiovascular collapse (shock) II) acute myocardial infarction III) septicemia including diabetic ketoacidosis, with or without coma. Withheld for iodinated contrast materials

Metformin Package Insert Black Box • LA fatal in 50% of cases • Unstable HF at risk of LA • Elderly – Careful monitoring of renal function – Over 80, do not initiated UNLESS measured Cr. Cl indicates nonreduced renal function • i. e. don’t assume adequate renal function • Withhold for – hypoxia – dehydration – sepsis • Avoided in hepatic disease • Avoid excessive drinking, potentiate metformin's lactate production

Phenformin vs Metformin • Biguanides inhibit gluconeogenesis from lactate – Phenformin more potent, affects hepatic and peripheral lactate production – Metformin is not believed to affect peripheral lactate production • Phenformin was withdrawn due to 40 -64 cases of LA per 100, 000 patient years

Metformin Kinetics Elderly subjects, mean age 71 years (range 65 -81 years)

ADA/ EASD Consensus Recommendations • Reference – Nathan, DM, Buse, JB, Davidson, MB, Ferrannini, E, Holman, RR, Sherwin, R, Zinman, B. Medical management of hyperglycaemia in type 2 diabetes mellitus: a consensus algorithm for the initiation and adjustment of therapy: A consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes Diabetologia (2009) 52: 17– 30 • “Renal dysfunction is considered a contraindication to metformin use because it may increase the risk of lactic acidosis… However, recent studies have suggested that metformin is safe unless the estimated glomerular filtration rate falls to <30 ml/min [52]. ”

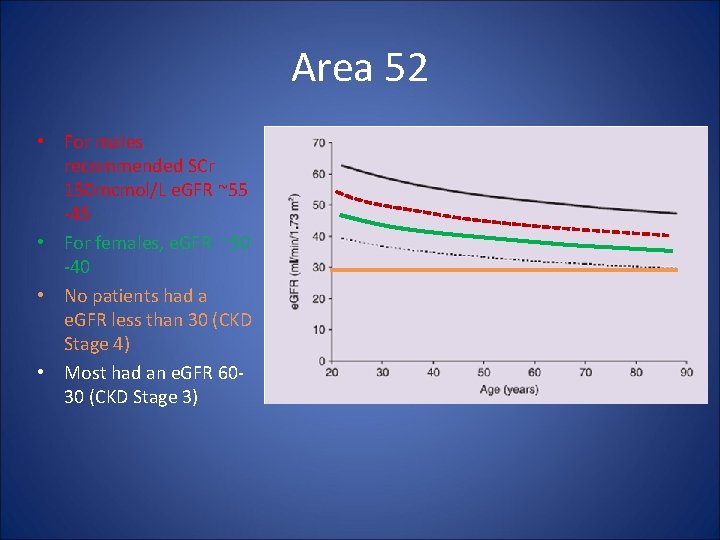
Area 52 • Reference – Shaw, JS, Wilmot, RL, Kilpatrick, ES. Establishing pragmatic estimated GFR thresholds to guide metformin prescribing. Diabetic Medicine 2007: 24; 1160– 1163. • Study Objective – establish “pragmatic” e. GFR cut-offs for metformin based on recommended serum creatinine (SCr) • Design – – Retrospective chart review n=12, 482 patients (6, 712 males, 5, 770 females) Median age 67 years Compare serum creatinine (SCr) cutoffs with e. GFR • 130μmol/L females (1. 47 mg/d. L) • 150 μmol/L males (1. 7 mg/d. L)

Area 52 • For males recommended SCr 150 mcmol/L e. GFR ~55 -45 • For females, e. GFR ~50 -40 • No patients had a e. GFR less than 30 (CKD Stage 4) • Most had an e. GFR 6030 (CKD Stage 3)

Exit 52 • Author’s Conclusions – Stage 4 CKD Absolute Contraindication – Stage 3 CKD Relative Contraindication, based on other risk factors • Safety – No intervention was performed in this study to validate the safety – The authors did not report if there were any documented cases of LA in their patient population – Authors cited Cochrane review (2006) for safety data

Cochrane Review • Salpeter, SR, Greyber, E, Pasternak, GA, Salpeter EE. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus (Review). Cochrane Collaboration 2006 (updated September 2007, re-published 2009) • Pooled data from 274 trials of metformin – 59, 321 patient years for metformin – 51, 627 patient years for non-metformin

Cochrane Review • No reported incidence of lactic acidosis in either group – Poisson statistics determined upper limit of the incidence of lactic acidosis 5. 1 in metformin, 5. 9 in non-metformin • Exclusions to studies – – – SCr >1. 5 mg/d. L (55%) Cardiovascular disease (45%) Liver disease (52%) Pulmonary disease (15%) Age >65 (14%) • No significant change in lactate levels between metformin and non-metformin groups in the studies which reported lactate levels

Safety Above 1. 5 mg/d. L • Rachmani, R, Slavachevski, I, Zohar, L, Bat-Sheva, Z, Kedar, Y, Mordachai, R. Metformin in patients with type 2 diabetes mellitus: reconsideration of traditional contraindications. European Journal of Internal Medicine 2002; 13: 428 -433) • Prospective study of patients admitted to a single facility already on metformin – – – n=393 Randomized, non-blinded Follow up for 4 years Mean SCr 1. 8 Meanu Albumin to Creatinine Ration (ACR) 46+/-10 • All patients had at least one additional risk factor for LA – – CAD 68% HF 24% COPD 23% Liver Disease 13% (Excluding Cirrhosis) • No incidence of LA in either group

Prediction of Metformin-Induced LA • Seidowsky, A, Saad, N, Houdret, N, Fourrier, F. Metformin-associated lactic acidosis: A prognostic and therapeutic study. Critical Care Medicine 2009; 30: 2191 -2196 • Ten year retrospective study – ICU patients for metformin-associated LA – n=42 • Group 1 (Intentional overdose) n=13 • Group 2 (All others) n=29

Prediction of Metformin-Induced LA • Patient Characteristics – 50% Shock – 45% Mechanical Ventilation – 75% Acute Renal Failure (ARF) • Group 2 – Admission reason circulatory or respiratory failure with multi-organ dysfunction – Mortality rate 48% • Predictors of survival – Age, Lactate, p. H, organ dysfunction, PT activity – Metformin levels not associated with mortality

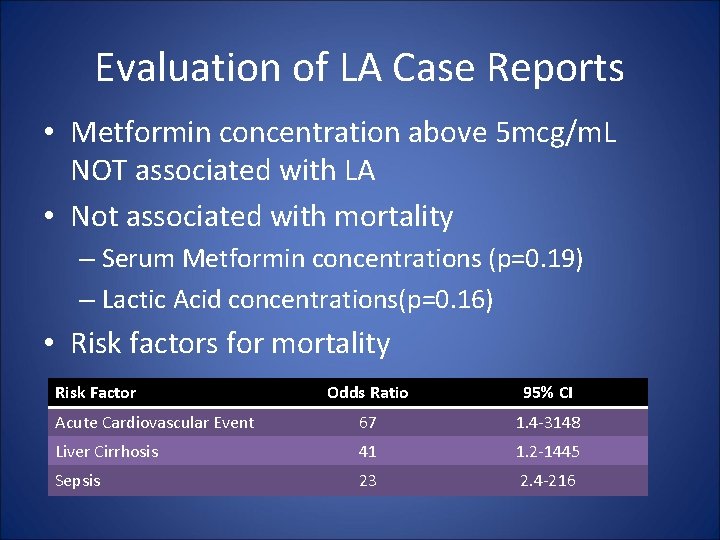
Evaluation of LA Case Reports • Stades, AME, Heikens, JT, Erkelens, SW, Holleman, F, Hoekstra, JBL. Metformin and lactic acidosis: Cause or coincidence? A review of case reports. • Literature search from 1959 -1999 identified 80 published case reports • 47 cases met inclusion criteria for review – One case had no additional risk factors – Three cases had two or more additional risk factors – 44 cases had one additional risk factor

Evaluation of LA Case Reports • Metformin concentration above 5 mcg/m. L NOT associated with LA • Not associated with mortality – Serum Metformin concentrations (p=0. 19) – Lactic Acid concentrations(p=0. 16) • Risk factors for mortality Risk Factor Odds Ratio 95% CI Acute Cardiovascular Event 67 1. 4 -3148 Liver Cirrhosis 41 1. 2 -1445 Sepsis 23 2. 4 -216

Metformin & Lactic Acidosis Summary 1. 2. Although renal impairment can increase metformin serum concentrations, there has been no evidence to show an association between metformin use or serum concentrations and the incidence of lactic acidosis Kinetic and epidemiological data suggests that metformin can be used safely in patients with diminished renal function – e. GFR is preferred over serum creatinine – e. GFR 30 -60 (KDOQI Stage 3) is a relative contraindication • suggest dose NTE 500 mg BID • This is more aggressive than FDA contraindications allows 3. – e. GFR <30 absolute contraindication (KDOQI Stage 3) Patients with multiple risk factors for lactic acidosis should be evaluated carefully, even if their renal function is acceptable – – Sepsis Congestive Heart Failure Severe Respiratory Disease Hepatic Disease

Back to our case…

Case Assessment • Patient had metformin held due to elevated creatinine during hospitalization, which is in accordance with the package insert, guidelines, and accepted practice • Diabetes has been well controlled on metformin and glyburide with A 1 C at goal (6. 4%) • Patient’s SCr of 1. 6 mg/d. L is a contraindication according to the package insert • e. GFR of 58 is a relative contraindication according to ADA Consensus Guidelines – No diagnosis of hypoxic LA risk factors – Stage 3 KD with e. GFR 58 is a risk factor for acidosis, but normal bicarbonate levels of 23 and 25

Case Plan • Medications – Recommend resume metformin at a reduced dose of 500 mg BID • Titrate dose based on response and any future renal function changes – Recommend resume glipizide 5 mg PO daily • If A 1 C not a goal, consider increasing glipizide to 5 mg PO BID • Monitoring – Reassess A 1 C in 3 months – Renal function: SCr, BUN, e. GFR, bicarbonate • NF for rosiglitazone not approved

Post Hoc Notes • In November 2009, AJHP published a similar review of the literature – Philbrick, et al. Metformin use in renal dysfunction: Is a serum creatinine threshold appropriate? AJHP 2009: 66: 2017 -2022